A Literature Review of the Pharmacological Effects of Jujube
Abstract
1. Introduction
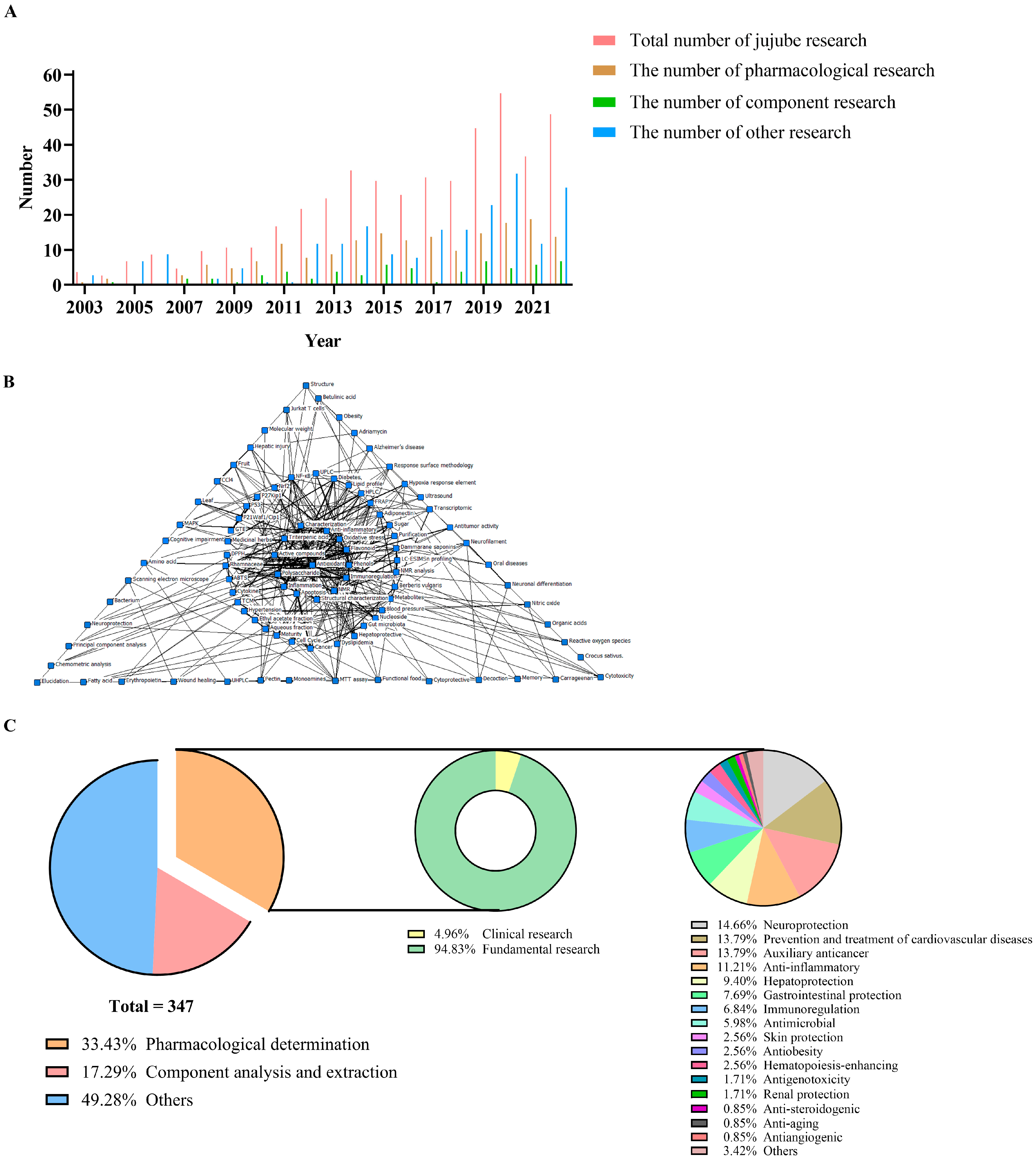
2. Data Collection
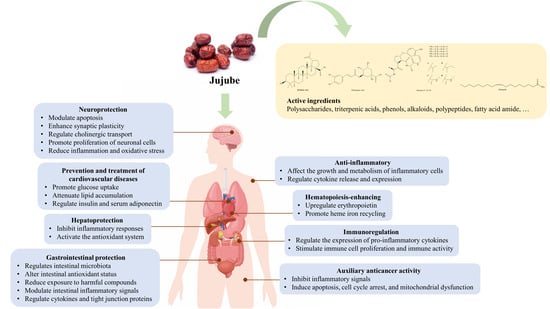
3. Active Ingredients and Pharmacological Effects
3.1. Neuroprotection
| Diseases | Models | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Cerebral ischemia | Rats and gerbils: I/R, MACo | In vivo | i.g.: 100 mg/kg 100, 250, 500 mg/kg | ↑SOD ↑BDNF, NeuN-immunoreactive neurons ↓Reactive gliosis ↓HNE, MDA, NO ↓Neurological deficit score, motor dysfunction, cerebral infarct volume | [29,30] |
| AD | Rats and mice: scopolamine, D-galactose, NBM | In vivo | p.o.: 16, 32 mg/d (oleamide) 1 14–16 mg/d 29, 57, 114 mg/kg | ↑Learning and memory ↑SOD, FRAP, GSH ↑ACh ↑Neurons ↓ALT, AST, AChE, BChE, GFAP, Iba-1 ↓Caspase3, 9 ↓IL-1β, TNF-α, IL-6, INF-β | [15,31,33] |
| Epilepsy | Rats: maximal electroshock, pentylenetetrazole | In vivo | i.p.: 100, 250, 500 mg/kg p.o.: 500 mg/kg | ↑Learning memory ↑GSH, AChE, BChE ↓MDA ↓THLE, GTCS | [34,35] |
3.2. Prevention and Treatment of Cardiovascular Diseases
3.3. Auxiliary Anticancer Activity
3.4. Anti-Inflammatory Effects
3.5. Hepatoprotection
3.6. Gastrointestinal Protection
3.7. Others
4. Discussion and Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, S.; Hu, X.; Guo, Y.; Wang, S.; Yang, X.; Wu, Z.; Huang, Y. A comparative plastomic analysis of Ziziphus jujuba var. spinosa (Bunge) Hu ex H. F. Chow and implication of the origin of Chinese jujube. AoB Plants 2023, 15, plad006. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, L.; Li, X. Treatise on Typhoid and Miscellaneous Diseases; Beijing Science and Technology Press: Beijing, China, 2014. [Google Scholar]
- Yang, S.S.; Xiao, Y.P.; Wang, H.T.; Li, Y. Yellow Emperor’s Classic of Internal Medicine; People’s Medical Publishing House: Beijing, China, 1955. [Google Scholar]
- Gu, G.G. Shennong’s Herbal; Lanzhou University Press: Lanzhou, China, 2004. [Google Scholar]
- Kou, X.; Chen, Q.; Li, X.; Li, M.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, X.; Yang, S.; Yu, L.; Gao, Q.; Yang, X.; Zhao, Y. Characterization of the antioxidative polysaccharides from Ziziphus jujube cv. Goutouzao and its tumor-inhibitory effects on human colorectal carcinoma LoVo cells via immunocyte activation. J. Food Biochem. 2020, 44, e13462. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H.; et al. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. [Google Scholar] [CrossRef]
- Kandimalla, R.; Dash, S.; Kalita, S.; Choudhury, B.; Malampati, S.; Kalita, K.; Kalita, B.; Devi, R.; Kotoky, J. Protective Effect of Bioactivity Guided Fractions of Ziziphus jujuba Mill. Root Bark against Hepatic Injury and Chronic Inflammation via Inhibiting Inflammatory Markers and Oxidative Stress. Front. Pharmacol. 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, M.; Lee, B.M.; Kim, O.; Tran, H.N.K.; Lee, S.; Hwangbo, C.; Min, B.S.; Lee, J.H. Triterpenoids from Ziziphus jujuba induce apoptotic cell death in human cancer cells through mitochondrial reactive oxygen species production. Food Funct. 2018, 9, 3895–3905. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Lee, K.T.; Athapaththu, A.; Choi, Y.H.; Hwang, J.; Sim, S.J.; Kang, S.; Kim, G.Y. Flavonoid Glycosides from Ziziphus jujuba var. inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis. Int. J. Mol. Sci. 2021, 22, 7701. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Jiang, X.; Sun, Y.; Zhao, Z.; Li, S. Protective Effect of Flavonoids from Ziziphus jujuba cv. Jinsixiaozao against Acetaminophen-Induced Liver Injury by Inhibiting Oxidative Stress and Inflammation in Mice. Molecules 2017, 22, 1781. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, X.; Liu, C.; Chen, G.; Wu, C.; He, X. Optimization of Extraction Technology and Oxidation Resistance Analysis of Alkaloids in Jujube. Mol. Plant Breed. 2019, 17, 972–977. [Google Scholar] [CrossRef]
