Simultaneous Analysis of Organic Acids, Glycerol and Phenolic Acids in Wines Using Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Wines
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Chromatographic Conditions
2.2.3. Linearity Range and Limits of Detection and Quantification
- -
- LOD (mg) = 3.3 × RMSE/slope of the curve;
- -
- LOQ (mg) = 10 × RMSE/slope of the curve.
2.2.4. Recovery and the Matrix Effect
2.2.5. Comparison of Our GC Method with Other Analytical Methods
2.3. Statistical Analysis
3. Results
3.1. Effects of Extraction Solvents
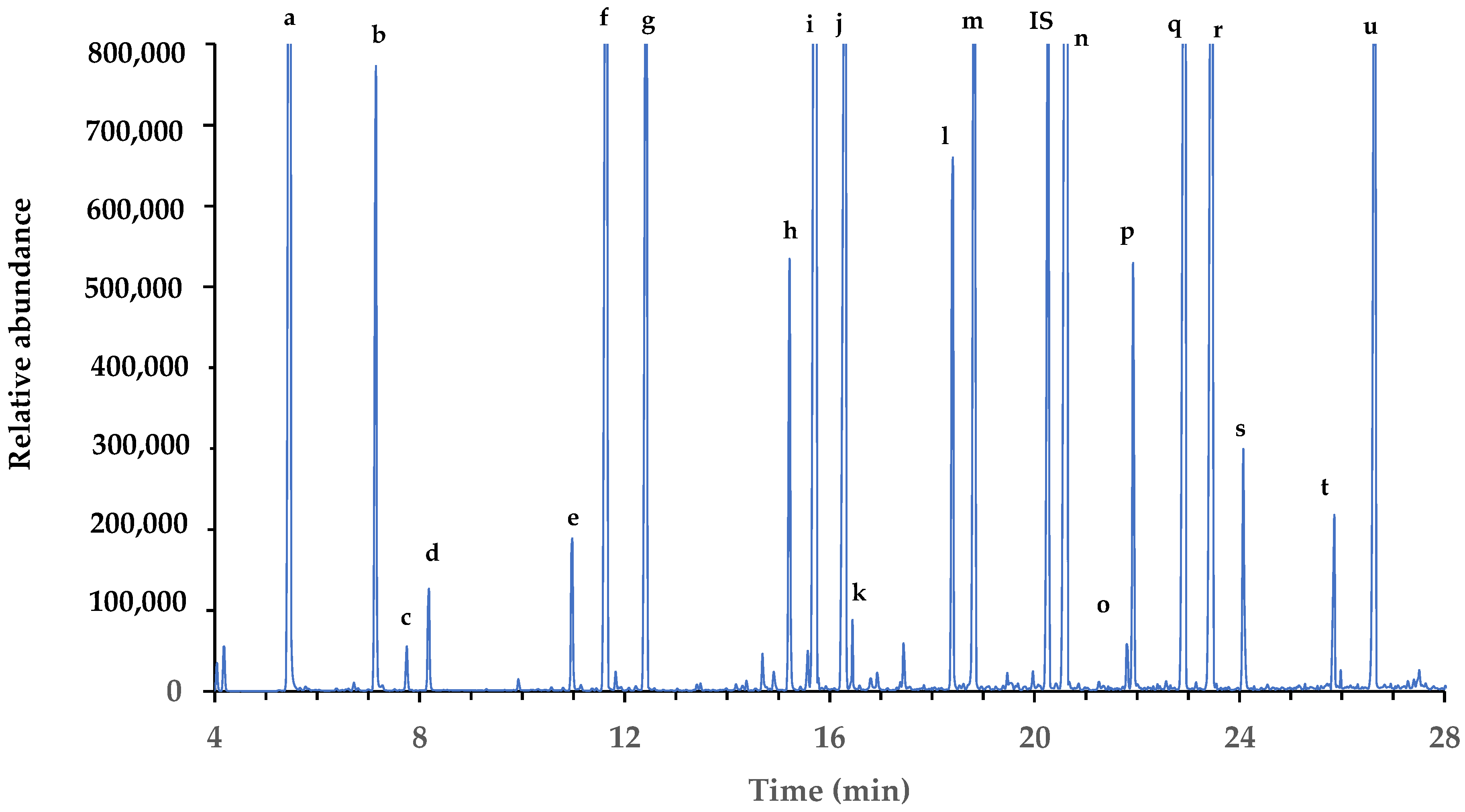
3.2. Detection of Organic and Phenolic Acids
3.3. Linearity
3.4. Limits of Detection (LOD) and Quantification (LOQ)
