Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Tempo Campylobacter Enumeration and Prevalence
2.3. BAX® Salmonella Enumeration and Prevalence
2.4. Limit of Quantification for Salmonella Results
2.5. Statistical Analysis
3. Results
3.1. Salmonella Detection and Enumeration
3.1.1. Salmonella Plant One
3.1.2. Salmonella Plant Two
3.1.3. Salmonella Plant Three
3.2. Campylobacter
4. Discussion
4.1. Salmonella Prevalence and Quantification Plant One
4.2. Salmonella Prevalence and Quantification Plant Two
4.3. Salmonella Prevalence and Quantification Plant Three
4.4. Campylobacter Quantification Plant One
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service. Livestock and Poultry: World Markets and Trade China Continues to Drive Red Meat Trade; United States Department of Agriculture: Ithaca, NY, USA, 2021.
- United States Department of Agriculture. National Agricultural Statistics Service Poultry-Production and Value 2020 Summary (April 2021); United States Department of Agriculture: Washington, DC, USA, 2021.
- Dohlman, E.; Hansen, J.; Boussios, D. USDA Agricultural Projections to 2030 Interagency Agricultural Projections Committee USDA Long-Term Projections; United States Department of Agriculture: Washington, DC, USA, 2021.
- Information Resources Inc. (IRI); 210 Analytics. Mid-Year Meat Department Performance Review Animal Protein Reigns High AMID Pandemic; What’s Next? 210 Analytics: Chicago, IL, USA, 2020. [Google Scholar]
- CDC Burden of Foodborne Illness: Findings. Available online: https://www.cdc.gov/foodborneburden/estimates-overview.html#:~:text=CDC%20estimates%2048%20million%20people,year%20in%20the%20United%20States (accessed on 18 December 2023).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- The Integracy Food Safety Analytics Collaboration. Foodborne Illness Source Attribution Estimates for 2019 for Salmonella, Escherichia Coli O157, Listeria Monocytogenes, and Campylobacter Using Multi-Year Outbreak Surveillance Data, United States; United States Department of Agriculture: Washington, DC, USA, 2021.
- Food Safety and Inspection Service. FSIS Guideline for Controlling Salmonella in Raw Poultry: FSIS-GD-2021-0005; United States Department of Agriculture: Washington, DC, USA, 2021.
- Department of Agriculture; Food Safety and Inspection Service. New Performance Standards for Salmonella and Campylobacter in Not-Ready-to-Eat Comminuted Chicken and Turkey Products and Raw Chicken Parts and Changes to Related Agency Verification Procedures: Response to Comments and Announcement of Implementation Schedule. Fed. Regist. 2016, 81, 7285–7300. [Google Scholar]
- Department of Agriculture Food Safety and Inspection Service. Modernization of Poultry Slaughter Inspection; Final Rule; Department of Agriculture Food Safety and Inspection Service: Washington, DC, USA, 2010.
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating Best Practices for Campylobacter and Salmonella Reduction in Poultry Processing Plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.M.; Bauermeister, L.J.; Bratcher, C.L.; Singh, M.; McKee, S.R. Salmonella and Campylobacter Reduction and Quality Characteristics of Poultry Carcasses Treated with Various Antimicrobials in a Post-Chill Immersion Tank. Int. J. Food Microbiol. 2013, 165, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.C.; Adams, B.W.; Parry, R.T. The Effectiveness of In-plant Chlorination in Poultry Processing. Br. Poult. Sci. 1975, 16, 517–526. [Google Scholar] [CrossRef]
- Kataria, J.; Vaddu, S.; Rama, E.N.; Sidhu, G.; Thippareddi, H.; Singh, M. Evaluating the Efficacy of Peracetic Acid on Salmonella and Campylobacter on Chicken Wings at Various PH Levels. Poult. Sci. 2020, 99, 5137–5142. [Google Scholar] [CrossRef]
- United States Department of Agriculture; Food Safety and Inspection Service. Sampling Instructions: Salmonella and Campylobacter Verification Program for Raw Poultry Products; United States Department of Agriculture: Washingtong, DC, USA, 2021.
