Effect of Microwave Intermittent Drying on the Structural and Functional Properties of Zein in Corn Kernels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microwave Intermittent Drying of Corn Kernels
2.3. Measurement of Protein Content
2.4. Treatments and Preparation of Zein
2.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Circular Binary Chromatography
2.7. Measurement of Free Sulfhydryl (-SH) Groups
2.8. Measurement of Endogenous Fluorescence Spectra
2.9. Measurement of Particle Size and Polydispersity Index
2.10. Measurement of Surface Hydrophobicity (H0)
2.11. Measurement of Solubility
2.12. Thermal Stability (DSC) Analysis
2.13. Measurement of the Emulsification and Emulsion Stability
2.14. Statistical Analysis
3. Results and Discussion
3.1. Effect of Microwave Power on Drying Characteristics of Corn Kernels
3.2. Changes in Total Protein Content and Extraction Rate of Zein from Corn Kernels
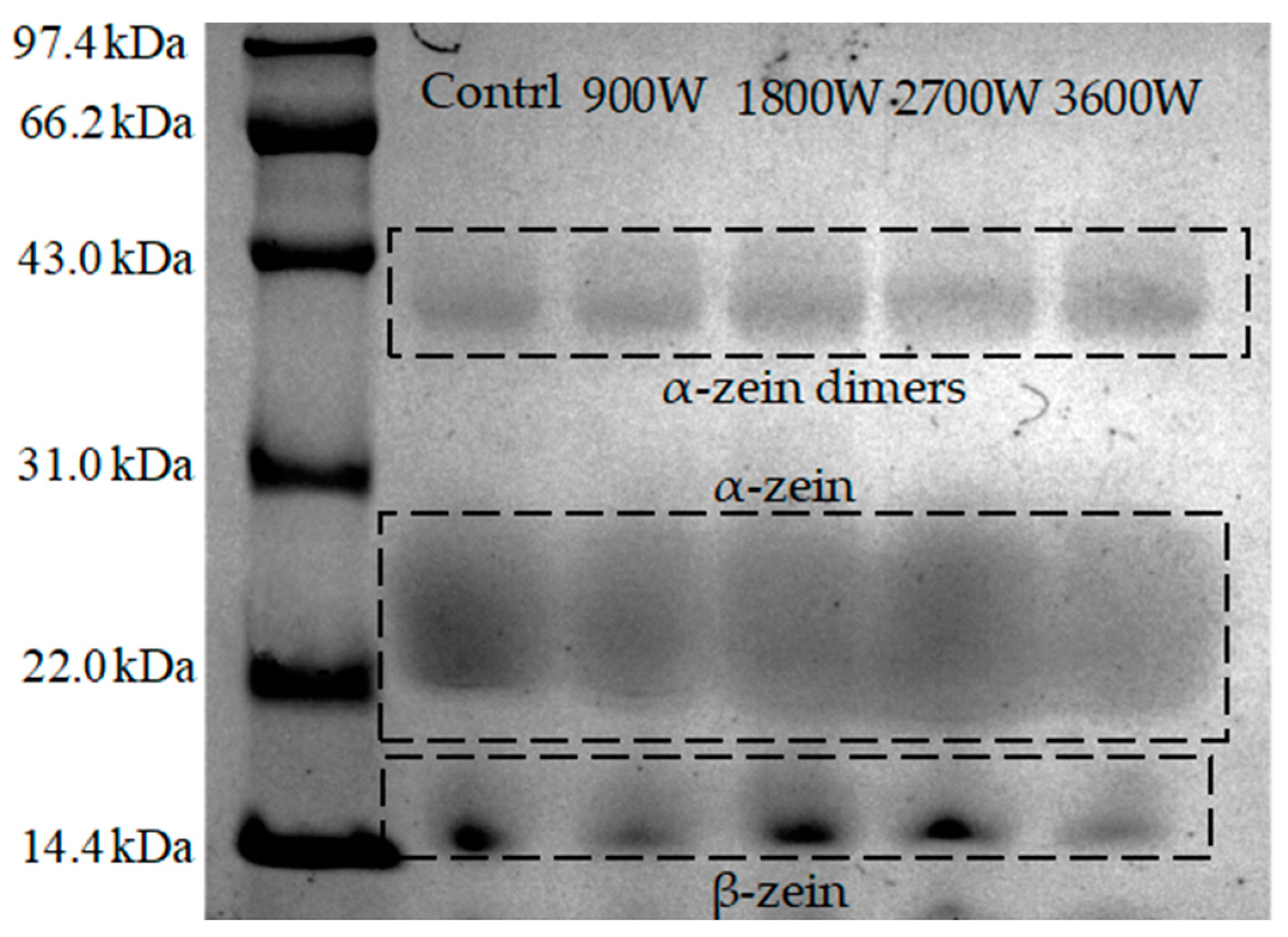
3.3. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis
3.4. Circular Binary Chromatography
3.5. Free Sulfhydryl (-SH) Groups
3.6. Endogenous Fluorescence Spectra
3.7. Particle Size and Polydispersity Index
3.8. Surface Hydrophobicity (H0)
3.9. Solubility
3.10. Thermal Stability (DSC)
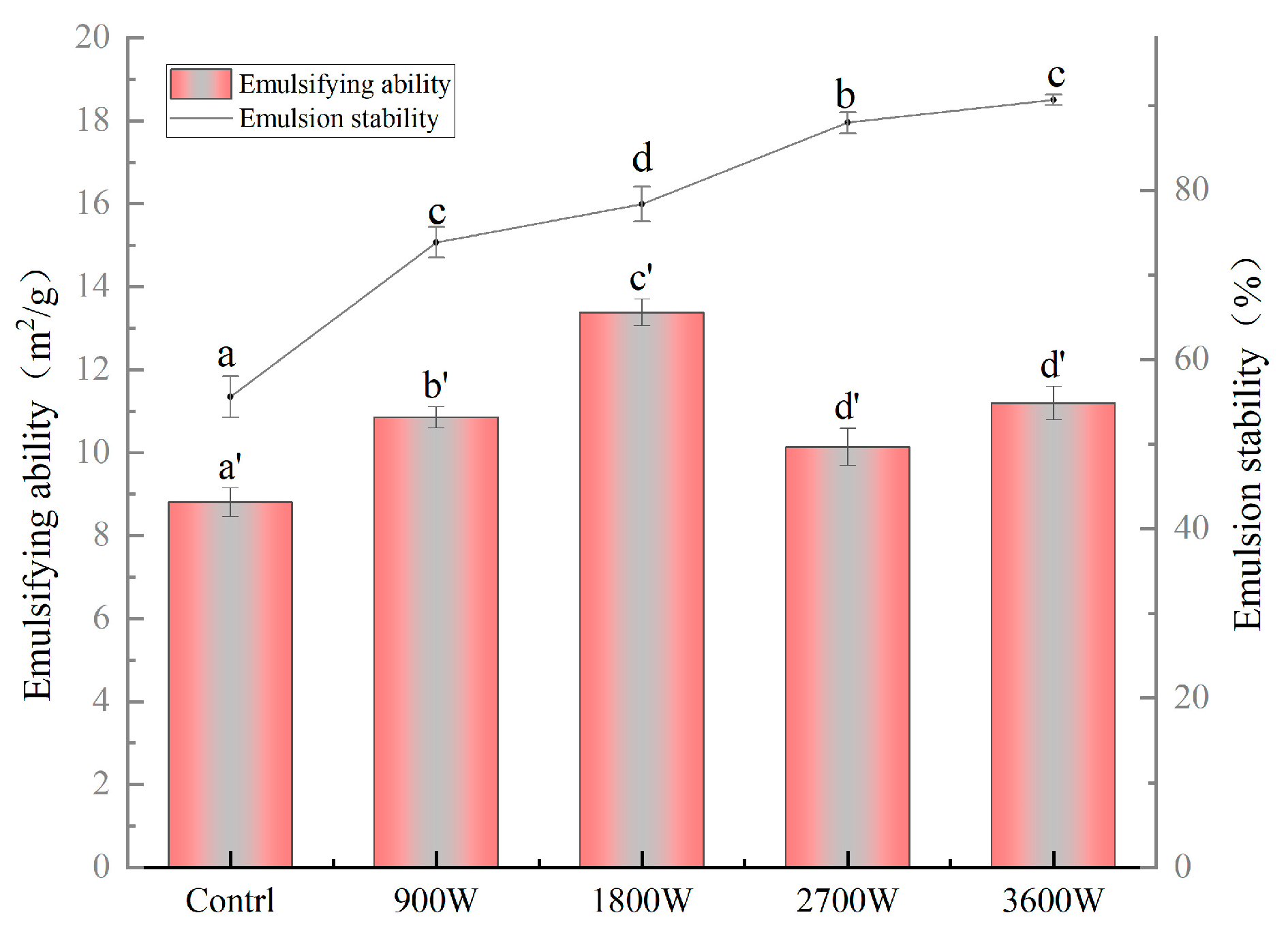
3.11. The Emulsification and Emulsion Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolomiets, T.M.; Kiseleva, M.I.; Zhemchuzhina, N.S.; Pankratova, L.F.; Elizarova, S.A. A characteristic of the species composition of pathogenic fungi of the genus Fusarium in corn biocenoses of the Voronezh region. Vavilovskii Zhurnal Genet. I Sel. 2022, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, Y.; Xie, Y.; Li, X.; Liu, Y.; Gao, Z. Radio frequency treatment accelerates drying rates and improves vigor of corn seeds. Food Chem. 2020, 319, 126597. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, M.; Mujumdar, A.S.; Xu, J.; Wang, W. Influence of drying methods on the drying kinetics, bioactive compounds and flavor of solid-state fermented okara. Dry. Technol. 2021, 39, 644–654. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A. Microwave-convective drying of food materials: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Behera, G.; Sutar, P.P. Effect of convective, infrared and microwave heating on drying rates, mass transfer characteristics, milling quality and microstructure of steam gelatinized Paddy. J. Food Process Eng. 2018, 41, e12900. [Google Scholar] [CrossRef]
- Ma, X.; Luo, G.; Li, Z.; Raghavan, G.; Chen, H.; Song, C. Microwave power control scheme for potatoes based on dielectric loss factor feedback. J. Food Eng. 2021, 288, 110134. [Google Scholar] [CrossRef]
- Dai, J.-W.; Xiao, H.-W.; Zhang, L.-H.; Chu, M.-Y.; Qin, W.; Wu, Z.-J.; Han, D.-D.; Li, Y.-L.; Liu, Y.-W.; Yin, P.-F. Drying characteristics and modeling of apple slices during microwave intermittent drying. J. Food Process Eng. 2019, 42, e13212. [Google Scholar] [CrossRef]
- Xu, F.; Chen, Z.; Huang, M.; Li, C.; Zhou, W. Effect of intermittent microwave drying on biophysical characteristics of rice. J. Food Process Eng. 2017, 40, e12590. [Google Scholar] [CrossRef]
- Kermani, A.M.; Khashehchi, M.; Kouravand, S.; Sadeghi, A. Effects of intermittent microwave drying on quality characteristics of pistachio nuts. Dry. Technol. 2017, 35, 1108–1116. [Google Scholar] [CrossRef]