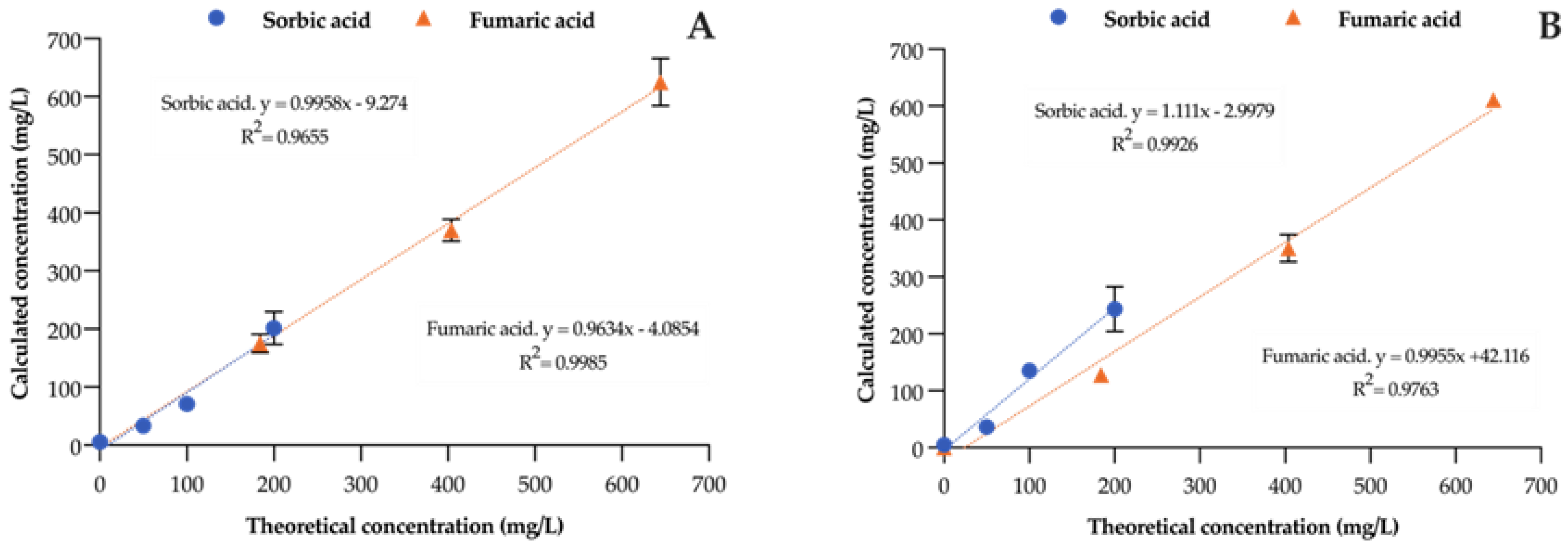
3.5. Recovery
3.6. Comparison of the Performance of the Proposed Method and Other Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The Microbiology of Wine and Vinifications. In Handbook of Enology, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 1, pp. 154–196. ISBN 0-470-01034-7. [Google Scholar]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity: A review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeast and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- OIV. RESOLUTION OIV-OENO 581A-2021. Treatment with Fumaric Acid in Wine to Inhibit Malolactic Fermentation. 2021. Available online: https://www.oiv.int/public/medias/8084/en-oiv-oeno-581a-2021.pdf (accessed on 6 June 2022).
