Vapor-Phase Essential Oils as Antifungal Agents against Penicillium olsonii Causing Postharvest Cherry Tomato Rot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Fruits
2.3. EOs
2.4. Antifungal Activity of Volatile EOs In Vitro
2.5. Minimal Inhibitory Concentration Determination
2.6. EO Antifungal Activity on Cherry Tomatoes
2.7. Effect of EOs on Spore Germination
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. Antifungal Effect of EOs In Vitro
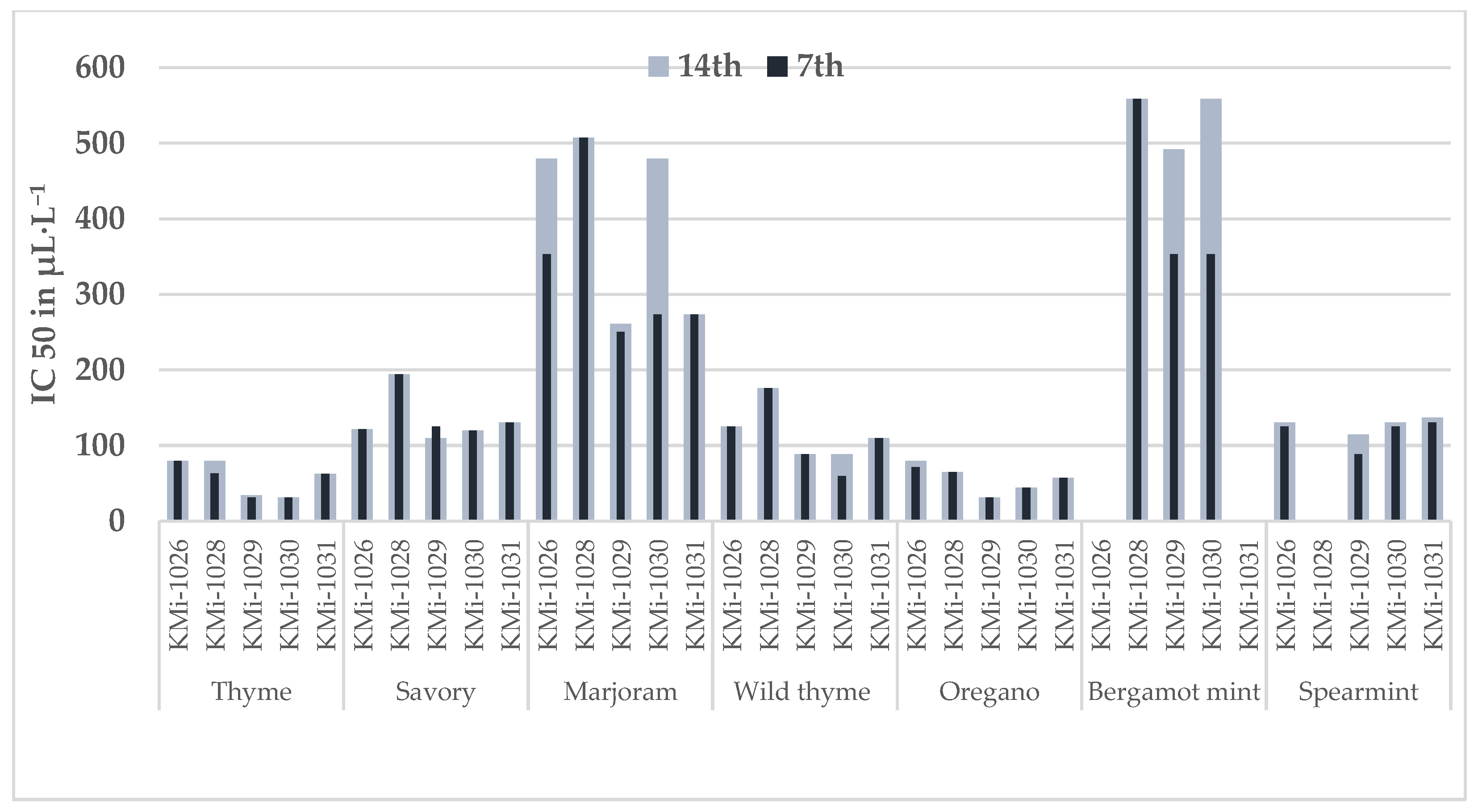
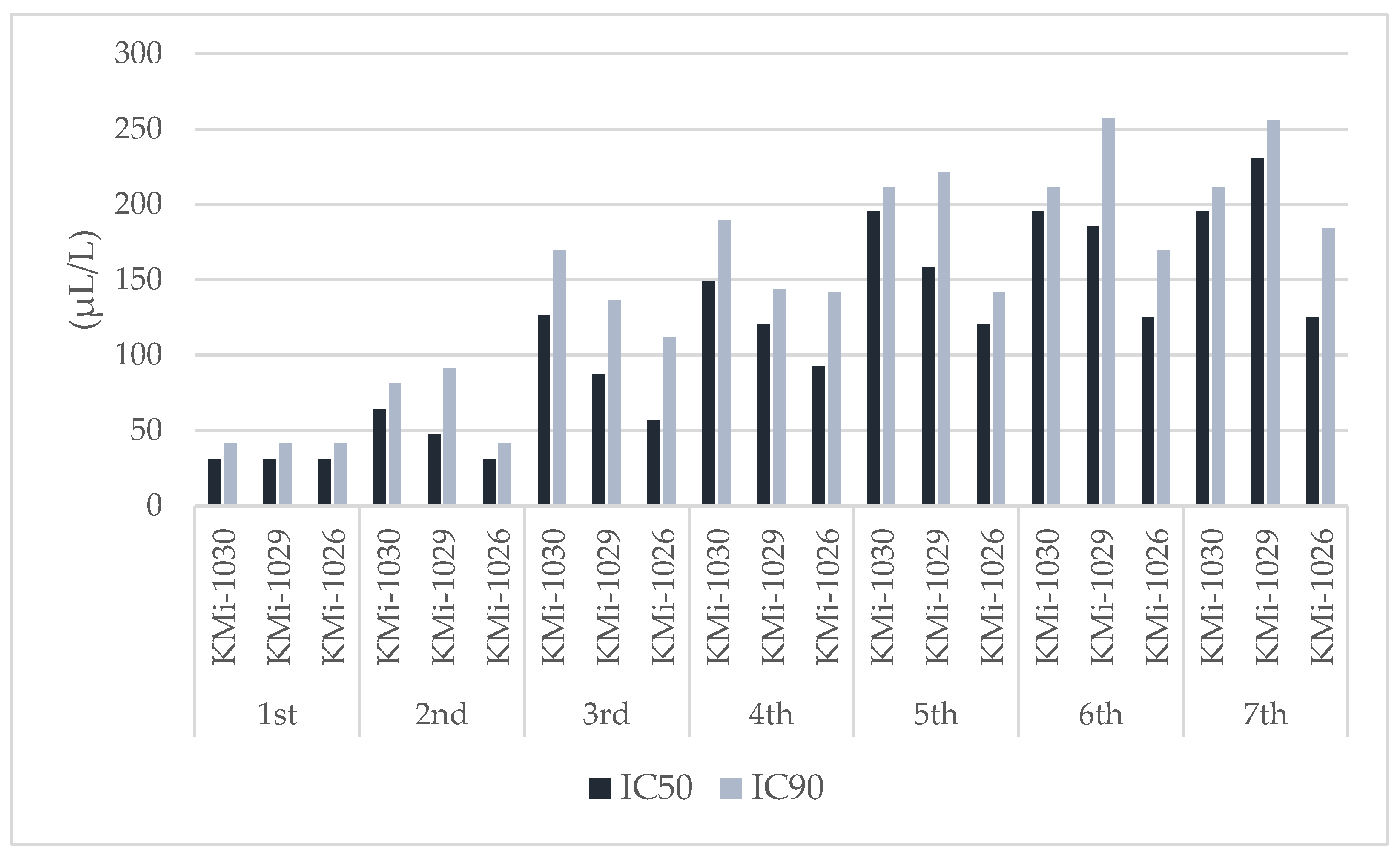
3.2. Minimal Inhibitory Concentration
3.3. EO Antifungal Activity on Cherry Tomatoes
3.4. Effect of EOs on Spore Germination
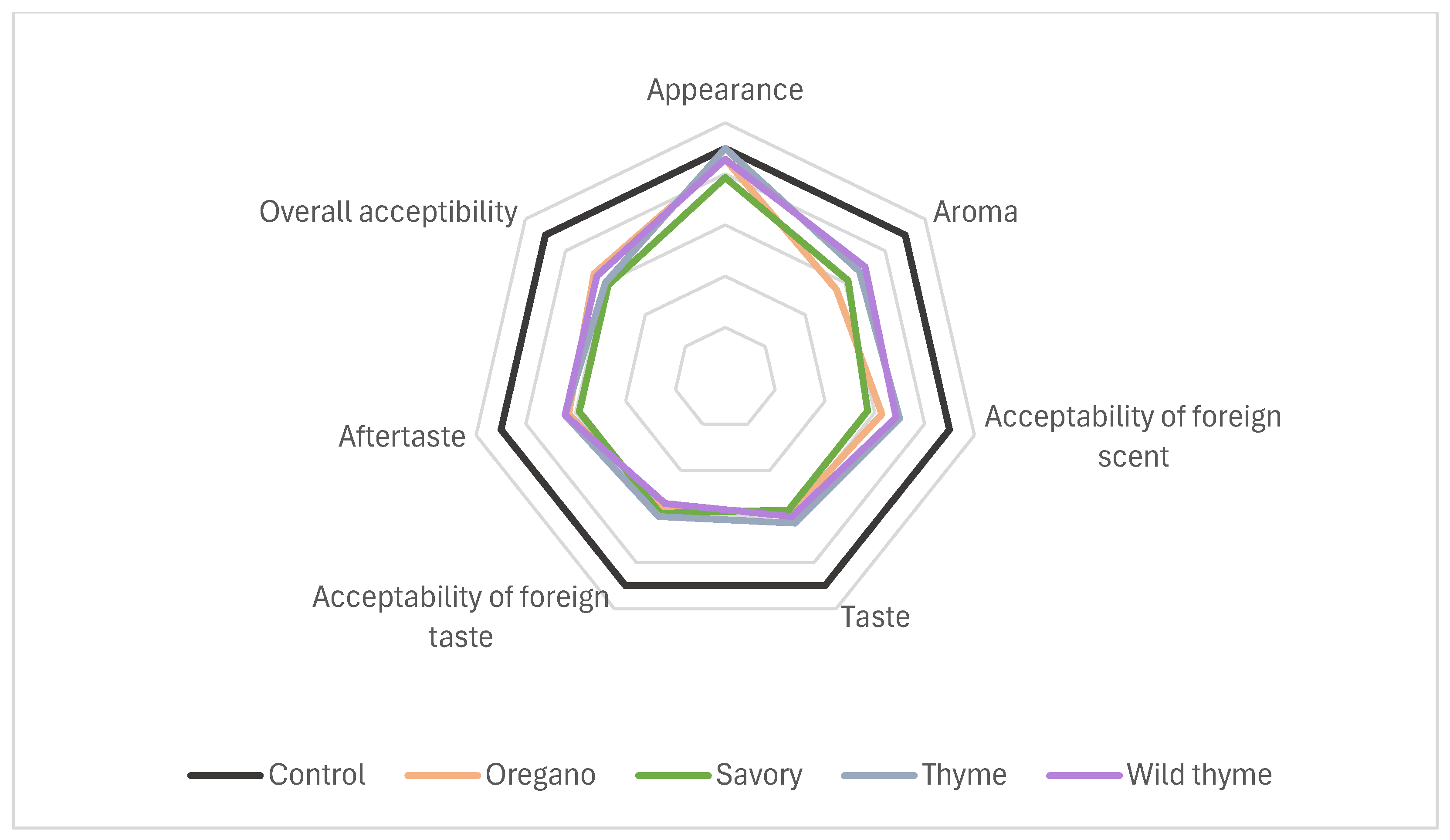
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appleton, K.M.; Boxall, L.R.; Adenuga-Ajayi, O.; Seyar, D.F. Does fruit and vegetable consumption impact mental health? Systematic review and meta-analyses of published controlled intervention studies. Br. J. Nutr. 2024, 131, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef] [PubMed]
- M-Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today’s world. Int. J. Microbiol. 2020, 1, 3029295. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of essential oils as antibacterial agents in minimally processed fruits and vegetables—A review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Aslam, M.F.; Irshad, G.; Naz, F.; Khan, M.A. Evaluation of the antifungal activity of essential oils against Alternaria alternata causing fruit rot of Eriobotrya japonica. Turk. J. Biochem. 2022, 47, 511–521. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Poitevin, C.G.; da Luz, T.S.; Mazarotto, E.J.; Furuie, J.L.; Martins, C.E.N.; do Amaral, W.; Cipriano, R.R.; da Rosa, J.M.; Pimentel, I.C.; et al. Antifungal activity of essential oils and their combinations against storage fungi. Environ. Sci. Pollut. Res. 2023, 30, 48559–48570. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.D.; da Cruz Silva, G.; de Aguiar, A.C.; Cipriano, L.; de Azeredo, H.M.C.; Bogusz Junior, S.; Ferreira, M.D. Chemical composition and antifungal activity of essential oils and their combinations against Botrytis cinerea in strawberries. J. Food Meas. Charact. 2021, 15, 1815–1825. [Google Scholar] [CrossRef]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; Romero-Peña, M.; et al. Conventional and non-conventional disinfection methods to prevent microbial contamination in minimally processed fruits and vegetables. LWT 2022, 165, 113714. [Google Scholar] [CrossRef]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Bangulzai, N.; Ahmed, S.F.; Kashif, M.; Fatima, M.; Ahmed, M.; Mushtaq, N. Antifungal activity of essential oils extracted from different plants against Penicillium digitatum causing green mold of citrus. Int. J. Agric. Biosci. 2022, 11, 75–83. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Pitt, J.I. PENICILLIUM|Penicillium and Talaromyces:: Introduction. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, A.C., Tortorello, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 3, p. 613. ISBN 9780123847300. [Google Scholar]
- Zhao, F.; Li, Q.; Wu, H.; Huang, J.; Ju, J. Synergistic antifungal mechanism of effective components from essential oil against Penicillium roqueforti. Eng. Microbiol. 2023, 3, 100057. [Google Scholar] [CrossRef]