- Kang, K.B.; Ming, G.; Kim, G.J.; Ha, T.K.; Choi, H.; Oh, W.K.; Sung, S.H. Jubanines F-J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry 2015, 119, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Park, Y.J.; Suh, Y.M.; Choi, S.J.; Kim, M.J.; Cho, H.Y.; Chang, Y.J.; Hong, B.; Kim, H.K.; Kim, E.; et al. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, F.; Zare-Zardini, H.; Ebrahimi, L. Investigation of the antimicrobial activities of Snakin-Z, a new cationic peptide derived from Zizyphus jujuba fruits. Nat. Prod. Res. 2013, 27, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimers Dis. Other Dement. 2013, 28, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Wang, Y.; Liu, G.; Zhang, Z.; Zhao, Z.; Cheng, H. In vitro antioxidative and immunological activities of polysaccharides from Zizyphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2017, 95, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Yang, Y.; Luo, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhang, W.; Wang, Q.; Zhang, A.; Mu, H.; Bai, H.; Duan, J. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohydr. Polym. 2014, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Hayashida, A.; Tsurushima, K.; Nagai, R.; Yoshitomi, M.; Daiguji, N.; Sakashita, N.; Takeya, M.; Tsukamoto, S.; Ikeda, T. Triterpenoids isolated from Zizyphus jujuba inhibit foam cell formation in macrophages. J. Agric. Food. Chem. 2011, 59, 4544–4552. [Google Scholar] [CrossRef]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids Isolated from Ziziphus jujuba Enhance Glucose Uptake Activity in Skeletal Muscle Cells. J. Nutr. Sci. Vitaminol. 2017, 63, 193–199. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.J.; Yang, H. Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef]
- Lee, D.; Kang, K.B.; Hwang, G.S.; Choi, Y.K.; Kim, T.K.; Kang, K.S. Antioxidant and Anti-Inflammatory Effects of 3-Dehydroxyceanothetric Acid 2-Methyl Ester Isolated from Ziziphus jujuba Mill. against Cisplatin-Induced Kidney Epithelial Cell Death. Biomolecules 2021, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, Y.; Furusawa, Y.; Aradate, T.; Zhao, Q.L.; Moniruzzaman, R.; Kanamori, M.; Noguchi, K.; Kondo, T. 3-O-trans-p-coumaroyl-alphitolic acid, a triterpenoid from Zizyphus jujuba, leads to apoptotic cell death in human leukemia cells through reactive oxygen species production and activation of the unfolded protein response. PLoS ONE 2017, 12, e0183712. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, L.; Huang, Q.; Luo, Y. Quantitative Assessment of Phenolic Acids, Flavonoids and Antioxidant Activities of Sixteen Jujube Cultivars from China. Plant Foods Hum. Nutr. 2020, 75, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Ma, Y.; Chen, J.; Yuan, H.M.; Zheng, Y.Z. Two new C-glucosyl flavonoids from Ziziphus jujube and their anti-inflammatory activity. J. Asian Nat. Prod. Res. 2017, 19, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, X.; Han, A.; Chen, P.; Bai, H. In vitro immunological and anti-complementary activities of two water-soluble lignins from Zizyphus jujube cv. Jinchangzao. Int. J. Biol. Macromol. 