- BioMeriux. Validation of Alternative Analytical Methods Application in Food Microbiology Summary Report Initial Validation Study TEMPO CAM Method (Certificate Number: BIO 12/43-04/20) for the Enumeration of Thermotolerant Campylobacter in Raw Poultry and Ready-to-Cook Poultry Products; BioMeriux: Paris, France, 2021. [Google Scholar]
- Becton, Dickinson and Company. BD GasPakTM EZ Container Systems; Becton, Dickinson and Company: Franklin Lakes, NJ, USA, 2022. [Google Scholar]
- Hygiena. BAX-System-SalQuant-Utilizing-BAX-System-Real-Time-PCR-Assay-for-Salmonella-AOAC-Summary-Extension; Hygiena: Camarilo, CA, USA, 2021. [Google Scholar]
- De Villena, J.F.; Vargas, D.A.; Bueno López, R.; Chávez-Velado, D.R.; Casas, D.E.; Jiménez, R.L.; Sanchez-Plata, M.X. Bio-Mapping Indicators and Pathogen Loads in a Commercial Broiler Processing Facility Operating with High and Low Antimicrobial Intervention Levels. Foods 2022, 11, 775. [Google Scholar] [CrossRef]
- Simonsen, B.; Bryan, F.L.; Christian, J.H.B.; Roberts, T.A.; Tompkin, R.B.; Silliker, J.H. Prevention and Control of Food-Borne Salmonellosis through Application of Hazard Analysis Critical Control Point (HACCP). Int. J. Food Microbiol. 1987, 4, 227–247. [Google Scholar] [CrossRef]
- Ebel, E.D.; Williams, M.S. When Are Qualitative Testing Results Sufficient to Predict a Reduction in Illnesses in a Microbiological Food Safety Risk Assessment? J. Food Prot. 2015, 78, 1451–1460. [Google Scholar] [CrossRef]
- Ebel, E.D.; Williams, M.S.; Golden, N.J.; Marks, H.M. Simplified Framework for Predicting Changes in Public Health from Performance Standards Applied in Slaughter Establishments. Food Control 2012, 28, 250–257. [Google Scholar] [CrossRef]