- Fincheira, P.; Jofré, I.; Espinoza, J.; Levío-Raimán, M.; Tortella, G.; Oliveira, H.C.; Diez, M.C.; Quiroz, A.; Rubilar, O. The efficient activity of plant essential oils for inhibiting Botrytis cinerea and Penicillium expansum: Mechanistic insights into antifungal activity. Microbiol. Res. 2023, 277, 127486. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 4th ed.; Springer: Cham, Switzerland, 2022; p. 645. ISBN 978-3-030-85638-0. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; Lavoisier S.A.S.: Utrecht, The Netherlands, 2019; p. 481. ISBN 978-9-4917-5118-9. [Google Scholar]
- Anjum, N.; Shahid, A.A.; Iftikhar, S.; Nawaz, K.; Haider, M.S. First report of postharvest fruit rot of tomato (Lycopersicum esculentum Mill.) caused by Penicillium olsonii in Pakistan. Plant Dis. 2018, 102, 451. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, T.; Wen, G.; Song, B.; Jiang, S. First Report of Penicillium olsonii causing postharvest fruit rot of grape (Vitis vinifera) in China. Plant Dis. 2022, 106, 1761. [Google Scholar] [CrossRef]
- Živković, S.; Ristić, D.; Stošić, S. First report of Penicillium olsonii causing postharvest fruit rot on tomato in Serbia. Plant Dis. 2021, 105, 2246. [Google Scholar] [CrossRef]
- Buonassisi, A.J. Biosecurity Guidelines for Post-Harvest Greenhouse Tomatoes: Prevention of Post-Harvest and Storage Rot; B.C. Greenhouse Growers’ Association: Abbotsford, BC, Canada, 2013; pp. 4–6. Available online: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/plant-health/biosecurity-tomato.pdf (accessed on 6 October 2024).