2017, 105, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.Y.; Li, H.; Hwang, I.K.; Choi, J.H.; Lee, C.H.; Kwon, D.Y.; Ryu, S.Y.; Kim, Y.S.; Kang, I.J.; Shin, H.C.; et al. Zizyphus attenuates ischemic damage in the gerbil hippocampus via its antioxidant effect. J. Med. Food 2010, 13, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, Y.K. Combination of Zizyphus jujuba and silymarin showed better neuroprotective effect as compared to single agent in MCAo-induced focal cerebral ischemia in rats. J. Ethnopharmacol. 2017, 197, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Djeuzong, E.; Kandeda, A.K.; Djiogue, S.; Stephanie, L.; Nguedia, D.; Ngueguim, F.; Djientcheu, J.P.; Kouamouo, J.; Dimo, T. Antiamnesic and Neuroprotective Effects of an Aqueous Extract of Ziziphus jujuba Mill. (Rhamnaceae) on Scopolamine-Induced Cognitive Impairments in Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 5577163. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Yoo, D.Y.; Choi, J.H.; Lee, C.H.; Kang, I.J.; Kwon, D.Y.; Kim, Y.S.; Kim, D.W.; Won, M.H. Zizyphus enhances cell proliferation and neuroblast differentiation in the subgranular zone of the dentate gyrus in middle-aged mice. J. Med. Food 2011, 14, 195–200. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Nguedia, D.; Ayissi, E.R.; Kouamouo, J.; Dimo, T. Ziziphus jujuba (Rhamnaceae) Alleviates Working Memory Impairment and Restores Neurochemical Alterations in the Prefrontal Cortex of D-Galactose-Treated Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 6610864. [Google Scholar] [CrossRef]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Joshi, S.; Gupta, Y.K. Hydroalcoholic extract of Zizyphus jujuba ameliorates seizures, oxidative stress, and cognitive impairment in experimental models of epilepsy in rats. Epilepsy Behav. 2011, 21, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, M.; Kleekal, T.; Reeta, K.H.; Tripathi, M.; Gupta, Y.K. Interaction profile of Zizyphus jujuba with phenytoin, phenobarbitone, and carbamazepine in maximal electroshock-induced seizures in rats. Epilepsy Behav. 2012, 25, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Ghadiri-Anari, A.; Mehrjardi, A.V.; Dehghani, A.; Zardini, H.Z.; Nadjarzadeh, A. Effect of Ziziphus jujube Fruit Infusion on Lipid Profiles, Glycaemic Index and Antioxidant Status in Type 2 Diabetic Patients: A Randomized Controlled Clinical Trial. Phytother. Res. 2017, 31, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Irannejad Niri, Z.; Shidfar, F.; Jabbari, M.; Zarrati, M.; Hosseini, A.; Malek, M.; Dehnad, A. The effect of dried Ziziphus vulgaris on glycemic control, lipid profile, Apo-proteins and hs-CRP in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. J. Food Biochem. 2021, 45, e13193. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Zohoori, E.; Mehrpour, O.; Karamian, M.; Asghari, S.; Zarban, A.; Nasouti, R. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. Excli. J. 2015, 14, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Kaeidi, A.; Taati, M.; Hajializadeh, Z.; Jahandari, F.; Rashidipour, M. Aqueous extract of Zizyphus jujuba fruit attenuates glucose induced neurotoxicity in an in vitro model of diabetic neuropathy. Iran. J. Basic. Med. Sci. 2015, 18, 301–306. [Google Scholar] [PubMed]
- Mohebbati, R.; Bavarsad, K.; Rahimi, M.; Rakhshandeh, H.; Khajavi Rad, A.; Shafei, M.N. Protective effects of long-term administration of Ziziphus jujuba fruit extract on cardiovascular responses in L-NAME hypertensive rats. Avicenna J. Phytomed. 2018, 8, 143–151. [Google Scholar]
- Zhao, Y.; Yang, X.; Ren, D.; Wang, D.; Xuan, Y. Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice. Food Funct. 2014, 5, 1771–1778. [Google Scholar] [CrossRef]