- Oscar, T. Salmonella Prevalence Alone Is Not a Good Indicator of Poultry Food Safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Stopforth, J.D.; O’connor, R.; Lopes, M.; Kottapalli, B.; Hill, W.E.; Samadpour, M. Validation of Individual and Multiple-Sequential Interventions for Reduction of Microbial Populations during Processing of Poultry Carcasses and Parts. J. Food Prot. 2007, 70, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How Do We Determine the Efficacy of an Antibacterial Surface? A Review of Standardised Antibacterial Material Testing Methods. Antibiotics 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Buege, D.; Ingham, S. Small Plant Intervention Treatments to Reduce Bacteria on Beef Carcasses at Slaughter; University of Wisconsin-Madison: Madison, WI, USA, 2003. [Google Scholar]
- Mohapatra, S. Sterilization and Disinfection. In Essentials of Neuroanesthesia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 929–944. [Google Scholar]
- Russell, S. Controlling Salmonella in Poultry Production and Processing; Taylor & Francis Ltd: London, UK, 2012. [Google Scholar]
- Geornaras, I.; De Jesus, A.E.; Van Zyl, E.; Von Holyl, A. Bacterial Populations of Different Sample Types from Carcasses in the Dirty Area of a South African Poultry Abattoir. J. Food Prot. 1997, 60, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Kollanoor Johny, A. Salmonella in Poultry Meat Production. In Food Safety in Poultry Meat Production; Springer International Publishing: Cham, Swizerland, 2019. [Google Scholar]