- Perrone, G.; Samson, R.A.; Frisvad, J.C.; Susca, A.; Gunde-Cimerman, N.; Epifani, F.; Houbraken, J. Penicillium salamii, a new species occurring during seasoning of dry-cured meat. Int. J. Food Microbiol. 2015, 193, 91–98. [Google Scholar] [CrossRef]
- Díaz, T.L.; González, C.J.; Moreno, B.; Otero, A. Effect of temperature, water activity, pH and some antimicrobials on the growth of Penicillium olsonii isolated from the surface of Spanish fermented meat sausage. Food Microbiol. 2002, 19, 1–7. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Parra, C.; Navarro, M.J.; Blanco, R.; Gea, F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal Activity of Select Essential Oils against Candida auris and Their Interactions with Antifungal Drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Zheng, Y.; Chun, H.S. Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics 2022, 11, 1727. [Google Scholar] [CrossRef]
- Brahmi, F.; Mokhtari, O.; Yahyaoui, M.I.; Zraibi, L.; Bentouhami, N.E.; Abdeslam, A.; Legssyer, B. Phytochemical composition, antioxidant, and antifungal activity of essential oil from Myrtus communis, L. Mater. Today Proc. 2023, 72, 3826–3830. [Google Scholar] [CrossRef]
- Thinh, B.B.; Hanh, D.H.; Hung, N.; Thin, D.B. Comparison of Yield, Chemical Composition and Antimicrobial Activity of Distichochlamys citrea Rhizome Essential Oils Obtained by Different Extraction Methods. Mosc. Univ. Chem. Bull. 2022, 77, 300–305. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; Palou, E.; López-Malo, A.; Ávila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1641–1650. [Google Scholar] [CrossRef]
- Tančinová, D.; Barboráková, Z.; Mašková, Z.; Mrvová, M.; Medo, J.; Golian, M.; Lakatošová, J.; Árvay, J. In vitro antifungal activity of essential oils (family Lamiaceae) against Cladosporium sp. Strains–postharvest pathogens of fruits. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9921. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 6 October 2024).
- Yang, J.; Chen, Y.Z.; Wu, Y.X.; Tao, L.; Zhang, Y.D.; Wang, S.R.; Zhang, G.C.; Zhang, J. Inhibitory effects and mechanisms of vanillin on gray mold and black rot of cherry tomatoes. Pestic. Biochem. Physiol. 2021, 175, 104859. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the biocontrol potentiality of Wickerhamomyces anomalus against postharvest gray mold decay in cherry tomatoes. Sci. Hortic. 2021, 285, 110137. [Google Scholar] [CrossRef]
- Tančinová, D.; Barboráková, Z.; Mašková, Z.; Uzsáková, V. Microsopic fungi causing cherry tomatoe rot in stores. In Food Mycology—Taxonomy, Spoilage and Mycotoxins; International Comission on Penicillium and Aspergillus Intenrational Commission on Food Mycology workskop 2022; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2022; 31p. [Google Scholar]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal activity of plant-derived essential oils on pathogens of pulse crops. Plant Dis. 2021, 105, 1692–1701. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cy-to/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef] [PubMed]
- Mkaddem Mounira, G.; Ahlem, Z.; Abdallah Mariem, B.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Madnay, M.M.; AbdElgayed, G.; Asard, H.; et al. Essential oil composition and antioxidant and antifungal activities of two varieties of Ocimum basilicum L.(Lamiaceae) at two phenological stages. Agronomy 2022, 12, 825. [Google Scholar] [CrossRef]
- Blejan, E.I.; Popa, D.E.; Costea, T.; CIOACĂ, A.; Olariu, L.; Ghica, M.; Georgescu, M.; Stancov, G.; Arsene, A.L. The in vitro antimicrobial activity of some essential oils from aromatic plants. Farmacia 2021, 69, 290–298. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of mint, basil, and lavender essential oil vapor-phase in antifungal protection and lemon fruit quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef]
- Zhakipbekov, K.; Turgumbayeva, A.; Akhelova, S.; Bekmuratova, K.; Blinova, O.; Utegenova, G.; Utegenova, G.; Shertaeva, K.; Sadykov, N.; Tastambek, K.; et al. Antimicrobial and other pharmacological properties of Ocimum basilicum, Lamiaceae. Molecules 2024, 29, 388. [Google Scholar] [CrossRef]