- Farhadnejad, H.; Asghari, G.; Hedayati, M.; Sahranavard, S.; Teymoori, F.; Mirmiran, P.; Azizi, F. Effect of Ziziphus jujube on cardiometabolic factors and systemic inflammation in type 2 diabetic patients: A randomized controlled trial. Clin. Nutr. ESPEN 2022, 49, 53–60. [Google Scholar] [CrossRef]
- Solati, J.; Soleimani, N. Antihyperglycemic and antihyperlipidemic effects of Ziziphus vulgaris L. on streptozocin-induced [corrected] diabetic adult male Wistar rats. Acta Diabetol. 2010, 47 (Suppl. S1), 219–223. [Google Scholar] [CrossRef]
- Mohebbati, R.; Kamkar-Del, Y.; Shafei, M.N. Effect of Aqueous and Ethyl Acetate Fractions of Ziziphus jujuba Mill Extract on Cardiovascular Responses in Hypertensive Rats. Malays. J. Med. Sci. 2020, 27, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Jing, N.; Wang, L.; Jiang, G.; Liu, Z. Jujube Powder Enhances Cyclophosphamide Efficiency against Murine Colon Cancer by Enriching CD8(+) T Cells While Inhibiting Eosinophilia. Nutrients 2021, 13, 2700. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Wu, W.H.; Chien, S.P.; Liu, C.T.; Liu, M.Y. Dietary Ziziphus jujuba Fruit Attenuates Colitis-Associated Tumorigenesis: A Pivotal Role of the NF-κB/IL-6/JAK1/STAT3 Pathway. Nutr. Cancer 2020, 72, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, R.; Li, H.; Wang, Y.; Luo, Y.; Zou, J.; Zhao, B.; Chen, S. New insight into the joint significance of dietary jujube polysaccharides and 6-gingerol in antioxidant and antitumor activities. RSC Adv. 2021, 11, 33219–33234. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Hsu, B.Y.; Chang, S.C.; Chen, B.H. Antiproliferation of melanoma cells by polysaccharide isolated from Zizyphus jujuba. Nutrition 2012, 28, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Taechakulwanijya, N.; Weerapreeyakul, N.; Barusrux, S.; Siriamornpun, S. Apoptosis-inducing effects of jujube (Zǎo) seed extracts on human Jurkat leukemia T cells. Chin. Med. 2016, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kojima-Yuasa, A.; Norikura, T.; Kennedy, D.O.; Hasuma, T.; Matsui-Yuasa, I. Mechanism of the anti-cancer activity of Zizyphus jujuba in HepG2 cells. Am. J. Chin. Med. 2007, 35, 517–532. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, B.P.; Luo, D.; Shen, X.C.; Guo, S.; Duan, J.A.; Tang, Y.P. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine 2012, 19, 239–244. [Google Scholar] [CrossRef]
- Ruan, J.; Sun, F.; Hao, M.; Han, L.; Yu, H.; Lin, F.; Wang, L.; Cao, G.; Zhang, Y.; Wang, T. Structurally diverse triterpenes obtained from the fruits of Ziziphus jujuba Mill. as inflammation inhibitors by NF-κB signaling pathway. Food Funct. 2021, 12, 4496–4503. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Yoon, J.I.; Kim, H.J.; Kim, J.S.; Kang, S.C. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem. Toxicol. 2010, 48, 639–643. [Google Scholar] [CrossRef]
- Goyal, R.; Sharma, P.L.; Singh, M. Possible attenuation of nitric oxide expression in anti-inflammatory effect of Ziziphus jujuba in rat. J. Nat. Med. 2011, 65, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Mesaik, A.M.; Poh, H.W.; Bin, O.Y.; Elawad, I.; Alsayed, B. In Vivo Anti-Inflammatory, Anti-Bacterial and Anti-Diarrhoeal Activity of Ziziphus jujuba Fruit Extract. Open Access Maced. J. Med. Sci. 2018, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, J.; Jang, C.H.; Lim, J.S.; Lee, J.S.; Kim, J.S. In Vivo Anti-inflammatory Potential of Viscozyme(®)-Treated Jujube Fruit. Foods 2020, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Koohi-Hosseinabadi, O.; Andisheh-Tadbir, A.; Bahadori, P.; Sepehrimanesh, M.; Mardani, M.; Tanideh, N. Comparison of the therapeutic effects of the dietary and topical forms of Zizyphus jujuba extract on oral mucositis induced by 5-fluorouracil: A golden hamster model. J. Clin. Exp. Dent. 2015, 7, e304–e309. [Google Scholar] [CrossRef] [PubMed]