- Morata, A.; Bañuelos, M.A.; López, C.; Song, C.; Vejarano, R.; Loira, I.; Palomerp, F.; Suarez Lepe, J.A. Use of fumaric acid to control pH and inhibit malolactic fermentation in wines. Food Addit. Contam. Part A 2020, 37, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Probl. Chall. Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. Hum. Nutr. Metab. 2001, 1, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Laccase and tyrosinase biosensors used in the determination of hydroxycinnamic acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Felipe Laurie, V. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses Andrew. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins—An overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Metabolites 2: Tri- and dicarboxylic acids, purines, pyrimidines and derivates, coenzymes, inorganic compounds. In Methods of Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1985; Volume VIII, pp. 78–85. [Google Scholar]
- Mato, I.; Suárez-Luque, S.; Huidobro, J.F. A review of the analytical methods to determine organic acids in grape juices and wines. Food Res. Int. 2005, 38, 1175–1188. [Google Scholar] [CrossRef]
- Milovanovic, M.; Žeravíka, J.; Obořila, M.; Pelcováa, M.; Lacinab, K.; Cakarc, U.; Petrovicd, A.; Glatza, Z.; Skládala, P. A novel method for classification of wine based on organic acids. Food Chem. 2019, 284, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E. Optimization of a Solid-Phase Extraction Method Using Copolymer Sorbents for Isolation of Phenolic Compounds in Red Wines and Quantification by HPLC. J. Agric. Food Chem. 2008, 56, 11560–11570. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcés, N.; Gionfriddo, E.; German Gómez-Ríos, G.A.; Alam, M.d.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions Advantages and disadvantages of selected microextraction techniques. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, J.; Lancioni, C.; Purcaro, G. Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis. Methods Protoc. 2022, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Monitoring of minor compounds in corn oil oxidation by direct immersionsolid phase microextraction-gas chromatography/mass spectrometry. New oil oxidation markers. Food Chem. 2019, 290, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Varughese, B.; Ramji, S.; Kapoor, S. Liquid-Liquid Extraction and Solid Phase Extraction for Urinary Organic Acids: A Comparative Study from a Resource Constraint Setting. Ind. J. Clin. Biochem. 2016, 31, 414–422. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef]
- Van der Werf, M.J.; Overkamp, K.M.; Muilwijk, B.; Coulier, L.; Hankemeier, T. Microbial metabolomics: Toward a platform with full metabolome coverage. Anal. Biochem. 2007, 370, 17–25. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Wittmann, B.M.; Evans, A.M.; Miller, L.A.; Toal, D.R.; Lonergan, S.; Elsea, S.H.; Pappan, K.L. Metabolomics in the clinic. A review of the shared and unique features of untargeted metabolomics for clinical research and clinical testing. J. Mass Spectrom. 2018, 53, 1143–1154. [Google Scholar] [CrossRef]
- Kell, D.B.; Brown, M.; Davey, H.M.; Dunn, W.B.; Spasic, I.; Oliver, S.G. Metabolic footprinting and systems biology. The medium is the message. Nat. Rev. Microbiol. 2005, 3, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.R.; Sousa Furtado, D.Z.; Vilela de Moura Leite, F.B.; Antonio de Assunção, N.; Carrilhoa, E. Metabolic profiling of organic acids in urine samples of Cri Du Chat syndrome individuals by gas chromatography-mass spectrometry. J. Chromatogr. B 2020, 1153, 122267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Lan, Y.B.; Zhu, B.Q.; Xiang, X.F.; Duan, C.Q.; Shia, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. J. Sci. Food Agric. 2018, 98, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D.; Held, H.W. Synthesis, Storage, and Stability of [4-¹⁴C] Oxaloacetic Acid. Anal Biochem. 1985, 145, 393–397. [Google Scholar] [CrossRef]
- Tanaka, K.; Hine, D.G. Compilation of gas chromatographic retention indices of 163 metabolically important organic acids, and their use in detection of patients with organic acidurias. J. Chromatogr. 1982, 239, 301–322. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline Q2(R2) on Validation of Analytical Procedures Step 2b. Committee for Medicinal Products for Human Use. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 30 September 2023).