- Bailey, J.S. Integrated Colonization Control of Salmonella in Poultry. Poult. Sci. 1988, 67, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Lahellec, C.; Colin, P.; Bennejean, G.; Paquin, J.; Guillerm, A.; Debois, J.C. Influence of Resident Salmonella on Contamination of Broiler Flocks. Poult. Sci. 1986, 65, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Kotula, K.L.; Pandya, Y. Bacterial Contamination of Broiler Chickens before Scalding. J. Food Prot. 1995, 58, 1326–1329. [Google Scholar] [CrossRef]
- Khalifa, A.; Mohamed, A. Studies on Salmonella spp. and Escherichia coli Contamination in Poultry Meat Carcasses at an Automatic Slaughter House in Khartoum State; Sudan University of Science and Technology: Khartoum, Sudan, 2015. [Google Scholar]
- Fluckey, W.M.; Sanchez, M.X.; Mckee, S.R.; Smith, D.; Pendleton, E.; Brashears, M.M. Establishment of a Microbiological Proo Le for an Air-Chilling Poultry Operation in the United States. J. Food Prot. 2003, 66, 272–279. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Kasuga, F.; Fazil, A.; Ogden, I.D.; Rotariu, O.; Strachan, N.J.C. Dose-Response Modeling of Salmonella Using Outbreak Data. Int. J. Food Microbiol. 2010, 144, 243–249. [Google Scholar] [CrossRef]
- Akil, L.; Ahmad, H.A. Quantitative Risk Assessment Model of Human Salmonellosis Resulting from Consumption of Broiler Chicken. Diseases 2019, 7, 19. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Bugarel, M.; Kumar, S.; Thippareddi, H.; Brashears, M.M.; Sanchez-Plata, M.X. Phenotypic and Genotypic Characterization of Antimicrobial Resistance in Salmonella Strains Isolated from Chicken Carcasses and Parts Collected at Different Stages during Processing. J. Food Prot. 2019, 82, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, T.J. The Effects of PH and Levels of Organic Matter on the Death Rates of Salmonellas in Chicken Scald-Tank Water. J. Appl. Bacteriol. 1981, 51, 27–39. [Google Scholar] [CrossRef] [PubMed]
- May, K.N. 3. Changes in Microbial Numbers During Final Washing and Chilling of Commercially Slaughtered Broilers. Poult. Sci. 1974, 53, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Ebel, E.D.; Williams, M.S.; Tameru, B. Relatedness of Salmonella Contamination Frequency on Chicken Carcasses and Parts When Processed in the Same Establishment. Food Control. 2019, 100, 198–203. [Google Scholar] [CrossRef]
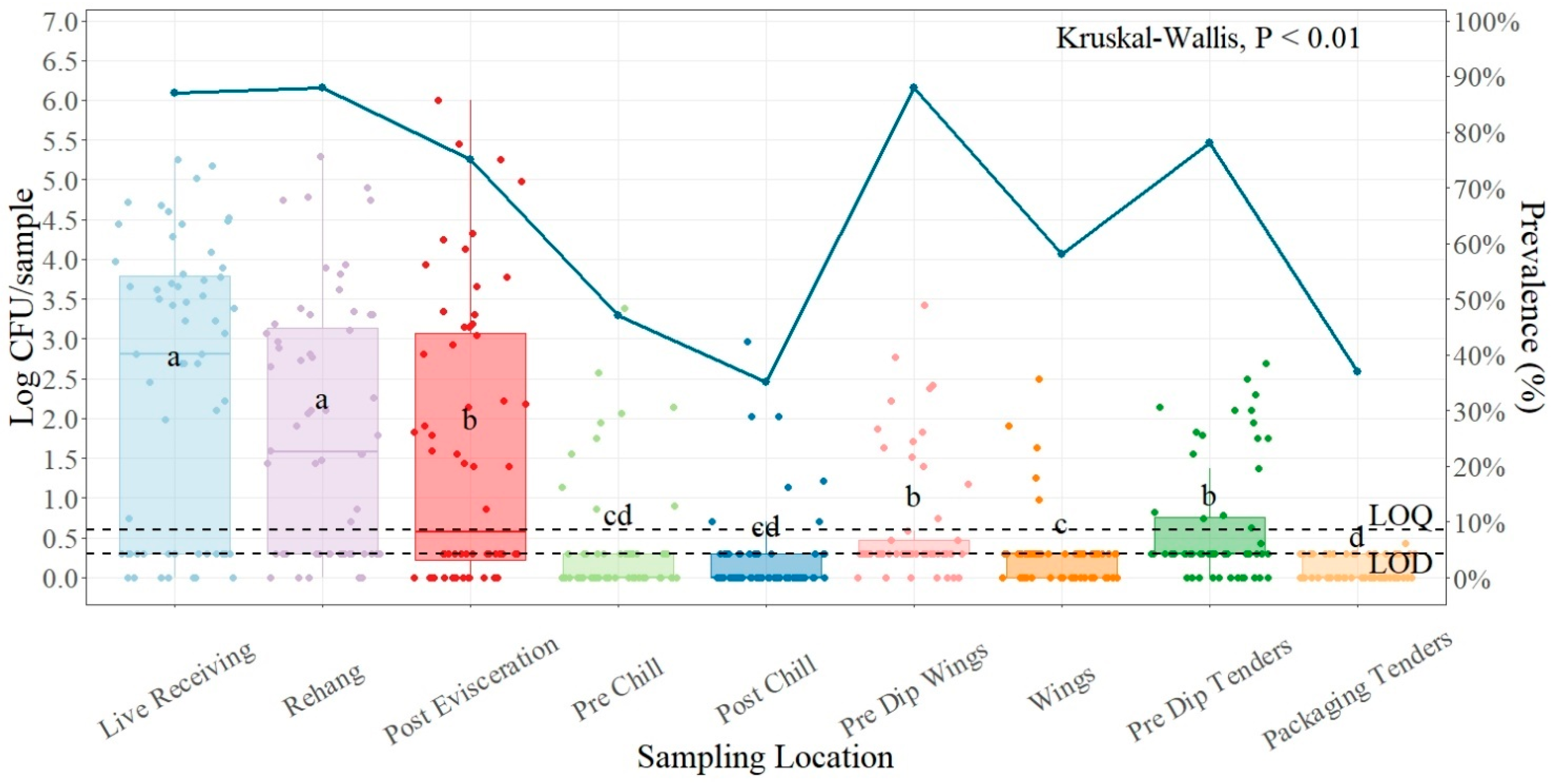
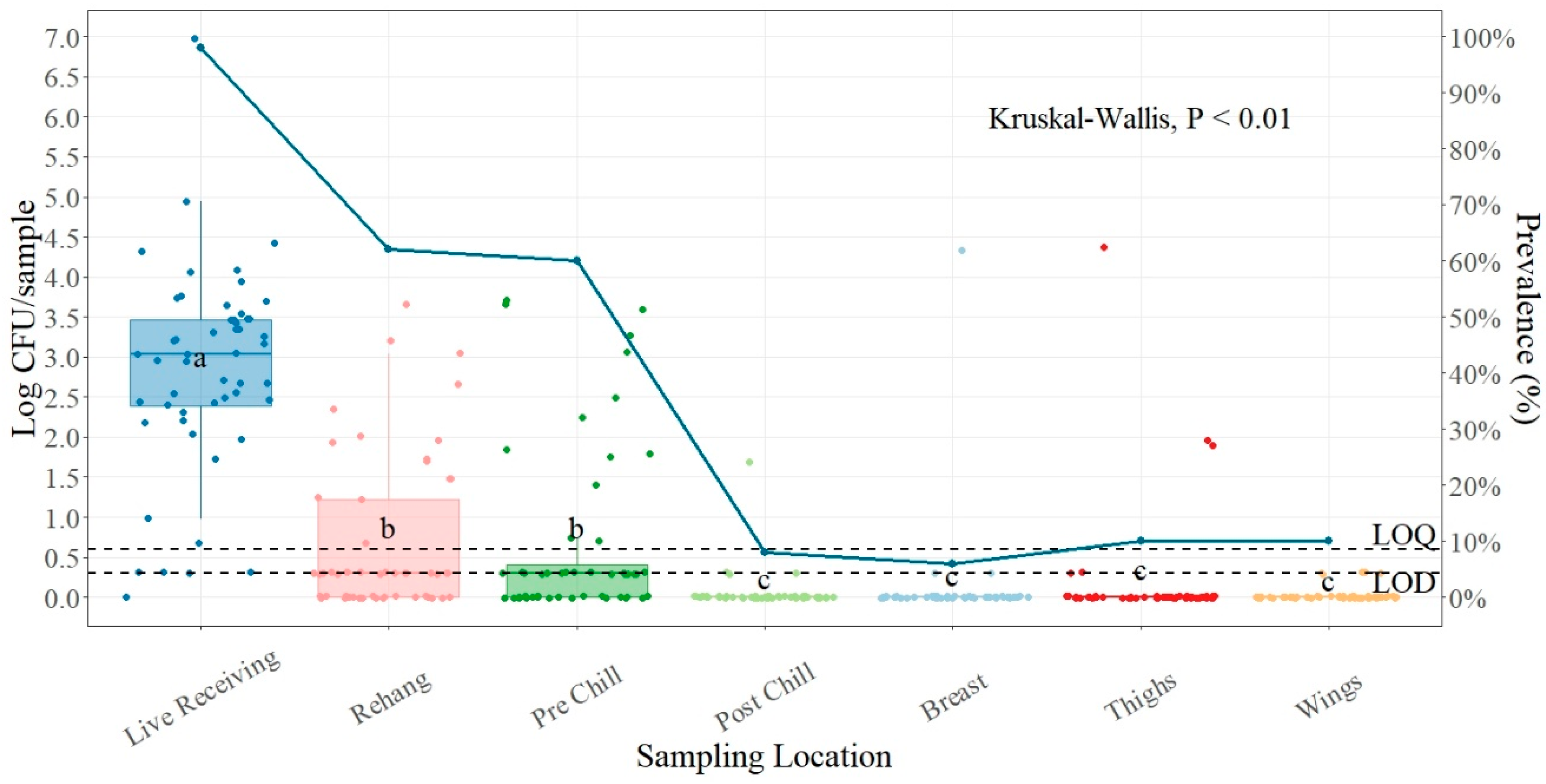
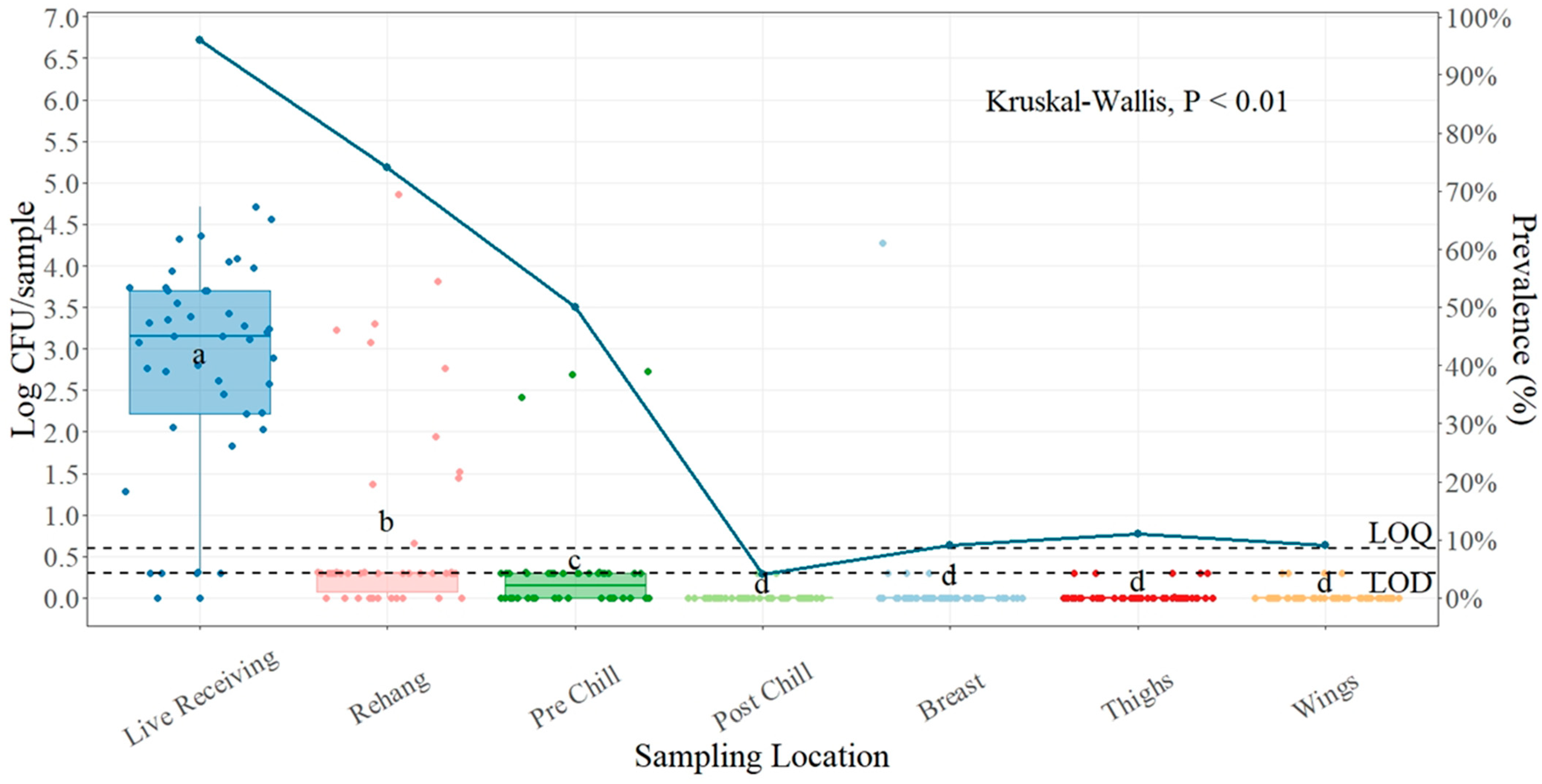
| Salmonella (n = 540) | ||
|---|---|---|
| Location | Counts (Log CFU/Sample ± SE 1) | Prevalence % |
| Live Receiving | 2.39 ± 0.23 a | 52/60 (86.67) |
| Rehanger | 1.85 ± 0.20 a | 53/60 (88.33) |
| Post Evisceration | 1.59 ± 0.23 b | 44/60 (75.00) |
| Pre-Chill | 0.40 ± 0.09 cd | 28/60 (46.67) |
| Post-Chill | 0.25 ± 0.07 cd | 21/60 (35.00) |
| Pre-Dip Wings | 0.63 ± 0.10 b | 53/60 (88.33) |
| Wings | 0.29 ± 0.06 c | 35/60 (58.33) |
| Pre-Dip Tenders | 0.63 ± 0.10 b | 47/60 (78.33) |
| Packaging Tenders | 0.11 ± 0.02 d | 22/60 (36.67) |
| Salmonella (n = 364) | ||
|---|---|---|
| Location | Counts (Log CFU/Sample ± SE 1) | Prevalence % |
| Live Receiving | 2.83 ± 0.18 a | 51/52 (98.08) |
| Rehanger | 0.68 ± 0.14 b | 32/52 (61.54) |