- Maddahi, S.Z.; Jokar, A.; Kamalinejad, M.; Behnampur, N. The efficacy of Jujube syrup on the prevention of drug-induced hepatotoxicity in pulmonary tuberculosis patients: A pilot randomized double-blind placebo-controlled clinical trial. Pharmacol. Res. Perspect. 2022, 10, e00902. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.P.; Kobos, R. Jaundice in the adult patient. Am. Fam. Physician 2004, 69, 299–304. [Google Scholar] [PubMed]
- Ebrahimimd, S.; Ashkani-Esfahani, S.; Poormahmudibs, A. Investigating the efficacy of Zizyphus jujuba on neonatal jaundice. Iran. J. Pediatr. 2011, 21, 320–324. [Google Scholar] [PubMed]
- Hong, S.; Kim, Y.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Jujube (Ziziphus jujuba Mill.) Protects Hepatocytes against Alcohol-Induced Damage through Nrf2 Activation. Evid. Based Complement. Altern. Med. 2020, 2020, 6684331. [Google Scholar] [CrossRef]
- Naftali, T.; Feingelernt, H.; Lesin, Y.; Rauchwarger, A.; Konikoff, F.M. Ziziphus jujuba extract for the treatment of chronic idiopathic constipation: A controlled clinical trial. Digestion 2008, 78, 224–228. [Google Scholar] [CrossRef]
- Huang, Y.L.; Yen, G.C.; Sheu, F.; Chau, C.F. Effects of water-soluble carbohydrate concentrate from Chinese jujube on different intestinal and fecal indices. J. Agric. Food. Chem. 2008, 56, 1734–1739. [Google Scholar] [CrossRef]
- Wang, B. Chemical characterization and Ameliorating effect of polysaccharide from Chinese jujube on intestine oxidative injury by ischemia and reperfusion. Int. J. Biol. Macromol. 2011, 48, 386–391. [Google Scholar] [CrossRef]
- Han, X.; Bai, B.; Zhou, Q.; Niu, J.; Yuan, J.; Zhang, H.; Jia, J.; Zhao, W.; Chen, H. Dietary supplementation with polysaccharides from Ziziphus jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct. 2020, 11, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Guo, C.A.; Yu, W.W.; Yan, X.Y.; Long, J.P.; Liu, Z.C.; Liang, X.Q.; Liu, H.B. Zizyphus jujuba cv. Muzao polysaccharides enhance intestinal barrier function and improve the survival of septic mice. J. Food Biochem. 2021, 45, e13722. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Arian, A.A.; Farzaei, M.H. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/Ethanol-induced gastric mucosal injury in rats. J. Tradit. Chin. Med. 2015, 35, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, J.; Jiang, D.; Liu, Y.; Gou, Z.; Li, J.; Shi, M.; Wang, X.; Guo, Y.; Ma, L.; et al. Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria. Int. J. Biol. Macromol. 2022, 208, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, H.; Wu, X.; Chang, H.; Gao, X.; Liu, M.; Tu, P. Characterization of a pectic polysaccharide from the fruit of Ziziphus jujuba. Chem. Nat. Compd. 2007, 43, 374–376. [Google Scholar] [CrossRef]
- Chi, A.; Kang, C.; Zhang, Y.; Tang, L.; Guo, H.; Li, H.; Zhang, K. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 2015, 122, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Morii, R.; Kojima-Yuasa, A.; Huang, X.; Yano, Y.; Matsui-Yuasa, I. Effect of Zizyphus jujuba extract on the inhibition of adipogenesis in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2009, 37, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lam, C.T.; Li, Z.; Yao, P.; Lin, H.; Dong, T.T.; Tsim, K.W. Extract of Ziziphus jujuba Fruit (Jujube) Stimulates Expression of Enzymes Responsible for Heme Recycle via Anti-oxidant Response Element in Cultured Murine Macrophages. Phytother. Res. 2016, 30, 267–271. [Google Scholar] [CrossRef]