- Ngome, M.T.; Alves, J.G.L.F.; de Oliveira, A.C.F.; da Silva Machado, P.; Mondragón-Bernal, O.L.; Piccoli, R.H. Linalool, citral, eugenol and thymol: Control of planktonic and sessile cells of Shigella flexneri. AMB Express 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Harcarova, M.; Conkova, E.; Proskovcova, M.; Váczi, P.; Marcincakova, D.; Bujnak, L. Comparison of antifungal activity of selected essential oils against Fusarium graminearum in vitro. Ann. Agric. Environ. Med. 2021, 28, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Elyemni, M.; El Ouadrhiri, F.; Lahkimi, A.; Elkamli, T.; Bouia, A.; Eloutassi, N. Chemical Composition and Antimicrobial Activity of Essential Oil of Wild and Cultivated Rosmarinus officinalis from Two Moroccan Localities. J. Ecol. Eng. 2022, 23, 214–222. [Google Scholar] [CrossRef]
- Stojiljkovic, J.; Trajchev, M.; Nakov, D.; Petrovska, M. Antibacterial activities of rosemary essential oils and their components against pathogenic bacteria. Adv. Cytol. Pathol. 2018, 3, 93–96. [Google Scholar] [CrossRef]
- Hendel, N.; Sarri, D.; Sarri, M.; Napoli, E.; Palumbo Piccionello, A.; Ruberto, G. Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. Int. J. Mol. Sci. 2024, 25, 7989. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kačániová, M. In Vitro Antimicrobial Activity of Lavender, Mint, and Rosemary Essential Oils and the Effect of Their Vapors on Growth of Penicillium spp. v systéme Bread Model System. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Erarslan, A.; Karakas, C.Y.; Bozkurt, F.; Sagdic, O. Enhanced antifungal activity of electrosprayed poly (vinyl alcohol)/chitosan nanospheres loaded with sage essential oil on the viability of Aspergillus niger and Botrytis cinerea. ChemistrySelect 2023, 8, e202300296. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Demirci, F.; Karadağ, A.E.; Biltekin, S.N.; Demirci, B. In vitro ACE2 and 5-LOX enzyme inhibition by menthol and three different mint essential oils. Nat. Prod. Commun. 2021, 16, 1934578X211055014. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Hussain, H.; Xiao, J. Recent advances in genus Mentha: Phytochemistry, antimicrobial effects, and food applications. Food Front. 2020, 1, 435–458. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Piątkowska, E.; Kuś, P.; Marijanović, Z.; Jerković, I.; Tuberoso, C.I.; Fecka, I. Volatile compounds and antibacterial effect of commercial mint cultivars-chemotypes and safety. Ind. Crops Prod. 2021, 166, 113430. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2021, 130, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2019, 35, 993–999. [Google Scholar] [CrossRef]
- Jahani, M.; Beheshti, M.; Aminifard, M.H.; Hosseini, A. Effects of essential oils to control Penicillium sp. in in vitro and in in vivo on grapevine (Vitis vinifera L.) fruit. Int. J. Fruit Sci. 2020, 20 (Suppl. S2), 812–826. [Google Scholar] [CrossRef]
- Hassan, H.A.; Genaidy, M.M.; Kamel, M.S.; Abdelwahab, S.F. Synergistic antifungal activity of mixtures of clove, cumin and caraway essential oils and their major active components. J. Herb. Med. 2020, 24, 100399. [Google Scholar] [CrossRef]
- Moumni, M.; Romanazzi, G.; Najar, B.; Pistelli, L.; Ben Amara, H.; Mezrioui, K.; Karous, O.; Chaieb, I.; Allagui, M.B. Antifungal Activity and Chemical Composition of Seven Essential Oils to Control the Main Seedborne Fungi of Cucurbits. Antibiotics 2021, 10, 104. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Rouissi, W.; Mougou-Hamdane, A.; Hannachi, I.; Nasraoui, B. Antifungal activity of essential oils of Origanum majorana and Lavender angustifolia against Fusarium wilt and root rot disease of melon plants. Tunis. J. Plant Prot. 2018, 13, 39–55. Available online: https://www.researchgate.net/publication/349763095_Antifungal_Activity_of_Essential_Oils_of_Origanum_majorana_and_Lavender_angustifolia_against_Fusarium_Wilt_and_Root_Rot_Disease_of_Melon_Plants (accessed on 6 October 2024).
- Kordali, S.; Usanmaz Bozhuyuk, A.; Komaki, A.; Ilhan, G.; Ercisli, S. Biological Control of Penicillium on Lemon Fruits by Essential Oils of Satureja Species. Erwerbs-Obstbau 2022, 64, 703–715. [Google Scholar] [CrossRef]