- Heyen, S.; Scholz-Böttcher, B.M.; Ralf Rabus, R.; Wilkes, H. Method development and validation for the quantification of organic acids in microbial samples using anionic exchange solid-phase extraction and gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 7491–7503. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Quantitative Chemical Analysis; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- Ruiz-de Villa, C.; Poblet, M.; Bordons, A.; Reguant, C.; Rozès, N. Comparative study of inoculation strategies of Torulaspora delbrueckii and Saccharomyces cerevisiae on the performance of alcoholic and malolactic fermentations in an optimized synthetic grape must. Int. J. Food Microbiol. 2023, 404, 110367. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, 73076. [Google Scholar] [CrossRef]
- Des Rosiers, C.; Fernandez, C.A.; David, F.; Brunengraber, H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. J. Biol. Chem. 1994, 269, 27179–27182. [Google Scholar] [CrossRef]
- Price, E.K.; Prakash, B.; Domino, M.M.; Pepich, B.V.; Munch, D.J. EPA Document #: 815-R-05-005. METHOD 527. Determination of Selected Pesticides and Flame Retardants in Drinking Water by Solid Phase Extraction and Capillary Column Gas Chromatography/Mass Spectrometry (GC/MS). 2005. Available online: https://nepis.epa.gov/Exe/ (accessed on 15 November 2023).
- Bortz, D.; Kirschner, M.I. Methods and Compositions for Enhancing Iron Absorption. US 2016/ 0022631 A1, 28 January 2016. [Google Scholar]
- Nagai, R.; M. Nagai, M.; Shimasaki, S.; Baynes, J.W.; Fujiwara, Y. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem. Biophys. Res. Commun. 2010, 393, 118e122. [Google Scholar] [CrossRef] [PubMed]
- Koeck, M.M.; Jellema, R.H.; van der Greef, J.; Tas, A.C.; Hankemeier, T. Quantitative metabolomics based on gas chromatography mass spectrometry: Status and perspectives. Metabolomics 2011, 7, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Dumlao, D.S.; Hertz, N.; Clarke, S. Secreted 3-isopropylmalate methyl ester signals invasive growth during amino acid starvation in Saccharomyces cerevisiae. Biochemistry 2008, 47, 698–709. [Google Scholar] [CrossRef] [PubMed]
| Sample (μL) | Standard (μL) | |
|---|---|---|
| Internal Standard (C13) | 10 | 10 |
| Sample | 400 | (10 to 400) |
| Ultrapure Milli-Q water | - | Q.S.* 400 |
| 30 μL of 30% (w/v) NaOH in ultrapure Milli-Q water | ||
| 80 μL of 2.5% (w/v) hydroxylamine-HCl in ultrapure Milli-Q water | ||
| 60 °C, 30 min | ||
| 80 μL of 6 N HCl in ultrapure Milli-Q water 2 × 400 μL of ethyl acetate | ||
| Sample dried for 40’ under vacuum at 45 °C | ||
| 50 μL of MTSFA | ||
| 70 °C, 30 min | ||
| Injection of 3 μL into the GC-MS system | ||
| Compounds | LOD (mg/L) | LOQ (mg/L) | Concentration Range (mg/L) | Linear Regression Equation | R2 |