- Chen, J.; Lam, C.T.; Kong, A.Y.; Zhang, W.L.; Zhan, J.Y.; Bi, C.W.; Chan, G.K.; Lam, K.Y.; Yao, P.; Dong, T.T.; et al. The extract of Ziziphus jujuba fruit (jujube) induces expression of erythropoietin via hypoxia-inducible factor-1α in cultured Hep3B cells. Planta Med. 2014, 80, 1622–1627. [Google Scholar] [CrossRef]
- Awad, D.S.; Ali, R.M.; Mhaidat, N.M.; Shotar, A.M. Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharm. Biol. 2014, 52, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Sabzghabaee, A.M.; Khayam, I.; Kelishadi, R.; Ghannadi, A.; Soltani, R.; Badri, S.; Shirani, S. Effect of Zizyphus jujuba fruits on dyslipidemia in obese adolescents: A triple-masked randomized controlled clinical trial. Med. Arch. 2013, 67, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, I.S.; Said, A.; Abdel-Wahhab, M.A. Zizyphus jujuba and Origanum majorana extracts protect against hydroquinone-induced clastogenicity. Environ. Toxicol. Pharmacol. 2008, 25, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mazumder, U.K.; Vamsi, M.L.; Sivakumar, T.; Kandar, C.C. Anti-steroidogenic activity of the two Indian medicinal plants in mice. J. Ethnopharmacol. 2004, 90, 21–25. [Google Scholar] [CrossRef]
- Ghimire, S.; Kim, M.S. Jujube (Ziziphus jujuba Mill.) fruit feeding extends lifespan and increases tolerance to environmental stresses by regulating aging-associated gene expression in Drosophila. Biogerontology 2017, 18, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xu, X.X.; Guo, S.; Xie, H.; Yan, H.; Ma, X.F.; Niu, Y.; Duan, J.A. Wild Jujube (Ziziphus jujuba var. spinosa): A Review of Its Phytonutrients, Health Benefits, Metabolism, and Applications. J. Agric. Food. Chem. 2022, 70, 7871–7886. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef]
- Shi, Q.; Han, G.; Liu, Y.; Jiang, J.; Jia, Y.; Li, X. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis. Food Chem. 2022, 385, 132627. [Google Scholar] [CrossRef]
| Ingredients | Representative Compounds | Pharmacological Activities | Refs. |
|---|---|---|---|
| Polysaccharides | Composed of nine monosaccharides in different ratios: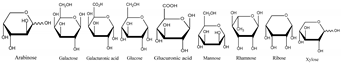 | Antioxidant, anti-inflammatory, auxiliary anticancer, immune-boosting, hypolipidemic, hypoglycemic, antibacterial | [7,18,19,20] |
| Triterpenic acids |  | Anti-inflammatory, auxiliary anticancer, foam cell formation inhibitory, hypoglycemic, antiviral, antioxidant | [21,22,23,24,25] |
| Phenols | 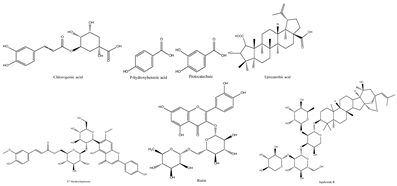 | Antioxidant, anti-inflammatory, immunoregulation | [26,27,28] |
| Alkaloids | 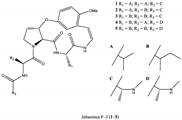 | Antiviral, antioxidant | [13,14] |
| Polypeptides | Snakin-Z | Antibacterial, antioxidant, cholinesterase inhibitory | [16,17] |
| Fatty acid amide | 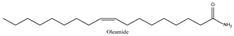 | Cholinesterase activation, antineurotoxicity | [15] |
| Diseases | Models | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Hyperglycemia/Diabetes | Rats: STZ | In vivo | i.g.: 0.25–2 g/kg | ↓FBG, TG, HDL-C ↓ROS ↓Caspase 3 activation | [36,37,39,42,43] |
| PC12 cells: glucose | In vitro | 300 μg/mL | |||
| Patients: type 2 diabetes | Clinical | 30 g/d | ↑ApoA-I, QUICKI ↓FBG, TC, LDL-C, TC/HDL-C, LDL-C/HDL-C, hs-CRP, insulin, HOMA-IR, ApoB100, HbA1c ↓Percentage change of weight, BMI | ||
| High blood pressure | Rats: L-NAME | In vivo | i.v.: 100, 200, 400 mg/kg i.g.: 150, 300 mg/kg | ↓∆SBP, ∆MAP, ∆HR, tachycardia | [40,44] |