- Kim, J.-E.; Lee, J.-E.; Huh, M.-J.; Lee, S.-C.; Seo, S.-M.; Kwon, J.H.; Park, I.-K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules 2019, 9, 561. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Salaria, D.; Rolta, R.; Patel, C.N.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in silico analysis of Thymus serpyllum essential oil as bioactivity enhancer of antibacterial and antifungal agents. J. Biomol. Struct. Dyn. 2021, 40, 10383–10402. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, X. Bactericidal and fungicidal activity of the wild thyme (Thymus serpyllum) essential oil. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2019, 350, 33–44. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Bounar, R.; Krimat, S.; Boureghda, H.; Dob, T. Chemical analyses, antioxidant and antifungal effects of oregano and thyme essential oils alone or in combination against selected Fusarium species. Int. Food Res. J. 2020, 27, 66–77. [Google Scholar]
- Walasek-Janusz, M.; Grzegorczyk, A.; Malm, A.; Nurzyńska-Wierdak, R.; Zalewski, D. Chemical Composition, and Antioxidant and Antimicrobial Activity of Oregano Essential Oil. Molecules 2024, 29, 435. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.H.; Ye, M.; Wang, K.B.; Fan, L.M.; Su, F.W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.M.; Crippa, B.L.; de Souza, V.V.M.A.; Alonso, V.P.P.; Júnior, E.D.M.S.; Picone, C.S.F.; Prata, A.S.; Silva, N.C.C. Antimicrobial action of Oregano, Thyme, Clove, Cinnamon and Black pepper essential oils free and encapsulated against foodborne pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- da Silva Bomfim, N.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Mikcha, J.M.G.; de Abreu Filho, B.A.; Machinski, M., Jr. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A 2019, 37, 153–161. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; De Oliveira Pereira, F.; De Oliveira, W.A.; Lima, I.O.; De Oliveira Lima, E. Antifungal Activity of Thymus vulgaris L. Essential Oil and Its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef]
- Wu, T.L.; Zhang, B.Q.; Luo, X.F.; Li, A.P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; Liu, Y.Q. Antifungal efficacy of sixty essential oils and mechanism of oregano essential oil against Rhizoctonia solani. Ind. Crops Prod. 2023, 191, 115975. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Lević, S.; Kalušević, A.; Cam, M.; Bugarski, B.; Rakić, V.; Pavlović, V.; Nedović, V. Characterization of peppermint (Mentha piperita L.) essential oil encapsulates. J. Microencapsul. 2019, 36, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Rashid, Z.; Khan, M.R.; Mubeen, R.; Hassan, A.; Saeed, F.; Afzaal, M. Exploring the effect of cinnamon essential oil to enhance the stability and safety of fresh apples. J. Food Process. Preserv. 2020, 44, e14926. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Hashemi, M.; Shahedi, M.; Mirzaalian Dastjerdi, A. Enhancing the Quality of Fresh Pistachio Fruit Using Sodium Alginate Enriched with Thyme Essential Oil. JAST J. Agric. Sci. Technol. 2021, 23, 65–82. [Google Scholar]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef]

| EO and Plant Source | Compound | Content in % |
|---|---|---|
| Thyme (Thymus vulgaris L.) | o-Xylene | 43.9 |
| Thymol | 33.7 | |
| Linalool | 7.1 | |
| α-Pinene | 3.5 | |
| Savory (Satureja hortensis L.) | γ-Terpinene | 45.1 |
| Thymol | 20.2 | |
| p-Cymene | 19.6 | |
| (+)-4-Carene | 3.8 | |
| Sage (Salvia officinalis L.) | Thujone | 22.4 |
| (+)-2-Bornanone | 19.7 | |
| Eucalyptol | 10.8 | |
| Humulene | 6.9 | |
| β-Thujone | 6.6 | |
| α-Pinene | 6.1 | |
| Camphene | 5.9 | |
| Caryophyllene | 5.6 | |
| endo-Borneol | 4.45 | |
| Spearmint (Mentha spicata L. var. crispa) | (-)-Carvone | 72.6 |
| D-Limonene | 15.2 | |
| Bergamot mint (Mentha citrata Erh.) | Linalyl acetate | 45.0 |
| Linalool | 34.0 | |
| Geranyl acetate | 5.9 | |
| Marjoram (Origanum majorana L.) | Terpinene-4-ol | 34.5 |
| γ-Terpinene | 16.9 | |
| cis-Sabinene hydrate | 15.1 | |
| (+)-4-Carene | 9.3 | |
| Sabinene | 6.9 | |
| o-Cymene | 6.3 | |
| Wild thyme (Thymus serpyllum L.) | Benzene, 4-ethyl-1,2-dimethyl- | 18.07 |
| Thymol | 12.13 | |
| Geraniol | 10.74 | |
| γ-Terpinene | 10.43 | |
| Linalool | 5.06 | |
| Geranyl acetate | 4.77 | |
| Oregano (Origanum vulgare L.) | Thymol | 60.37 |
| Benzene, 4-ethyl-1,2-dimethyl- | 13.14 | |
| gamma-Terpinene | 7.91 | |
| Basil (Ocinum basilicum L.) | Estragole | 84.89 |
| Eucalyptol | 4.1 | |
| Rosemary (Rosmarinus officinalis L.) | Eucalyptol | 43.17 |