|---|---|---|---|---|---|
| Lactic acid | 8.20 | 24.84 | 2.69–4292 | y = 0.0044x + 0.4166 | 0.9939 |
| Glyoxylic acid | 0.58 | 1.74 | 0.38–600 | y = 0.0242x − 0.0119 | 0.9984 |
| Pyruvic acid | 2.85 | 8.63 | 0.90–718 | y = 0.0007x + 0.0084 | 0.9933 |
| Sorbic acid | 7.14 | 21.64 | 0.20–312 | y = 0.0126x − 0.0399 | 0.9955 |
| Glycerol | 41.07 | 124.44 | 4.46–8600 | y = 0.0009x − 0.0914 | 0.9916 |
| Succinic acid | 1.43 | 4.33 | 0.81–1287 | y = 0.0195x + 0.2219 | 0.9958 |
| Fumaric acid | 4.16 | 12.61 | 0.72–1147 | y = 0.0315x + 0.0705 | 0.9964 |
| Citramalic acid | 1.88 | 5.70 | 0.30–474 | y = 0.0099x + 0.0485 | 0.9973 |
| Malic acid | 11.98 | 36.31 | 3.11–4976 | y = 0.0048x + 0.4983 | 0.9907 |
| Cinnamic acid | 6.76 | 20.48 | 0.69–228 | y = 0.0242x − 0.0385 | 0.9980 |
| Oxaloacetic acid | 2.51 | 7.61 | 0.68–540 | y = 0.0131x + 0.0112 | 0.9997 |
| α-Ketoglutaric acid | 1.03 | 3.11 | 0.33–519 | y = 0.0225x + 0.0765 | 0.9938 |
| Tartaric acid | 5.87 | 17.77 | 4.42–7042 | y = 0.0018x − 0.0076 | 0.9990 |
| Vanillic acid | 3.50 | 10.61 | 0.59–193 | y = 0.0392x + 0.0372 | 0.9966 |
| Shikimic acid | 30.13 | 91.31 | 5.00–200 | y = 0.0019x − 0.0198 | 0.9924 |
| Citric acid | 11.80 | 35.76 | 0.76–1207 | y = 0.0035x + 0.0305 | 0.9980 |
| Syringic acid | 5.37 | 16.27 | 0.74–243 | y = 0.0366x − 0.3418 | 0.9921 |
| p-Coumaric acid | 8.16 | 24.72 | 0.72–238 | y = 0.0305x − 0.0441 | 0.9989 |
| Gallic acid | 5.82 | 17.62 | 0.75–252 | y = 0.0084x − 0.0814 | 0.9915 |
| Ferulic acid | 9.29 | 28.16 | 0.57–189 | y = 0.0040x − 0.0075 | 0.9985 |
| Caffeic acid | 1.39 | 4.21 | 0.75–248 | y = 0.0184x − 0.0432 | 0.9967 |
| White Wine | Aged Red Wine | |||||
|---|---|---|---|---|---|---|
| Compounds | Equation | R2 | % Recovery | Equation | R2 | % Recovery |
| Lactic acid | y = 4.8462x + 310.93 | 0.9519 | 485 | y = 9.2146x + 6625.7 | 0.9533 | 921 |
| Glyoxylic acid | y = 0.8179x + 4.1975 | 0.983 | 82 | y = 0.5982x + 5.8733 | 0.9804 | 60 |
| Pyruvic acid | y = 1.0722x + 37.524 | 0.9932 | 107 | y = 1.7071x + 17.13 | 0.9927 | 170 |
| Sorbic acid | y = 0.9958x − 9.274 | 0.9655 | 99 | y = 1.111x − 2.9979 | 0.9926 | 111 |
| Glycerol | y = 0.5778x + 364.03 | 0.9657 | 58 | y = 0.3196x + 849.95 | 0.9503 | 32 |
| Succinic acid | y = 1.5125x + 477.31 | 0.9917 | 151 | y = 1.5164x + 952.79 | 0.9868 | 152 |
| Fumaric acid | y = 0.9634x − 4.0854 | 0.9985 | 96 | y = 0.9955x + 42.116 | 0.9763 | 99 |
| Citramalic acid | y = 1.061x + 0.6636 | 0.9982 | 106 | y = 1.4497x + 5.7235 | 0.9877 | 145 |
| Malic acid | y = 2.262x + 886.46 | 0.9929 | 262 | y = 1.3465x − 35.462 | 0.9806 | 135 |
| Cinnamic acid | y = 0.769x + 1.792 | 0.9855 | 77 | y = 0.7845x + 5.3769 | 0.9256 | 78 |
| Oxaloacetic acid | y = 0.2647x − 0.1312 | 0.999 | 26 | y = 0.0368x + 9.3218 | 0.8198 | 4 |