| High blood cholesterol | Mice: dyslipidemia | In vivo | i.g.: 200, 400 mg/kg | ↑HDL-C ↓TG, ALT, TC, LDL-C, VLDL-C, hepatic steatosis, AI | [7,41] |
| LO2 cells: oleic acid | In vitro | 100, 200, 300 μg/mL (polysaccharides) | |||
| Atherosclerosis | HMDM cells: Ac-LDL | In vitro | 50 μM (triterpenoids) | ↓Foam cells, CE, ACAT | [21] |
| Hyperinsulinemia | Mice: insulin resistance | In vivo | i.g.: 200, 400 mg/kg (polysaccharides) | ↓Insulin, HOMA-IR, HOMA-β | [41] |
| Diseases | Models | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Colon Cancer | Mice: AOM/DSS | In vivo | p.o.: 5%, 10% (w/w) | ↑ROS ↑Apoptosis, cell cycle arrest ↓Cell proliferation ↓NF-κB/IL-6/JAK1/STAT3 ↓Leukocytes, IL1-β, TNF-α, IL-7, GM-CSF ↓Fecal blood, diarrhea, DAI, spleen weight | [6,10,46,47] |
| Cells: LoVo cells, SW620 cells, HTH29 cells | In vitro | 100, 200, 400 μg/mL (polysaccharides) 25–800 μg/mL (polysaccharides) 100 μg/mL | |||
| Breast Cancer | MDA-MB-231 cells | In vitro | 3, 10, 30 μM (triterpenoids) | ↑Apoptosis | [10] |
| Leukemia | U937 cells, Molt-4 cells, Jurkat cells | In vitro | 10–500 μg/mL (seed extract) 40 μM (triterpenoid) | ↑Apoptosis ↑UPR, ROS ↑Caspase 3, 8, 9 activities ↑CHOP, p38 MAPK, t-BID, XBP1s, BCL2 ↓Bcl2 ↓Cell proliferation ↓Mitochondrial membrane potential | [25,49] |
| Cervical Cancer | HeLa cells, | In vitro | 25–400 μg/mL (polysaccharides) | ↑Apoptosis ↓Cell proliferation | [19] |
| Lung Cancer | A549 cells | In vitro | 1, 10, 50, 100 μg/mL 3, 10, 30 μM (triterpenoids) | ↑Apoptosis ↓Cell proliferation | [10] |
| Liver Cancer | HepG2 cells | In vitro | 100, 200 µg/mL | ↑Apoptosis, cycle block ↑ROS ↑RB, p27Kip1 ↓Mitochondrial membrane potential | [50] |
| Skin Cancer | Melanoma cells | In vitro | 2.5, 3.75, 4.25, 5 mg/mL | ↑Cell cycle arrest ↑Caspase 3, 9 activity ↓Cell proliferation | [48] |
| Prostate Cancer | PC-3 cells | In vitro | 3, 10, 30 μM (triterpenoids) | ↑ROS ↑Apoptosis ↑Cleaved caspase 3, 7, 8, BID, PARP, p38 MAPK activation ↓Mitochondrial membrane potential | [10] |
| Diseases | Models | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Plantar Fasciitis | Rats and mice: foot swelling | In vivo | i.g.: 200, 400 mg/kg 800, 1200, 1600 mg/kg 50, 100, 200, 400 mg/kg (root bark extract) | ↓Paw oedema ↓TNF-α, IL-1β | [9,54,55] |
| Granulomatous inflammation | Rats: granulomas | In vivo | i.g.: 100, 200, 400 mg/kg | ↓Granuloma ↓Nitrite/nitrate | [54] |
| Dermatitis | Mice: TPA | In vivo | 1%, 10% (seed essential oil) | ↓Ear thickness, water content | [53] |
| Pneumonia | Mice: benzo(a)pyrene | In vivo | p.o.: 1.5 g/kg 0.75 g/kg | ↑NRF2, HO-1 ↑PGE2, GSH/GSSG ↓iNOS, COX-2 ↓MDA, 8-OHdG ↓lung tissue injury ↓NF-κB, TNF-α, IL-1β | [56] |
| A549 cells: TPA | In vitro | 500 μg/mL | |||
| Mucositis of the oral cavity | Golden hamsters | In vivo | p.o: 300 mg/kg Application: 20% | ↑MPO, SOD ↓MDA ↓Histopathology score | [57] |
| Diseases | Model | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Non-alcoholic liver injury | Mice: CCl4, acetaminophen | In vivo | i.g.: 100, 200, 400 mg/kg (flavonoids) p.o.: 100, 200, 400 mg/kg (polysaccharides) | ↑CAT ↑NRF2 ↑SOD, GSH-Px, GSH, NQO1 ↓NF-κB ↓MDA, lipid peroxidation ↓ALT, AST, ALP, TB, LDH ↓TNF-α, IL-6, IL-1β, IL-10 | [8,9,12,58] |
| HepG2 cells: CCl4 | In vitro | 100 μg/mL (root bark extract) | |||
| Patients: tuberculosis | Clinical | 10 mL/d (jujube syrup) | ↑QOL ↓Cough ↓Hepatotoxicity | ||
| Alcoholic liver disease | Mice: alcohol | In vivo | i.g.: 0.02 g/kg | ↑GSH ↑Cell viability ↑NRF2, HO-1, NQO1, GCLC ↓AST, ALT ↓MDA, ROS ↓CYP2E1, TNF-α ↓Histological lesions | [61] |
| HepG2 cells: alcohol | In vitro | 100 μg/mL | |||