| (+)-2-Bornanone | 12.8 | |
| α-Pinene | 10.74 | |
| β-Pinene | 7.43 | |
| Camphene | 4.66 | |
| endo-Borneol | 3.83 | |
| Caryophyllene | 3.78 |
| Strain | EOs | Av in mm | Day of Cultivation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RI (%) | 2nd | 3rd | 4th | 7th | 9th | 11th | 14th | ||
| KMi-1026 | Control | Av ± sd | 10.26 ± 0.36 | 15.02 ± 0.34 | 22.01 ± 0.24 | 38.24 ± 0.65 | 47.54 ± 0.38 | 47.54 ± 0.38 | 72.95 ± 0.31 |
| Basil | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.92 ± 0.16 | 12.78 ± 0.79 | 19.46 ± 0.37 | 24.24 ± 0.21 | 44.89 ± 0.36 | |
| RI | 100 | 100 | 90.51 | 82.48 | 73.32 | 66.77 | 38.46 | ||
| Rosemary | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.04 ± 0.19 | 15.46 ± 0.50 | 22.72 ± 0.31 | 27.61 ± 0.43 | 49.19 ± 0.28 | |
| RI | 100 | 100 | 90.35 | 78.81 | 68.86 | 62.15 | 32.57 | ||
| Sage | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.46 ± 0.26 | 14.45 ± 0.22 | 18.59 ± 0.09 | 34.58 ± 0.65 | |
| RI | 100 | 100 | 100 | 85.66 | 80.19 | 74.52 | 52.60 | ||
| Bergamot mint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.76 ± 0.26 | 12.02 ± 0.33 | |
| RI | 100 | 100 | 100 | 100 | 100 | 93.47 | 83.52 | ||
| Spearmint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| KMi-1028 | Control | Av ± sd | 9.40 ± 0.13 | 13.22 ± 0.46 | 20.28 ± 0.19 | 32.74 ± 0.61 | 44.01 ± 0.91 | 54.38 ± 0.47 | 70.17 ± 0.27 |
| Basil | Av ± sd | 4.64 ± 0.20 | 6.07 ± 0.20 | 8.42 ± 0.24 | 10.29 ± 0.21 | 13.29 ± 0.26 | 18.96 ± 0.29 | 32.42 ± 3.73 | |
| RI | 93.39 | 91.35 | 88 | 85.34 | 81.06 | 72.98 | 53.80 | ||
| Rosemary | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.65 ± 0.40 | 13.80 ± 0.20 | 18.50 ± 0.28 | 36.97 ± 0.29 | |
| RI | 100 | 100 | 100 | 87.67 | 80.33 | 73.64 | 47.31 | ||
| Sage | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.50 ± 0.43 | 11.84 ± 0.23 | 14.06 ± 0.40 | 27.23 ± 0.69 | |
| RI | 100 | 100 | 100 | 87.89 | 83.13 | 79.96 | 61.19 | ||
| Bergamot mint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Spearmint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.94 ± 0.18 | 7.40 ± 0.32 | 8.39 ± 0.19 | 9.94 ± 0.23 | |
| RI | 100 | 100 | 100 | 90.11 | 84.45 | 88.04 | 85.83 | ||
| KMi-1029 | Control | Av ± sd | 10.11 ± 0.11 | 18.94 ± 0.62 | 21.72 ± 0.53 | 32.99 ± 0.80 | 43.40 ± 0.49 | 48.63 ± 0.42 | 61.28 ± 0.48 |
| Basil | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.48 ± 0.71 | 11.06 ± 0.91 | 14.65 ± 0.63 | 18.62 ± 0.35 | 30.28 ± 0.68 | |
| RI | 100 | 100 | 86.16 | 81.95 | 76.09 | 69.61 | 50.59 | ||
| Rosemary | Av ± sd | 0.00 ± 0.00 | 8.66 ± 0.13 | 14.96 ± 0.53 | 22.92 ± 0.37 | 27.01 ± 0.30 | 30.71 ± 0.41 | 45.07 ± 0.54 | |
| RI | 100 | 85.87 | 75.59 | 62.60 | 55.92 | 49.89 | 26.45 | ||
| Sage | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.12 ± 0.58 | 19.12 ± 0.62 | 22.08 ± 0.65 | 29.36 ± 0.65 | |
| RI | 100 | 100 | 100 | 81.85 | 68.80 | 63.97 | 52.09 | ||
| Bergamot mint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Spearmint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| KMi-1030 | Control | Av ± sd | 9.78 ± 0.40 | 17.49 ± 0.58 | 19.72 ± 0.62 | 33.68 ± 0.64 | 40.93 ± 1.11 | 48.80 ± 0.24 | 61.29 ± 0.73 |
| Basil | Av ± sd | 0.00 ± 0.00 | 4.12 ± 0.11 | 4.90 ± 0.18 | 5.69 ± 0.56 | 7.79 ± 0.57 | 11.23 ± 0.28 | 21.49 ± 0.55 | |
| RI | 100 | 93.28 | 92 | 90.72 | 87.29 | 81.68 | 64.94 | ||
| Rosemary | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.29 ± 0.12 | 20.92 ± 0.37 | 24.57 ± 0.39 | 29.01 ± 0.18 | 38.88 ± 0.45 | |
| RI | 100 | 100 | 88.11 | 65.87 | 59.91 | 52.67 | 36.56 | ||
| Sage | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.09 ± 0.42 | 13.00 ± 0.25 | 19.89 ± 0.67 | |
| RI | 100 | 100 | 100 | 100 | 85.17 | 78.79 | 67.55 | ||
| Bergamot mint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Spearmint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| KMi-1031 | Control | Av ± sd | 11.64 ± 0.50 | 20.42 ± 0.45 | 26.94 ± 0.44 | 36.77 ± 0.28 | 44.16 ± 0.65 | 50.94 ± 0.60 | 65.79 ± 0.51 |
| Basil | Av ± sd | 0.00 ± 0.00 | 7.17 ± 0.24 | 12.90 ± 0.44 | 20.41 ± 0.70 | 31.01 ± 0.35 | 36. 48 ± 0.60 | 51.21 ± 0.26 | |
| RI | 100 | 89.10 | 80.39 | 68.98 | 52.87 | 44.55 | 22.16 | ||
| Rosemary | Av ± sd | 0.00 ± 0.00 | 10.01 ± 0.52 | 11.90 ± 0.29 | 20.19 ± 0.76 | 29.09 ± 0.24 | 36.94 ± 0.53 | 52.49 ± 0.53 | |