| Pre-Chill | 0.69 ± 0.15 b | 31/52 (59.62) |
| Post-Chill | 0.05 ± 0.03 c | 4/52 (7.69) |
| Breast | 0.10 ± 0.08 c | 3/52 (5.77) |
| Thighs | 0.17 ± 0.10 c | 5/52 (9.62) |
| Wings | 0.03 ± 0.01 c | 5/52 (9.62) |
| Salmonella (n = 322) | ||
|---|---|---|
| Location | Counts (Log CFU/Sample ± SE 1) | Prevalence % |
| Live Receiving | 2.78 ± 0.19 a | 44/46 (95.65) |
| Rehanger | 0.76 ± 0.17 b | 34/46 (73.91) |
| Pre-Chill | 0.30 ± 0.09 c | 23/46 (50.00) |
| Post-Chill | 0.01 ± 0.01 d | 2/46 (4.38) |
| Breast | 0.11 ± 0.09 d | 4/46 (8.70) |
| Thighs | 0.03 ± 0.01 d | 5/46 (10.67) |
| Wings | 0.03 ± 0.01 d | 4/46 (8.70) |
| Campylobacter (n = 540) | |
|---|---|
| Location | Counts (Log CFU/mL ± SE) |
| Live Receiving | 0.20 ± 0.61 |
| Rehanger | 0.03 ± 0.14 |
| Post-Evisceration | 0.01 ± 0.07 |
| Pre-Chill | 0.00 ± 0.00 |
| Post-Chill | 0.00 ± 0.00 |
| Pre-Dip Wings Portions | 0.00 ± 0.00 |
| Wings | 0.00 ± 0.00 |
| Pre-Dip Tenders | 0.00 ± 0.00 |
| Packaging Tenders | 0.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez-Velado, D.R.; Vargas, D.A.; Sanchez-Plata, M.X. Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points. Foods 2024, 13, 180. https://doi.org/10.3390/foods13020180
Chavez-Velado DR, Vargas DA, Sanchez-Plata MX. Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points. Foods. 2024; 13(2):180. https://doi.org/10.3390/foods13020180
Chicago/Turabian StyleChavez-Velado, Daniela R., David A. Vargas, and Marcos X. Sanchez-Plata. 2024. "Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points" Foods 13, no. 2: 180. https://doi.org/10.3390/foods13020180