| Jaundice | Patients: jaundiced newborns | Clinical | 1 mg/kg | ↓Bilirubin | [60] |
| Diseases | Models | Type | Administration | Effects | Refs. |
|---|---|---|---|---|---|
| Intestinal mucosal injury | Rabbits: I/R Golden hamsters | In vivo | p.o.: 200, 400 mg/kg (polysaccharides) 1.7 g, 5.0 g, 15 g/kg diet | ↑SCFAs ↑Fecal moisture ↑GSH, GSH-Px, SOD, CAT, ↓MDA ↓Cecal ammonia ↓Bacterial enzymes ↓Gastrointestinal transit time | [63,64] |
| Intestinal barrier dysfunction | Mice: sepsis, cyclophosphamide | In vivo | i.g.: 150, 300, 600 mg/kg (polysaccharides) 200, 500, 1000 mg/kg (polysaccharides) | ↑Survival ↑IgA, SIgA ↑Splenic lymphocytes ↑Firmicutes/Bacteroidetes ↑ZO-1, claudin-1, occluding ↑IL-2, IL-4, IL-10, INF-γ, TNF-α ↓DAO ↓BCL2, BAX, caspase 3 ↓TLR4/NF-κB signaling ↓CD3+ and CD4+ spleen T lymphocytes, CD4+/CD8+ ↓intestinal mucosal damage | [65,66] |
| Gastric ulcer | Mice: gastric ulcer | In vivo | 100, 200, 400 mg/kg (stem bark extract) | ↓Ulcer area, submucosal edema, interstitial hemorrhage | [67] |
| Diarrheal disease | Mice: acute diarrhea | In vivo | 800, 1200, 1600 mg/kg | ↓Diarrhea | [55] |
| Chronic constipation | Patients: chronic constipation | Clinical | 20–40 drops | ↑QOL score ↓Transit time ↓Symptom severity ratings | [62] |
| Effects | Models | Type | Administration | Findings | Refs. |
|---|---|---|---|---|---|
| Immunoregulation | Rat: chronic fatigue Syndrome | In vivo | p.o.: 100, 200, 400 mg/kg (polysaccharides) | ↑IL-2 ↓IL-10 ↑CD4+/CD8+, T cell proliferation, NK cell activity | [70] |
| Antimicrobial | Oral pathogenic bacteria: streptococcus mutans, MRSA, porphyromonas gingivalis | In vitro | (Polysaccharides) | ↓Biofilm formation, host cell adhesion, host cell invasion, cytotoxicity | [68] |
| Skin protection | Zebrafish larvae | In vivo | 20 μM (flavonoid glycosides) | ↓Melanogenesis ↓Tyrosinase activity ↓cAMP/CREB/MITF | [11] |
| B16F10 cells | In vitro | ||||
| Anti-obesity | Obese adolescents | Clinical | 15 g/day | ↓LDL-C, TC | [75] |
| Hematopoiesis-enhancing | RAW 264.7 cells | In vitro | 0.187–3.0 mg/mL | ↑HO-1, biliverdin reductase A and B, ferroportin | [72] |
| Antigenotoxicity | Mice: hydroquinone | In vivo | p.o.: 0.5 g/kg (leaf extract) | ↓Chromosomal aberrations | [76] |
| Renal protection | Rats: ibuprofen | In vivo | p.o.: 500 mg/kg | ↑Albumin, total protein ↑Body weight ↓Urea, creatinine ↓Hypercellularity and shrinkage in glomeruli lines, ischemia in proximal convoluted tubules and congestion | [74] |
| Anti-steroidogenic | Adult female mice | In vivo | p.o.: 60, 120, 240 mg/kg (bark extract) | ↓Estrus cycle ↓Wet weight of ovaries ↓Cholesterol, ascorbic acid, Δ5-3β-hydroxysteroid dehydrogenase, glucose-6-phosphate dehydrogenase | [77] |
| Anti-aging | Drosophila | In vivo | p.o.: 30, 150 mg/mL | ↓lifespan, healthspan ↑d4E-BP mRNA transcript ↓mRNA levels of 14-3-3ε | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Jiang, N.; Wang, N.; Zhao, Y.; Liu, X. A Literature Review of the Pharmacological Effects of Jujube. Foods 2024, 13, 193. https://doi.org/10.3390/foods13020193
Zhu D, Jiang N, Wang N, Zhao Y, Liu X. A Literature Review of the Pharmacological Effects of Jujube. Foods. 2024; 13(2):193. https://doi.org/10.3390/foods13020193
Chicago/Turabian StyleZhu, Deqi, Ning Jiang, Ning Wang, Yufen Zhao, and Xinmin Liu. 2024. "A Literature Review of the Pharmacological Effects of Jujube" Foods 13, no. 2: 193. https://doi.org/10.3390/foods13020193
APA StyleZhu, D., Jiang, N., Wang, N., Zhao, Y., & Liu, X. (2024). A Literature Review of the Pharmacological Effects of Jujube. Foods, 13(2), 193. https://doi.org/10.3390/foods13020193