| RI | 100 | 84.78 | 81.91 | 69.31 | 55.78 | 43.85 | 20.22 | ||
| Sage | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.30 ± 0.54 | 18.53 ± 0.80 | 24.68 ± 0.29 | 37.05 ± 0.38 | |
| RI | 100 | 100 | 100 | 88.90 | 71.83 | 62.49 | 43.68 | ||
| Bergamot mint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 7.02 ± 0.26 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 89.11 | ||
| Spearmint | Av ± sd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| RI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| EOs | Day | Penicillium olsonii Strains | |||||
|---|---|---|---|---|---|---|---|
| 1026 | 1029 | 1030 | |||||
| Concentration of EOs (μL/L) | |||||||
| 250 | 125 | 250 | 125 | 250 | 125 | ||
| Thyme | 1. | 0/9 * | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 2. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 3. | 0/9 | 2/9 | 0/9 | 1/9 | 0/9 | 0/9 | |
| 5. | 0/9 | 72/9 ** | 0/9 | 4/9 | 0/9 | 4/9 | |
| 6. | 0/9 | 92/9 | 0/9 | 5/9 | 0/9 | 7/9 | |
| 7. | 0/9 | 92/9 | 0/9 | 7/9 | 0/9 | 7/9 | |
| 8. | 1/9 | 92/9 | 0/9 | 8/9 | 0/9 | 8/9 | |
| 9. | 1/9 | 92/9 | 0/9 | 9/9 | 0/9 | 8/9 | |
| 10. | 1/9 | 92/9 | 0/9 | 91/9 | 1/9 | 9/9 | |
| 12. | 1/9 | 92/9 | 0/9 | 91/9 | 2/9 | 9/9 | |
| Oregano | 1. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 2. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 3. | 0/9 | 2/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 5. | 0/9 | 3/9 | 0/9 | 0/9 | 0/9 | 2/9 | |
| 6. | 0/9 | 5/9 | 0/9 | 2/9 | 0/9 | 3/9 | |
| 7. | 0/9 | 7/9 | 0/9 | 2/9 | 0/9 | 4/9 | |
| 8. | 0/9 | 7/9 | 0/9 | 6/9 | 0/9 | 5/9 | |
| 9. | 0/9 | 71/9 | 0/9 | 6/9 | 0/9 | 5/9 | |
| 10. | 0/9 | 71/9 | 0/9 | 6/9 | 0/9 | 5/9 | |
| 12. | 0/9 | 71/9 | 0/9 | 7/9 | 0/9 | 5/9 | |
| Wild thyme | 1. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 2. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 3. | 0/9 | 1/9 | 0/9 | 0/9 | 1/9 | 0/9 | |
| 5. | 4/9 | 5/9 | 0/9 | 3/9 | 4/9 | 2/9 | |
| 6. | 6/9 | 71/9 | 0/9 | 5/9 | 6/9 | 3/9 | |
| 7. | 9/9 | 81/9 | 0/9 | 8/9 | 7/9 | 51/9 | |
| 8. | 9/9 | 83/9 | 2/9 | 9/9 | 7/9 | 83/9 | |
| 9. | 9/9 | 83/9 | 2/9 | 9/9 | 8/9 | 93/9 | |
| 10. | 9/9 | 83/9 | 2/9 | 9/9 | 8/9 | 93/9 | |
| 12. | 9/9 | 93/9 | 2/9 | 9/9 | 8/9 | 93/9 | |
| Savory | 1. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 2. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 3. | 1/9 | 4/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 5. | 3/9 | 82/9 | 2/9 | 6/9 | 6/9 | 62/9 | |
| 6. | 5/9 | 82/9 | 4/9 | 82/9 | 6/9 | 73/9 | |
| 7. | 6/9 | 83/9 | 5/9 | 93/9 | 8/9 | 83/9 | |
| 8. | 6/9 | 84/9 | 5/9 | 95/9 | 8/9 | 93/9 | |
| 9. | 6/9 | 84/9 | 6/9 | 95/9 | 8/9 | 93/9 | |
| 10. | 6/9 | 94/9 | 6/9 | 95/9 | 8/9 | 93/9 | |
| 12. | 6/9 | 94/9 | 7/9 | 95/9 | 8/9 | 93/9 | |
| Spearmint | 1. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 2. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 3. | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | |
| 5. | 0/9 | 6/9 | 0/9 | 2/9 | 0/9 | 1/9 | |
| 6. | 0/9 | 94/9 | 0/9 | 7/9 | 0/9 | 8/9 | |
| 7. | 0/9 | 95/9 | 0/9 | 8/9 | 0/9 | 91/9 | |
| 8. | 0/9 | 97/9 | 0/9 | 91/9 | 0/9 | 94/9 | |
| 9. | 0/9 | 98/9 | 0/9 | 91/9 | 0/9 | 97/9 | |
| 10. | 1/9 | 98/9 | 2/9 | 91/9 | 2/9 | 97/9 | |
| 12. | 4/9 | 98/9 | 2/9 | 91/9 | 3/9 | 97/9 | |
| Control | 1. | 7/9 | 5/9 | 4/9 | |||
| 2. | 9/9 | 9/9 | 61/9 | ||||
| 3. | 99/9 | 9/9 | 91/9 | ||||
| 5. | 99/9 | 98/9 | 98/9 | ||||
| 6.–12. | 99/9 | 99/9 | 99/9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrvová, M.; Medo, J.; Lakatošová, J.; Barboráková, Z.; Golian, M.; Mašková, Z.; Tančinová, D. Vapor-Phase Essential Oils as Antifungal Agents against Penicillium olsonii Causing Postharvest Cherry Tomato Rot. Foods 2024, 13, 3202. https://doi.org/10.3390/foods13193202
Mrvová M, Medo J, Lakatošová J, Barboráková Z, Golian M, Mašková Z, Tančinová D. Vapor-Phase Essential Oils as Antifungal Agents against Penicillium olsonii Causing Postharvest Cherry Tomato Rot. Foods. 2024; 13(19):3202. https://doi.org/10.3390/foods13193202
Chicago/Turabian StyleMrvová, Monika, Juraj Medo, Jana Lakatošová, Zuzana Barboráková, Marcel Golian, Zuzana Mašková, and Dana Tančinová. 2024. "Vapor-Phase Essential Oils as Antifungal Agents against Penicillium olsonii Causing Postharvest Cherry Tomato Rot" Foods 13, no. 19: 3202. https://doi.org/10.3390/foods13193202