- Malumba, P.; Odjo, S.; Boudry, C.; Danthine, S.; Bindelle, J.; Beckers, Y.; Béra, F. Physicochemical characterization and in vitro assessment of the nutritive value of starch yield from corn dried at different temperatures. Starch-Stärke 2014, 66, 738–748. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S. Effect of Drying Temperature on Mechanical Properties of Dried Corn. Dry. Technol. 2014, 32, 774–780. [Google Scholar] [CrossRef]
- Odjo, S.; Béra, F.; Beckers, Y.; Foucart, G.; Malumba, P. Influence of variety, harvesting date and drying temperature on the composition and the in vitro digestibility of corn grain. J. Cereal Sci. 2018, 79, 218–225. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimenez, E.; Lagaron, J.M. Characterization of the morphology and thermal properties of Zein Prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Sun, X.; Ohanenye, I.C.; Ahmed, T.; Udenigwe, C.C. Microwave treatment increased protein digestibility of pigeon pea (Cajanus cajan) flour: Elucidation of underlying mechanisms. Food Chem. 2020, 329, 127196. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xiang, X.; Chen, T.; Zou, P.; Liu, Y.; Ye, J.; Luo, S.; Wu, J.; Liu, C. Accelerated aging of rice by controlled microwave treatment. Food Chem. 2020, 323, 126853. [Google Scholar] [CrossRef]
- Rao, M.V.; Sunil, C.K.; Venkatachalapathy, N. Effect of microwave and hot air radiofrequency treatments on physicochemical and functional properties of foxtail millet flour and its protein isolate. J. Cereal Sci. 2023, 114, 103774. [Google Scholar] [CrossRef]
- GB/T 10362-2008; Inspection of Grain and Oils—Determination of Moisture Content of Maize. Standards Press of China: Beijing, China, 2008.