| α-Ketoglutaric acid | y = 0.9588x + 12.697 | 0.9947 | 96 | y = 0.8811x + 33.45 | 0.9963 | 88 |
| Tartaric acid | y = 0.1639x + 290.13 | 0.932 | 16 | y = 1.477x + 3143.3 | 0.9791 | 148 |
| Vanillic acid | y = 0.9177x + 1.2922 | 0.9802 | 92 | y = 1.1262x + 7.347 | 0.9524 | 113 |
| Shikimic acid | y = 0.5808x + 41.308 | 0.3349 | 58 | y = 0.0250x − 0.0680 | 0.5273 | 2 |
| Citric acid | y = 1.2404x + 279.13 | 0.9896 | 124 | y = 1.4104x + 31.261 | 0.9914 | 141 |
| Syringic acid | y = 0.816x + 9.287 | 0.9947 | 82 | y = 1.5691x + 22.363 | 0.938 | 157 |
| p-Coumaric acid | y = 1.4311x + 10.695 | 0.9981 | 143 | y = 1.6319x + 66.095 | 0.9028 | 163 |
| Gallic acid | y = 4.7908x + 5.476 | 0.9905 | 479 | y = 8.9203x + 277.96 | 0.9949 | 892 |
| Ferulic acid | y = 8.491x + 50.09 | 0.9957 | 849 | y = 10.43x + 61.19 | 0.9540 | 1043 |
| Caffeic acid | y = 2.9775x + 6.211 | 0.9955 | 298 | y = 5.8921x + 43.071 | 0.9849 | 589 |
| White Wine | Aged Red Wine | |||||
|---|---|---|---|---|---|---|
| Compounds | Equation | R2 | Concentration | Equation | R2 | Concentration |
| Lactic acid * 52; 105; 210 | y = 0.0206x + 1.1354 | 0.9808 | 65.7 ± 7.7 | y = 0.0907x + 25.043 | 0.9942 | 276.1 ± 9.1 |
| Glyoxylic acid 12.15; 24.3; 48.6 | y = 0.0195x + 0.1105 | 0.9931 | 5.7 ± 0.9 | y = 0.0145x + 0.1617 | 0.9938 | 11.2 ± 0.9 |
| Pyruvic acid 28.8; 57.6; 115.2 | y = 0.0034x + 0.0109 | 0.9955 | 3.3 ± 1.5 | y = 0.0031x + 0.269 | 0.9937 | 87.5 ± 3.6 |
| Sorbic acid 34; 68; 136 | y = 0.0243x + 0.482 | 0.9948 | 19.8 ± 2.2 | y = 0.0134x − 0.0325 | 0.9956 | 2.4 ± 1.6 |
| Glycerol 102; 204; 408 552; 1104; 1656 | y = 0.0029x + 0.0363 y = 0.042x + 0.0121 | 0.9939 0.9846 | 12.6 ± 5.5 3460 ± 280 | y = 0.0046x + 7.6979 y = 0.067x + 0.012 | 0.987 0.9711 | 1673.5 ± 66.9 5667 ± 313 |
| Succinic acid 49.7; 99.4; 198.8 | y = 0.0352x + 9.2003 | 0.9933 | 261.4 ± 9.2 | y = 0-0356x + 18.754 | 0.9933 | 526.8 ± 16.0 |
| Fumaric acid 33.2; 66.4; 132.8 | y = 0.0389x − 0.0235 | 0.9912 | 0.6 ± 0.2 | y = 0.0388x + 0.0394 | 0.9904 | 1.0 ± 0.2 |
| Citramalic acid 25.4; 50.8; 101.6 | y = 0.0121x + 0.023 | 0.9965 | 2.1 ± 1.1 | y = 0.015x + 0.0449 | 0.9943 | 3.0 ± 1.5 |
| Malic acid 105; 210; 420 | y = 0.0123x + 4.2333 | 0.9918 | 344.2 ± 15.8 | y = 0.0076x + 0.1612 | 0.9910 | 21.2 ± 7.8 |
| Cinnamic acid 11.4; 22.8; 45.6 | y = 0.0201x + 0.017 | 0.9976 | 0.08 ± 0.03 | y = 0.0208x + 0.1251 | 0.9982 | 6.01 ± 0.40 |
| Oxaloacetic acid 15; 30; 60 | y = 0.0037x − 0.0008 | 0.9981 | 5.6 ± 2.5 | y = 0.0022x + 01258 | 0.9845 | 55.5 ± 3.4 |
| α-Ketoglutaric acid 25.5; 51; 102 | y = 0.0215x + 0.3624 | 0.9952 | 16.9 ± 1.6 | y = 0.023x + 0.8483 | 0.9976 | 38.0 ± 1.4 |
| Tartaric acid 102.5; 205; 410 | y = 0.0103x + 8.1485 | 0.9918 | 789.6 ± 28.3 | y = 0.0077x + 5.5463 | 0.9908 | 722.5 ± 27.8 |
| 552; 1104; 1656 | y = 0.0031x + 5.7264 | 0.9931 | 1807 ± 320 | y = 0.0032x + 9.7186 | 0.9717 | 3019 ± 210 |
| Vanillic acid 9.7; 19.4; 38.8 | y = 0.0377x + 0.0844 | 0.9903 | 2.24 ± 0.63 | y = 0.0394x + 0.3522 | 0.9957 | 8.94 ± 0.52 |
| Citric acid 50.5; 101; 202 | y = 0.0043x + 1.0243 | 0.9926 | 237.1 ± 9.2 | y = 0.005x + 0.1555 | 0.9971 | 31.4 ± 2.4 |
| Syringic acid 12.2; 24.4; 48.6 | y = 0.03x + 0.0714 | 0.9962 | 2.38 ± 0.49 | y = 0.0429x + 0.7869 | 0.9949 | 18.35 ± 0.83 |
| p-Coumaric acid 11.9; 23.8; 47.6 | y = 0.053x + 0.1340 | 0.9981 | 2.54 ± 0.34 | y = 0.0570x + 2.2733 | 0.9876 | 39.91 ± 1.89 |
| Gallic acid 12.6; 25.2; 50.4 | y = 0.0164x + 0.1751 | 0.9946 | 10.67 ± 0.74 | y = 0.0709x + 2.0484 | 0.9983 | 30.51 ± 0.62 |
| Ferulic acid 9.4; 18.8; 37.6 | y = 0.03751x + 0.10054 | 0.9936 | 2.68 ± 0.63 | y = 0.0269x + 0.1944 | 0.9929 | 7.23 ± 0.75 |
| Caffeic acid 12.4; 24.8; 49.6 | y = 0.0315x + 0.1202 | 0.9939 | 3.82 ± 0.65 | y = 0.0925x + 0.9057 | 0.9969 | 9.79 ± 0.54 |
| White Wine | Aged Red Wine | |||
|---|---|---|---|---|
| Enzymatic | GC | Enzymatic | GC | |
| Glycerol | 4.04 ± 0.47 | 3.46 ± 0.28 | 10.47 ± 0.20 | 5.67 ± 0.31 |
| p-value | 0.016 | <0.0001 | ||
| Colorimetric | GC | Colorimetric | GC | |
| Tartaric acid | 1.92 ± 0.09 | 1.81 ± 0.32 | 3.13 ± 0.07 | 3.02 ± 0.21 |
| p-value | 0.2607 | 0.0992 | ||
| HPLC | GC | HPLC | GC | |
| p-Coumaric acid | 2.11 ± 1.26 | 2.54 ± 0.34 | 1.30 ± 0.69 | 39.91 ± 1.89 |
| p-value | 0.1975 | <0.0001 | ||
| Ferulic acid | 2.59 ±0.82 | 2.68 ± 0.63 | 2.64 ± 0.23 | 7.23 ± 0.75 |
| p-value | 0.7302 | <0.0001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Viñola, V.; Ruiz-de-Villa, C.; Gombau, J.; Poblet, M.; Bordons, A.; Reguant, C.; Rozès, N. Simultaneous Analysis of Organic Acids, Glycerol and Phenolic Acids in Wines Using Gas Chromatography-Mass Spectrometry. Foods 2024, 13, 186. https://doi.org/10.3390/foods13020186
Garcia-Viñola V, Ruiz-de-Villa C, Gombau J, Poblet M, Bordons A, Reguant C, Rozès N. Simultaneous Analysis of Organic Acids, Glycerol and Phenolic Acids in Wines Using Gas Chromatography-Mass Spectrometry. Foods. 2024; 13(2):186. https://doi.org/10.3390/foods13020186
Chicago/Turabian StyleGarcia-Viñola, Violeta, Candela Ruiz-de-Villa, Jordi Gombau, Montse Poblet, Albert Bordons, Cristina Reguant, and Nicolas Rozès. 2024. "Simultaneous Analysis of Organic Acids, Glycerol and Phenolic Acids in Wines Using Gas Chromatography-Mass Spectrometry" Foods 13, no. 2: 186. https://doi.org/10.3390/foods13020186
APA StyleGarcia-Viñola, V., Ruiz-de-Villa, C., Gombau, J., Poblet, M., Bordons, A., Reguant, C., & Rozès, N. (2024). Simultaneous Analysis of Organic Acids, Glycerol and Phenolic Acids in Wines Using Gas Chromatography-Mass Spectrometry. Foods, 13(2), 186. https://doi.org/10.3390/foods13020186