- Esturk, O. Intermittent and Continuous Microwave-Convective Air-Drying Characteristics of Sage (Salvia officinalis) Leaves. Food Bioprocess. Technol. 2012, 5, 1664–1673. [Google Scholar] [CrossRef]
- GB/T 10463-2008; Maize (Corn) Flour. Standards Press of China: Beijing, China, 2008.
- GB 5009.5-2016; National Food Safety Standard-Determination of Protein in Foods. Standards Press of China: Beijing, China, 2016.
- Zhao, C.; Li, Q.; Hu, N.; Yin, H.; Wang, T.; Dai, X.; Zheng, M.; Liu, J. Improvement of structural characteristics and in vitro digestion properties of zein by controlling postharvest ripening process of corn. Food Control 2022, 142, 109221. [Google Scholar] [CrossRef]
- Baloyi, J.T.; Taylor, J.; Taylor, J.R.N. Bioplastic film making properties of quality protein maize (QPM) zein. Cereal Chem. 2023, 100, 805–815. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.; Nakai, S. Determination of SH and SS groups in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhang, Z.; Chen, Z.; Jing, X.; Wang, X. Effect of high hydrostatic pressure treatment on the structure and physicochemical properties of millet gliadin. LWT 2022, 154, 112755. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; He, X.; Liu, F.; Yuan, F.; Gao, Y. Effect of heat treatment on physical, structural, thermal and morphological characteristics of zein in ethanol-water solution. Food Hydrocoll. 2016, 58, 11–19. [Google Scholar] [CrossRef]
- Smith, B.M.; Bean, S.R.; Selling, G.; Sessa, D.; Aramouni, F.M. Role of non-covalent interactions in the production of visco-elastic material from zein. Food Chem. 2014, 147, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Paraman, I.; Lamsal, B.P. Recovery and Characterization of α-Zein from Corn Fermentation Coproducts. J. Agric. Food Chem. 2011, 59, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, G.; Lu, W.; Wu, Y.; Hu, B.; Xu, L.; Fang, Y.; Nishinari, K.; Phillips, G.O. Interfacial and emulsion-stabilizing properties of zein nanoparticles: Differences among zein fractions (α-, β-, and γ-zein). Food Funct. 2021, 12, 1361–1370. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Calix-Rivera, C.S.; Villanueva, M.; Ronda, F. Microwave radiation induces modifications in the protein fractions of tef flours and modulates their derived techno-functional properties. Int. J. Biol. Macromol. 2023, 253, 126908. [Google Scholar] [CrossRef]
- Shen, L.; Gao, M.; Zhu, Y.; Liu, C.; Wang, L.; Kamruzzaman, M.; Liu, C.; Zheng, X. Microwave drying of germinated brown rice: Correlation of drying characteristics with the final quality. Innov. Food Sci. Emerg. Technol. 2021, 70, 102673. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga. Agriculture 2023, 13, 221. [Google Scholar] [CrossRef]
- Taylor, J.; Anyango, J.O.; Taylor, J.R.N. Developments in the Science of Zein, Kafirin, and Gluten Protein Bioplastic Materials. Cereal Chem. 2013, 90, 344–357. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Liu, A.; Wu, L.; Yan, W.; Tong, Y.; Wang, P. Circular Extraction: Innovative Use of a Switchable Composite Extractant for Prolamin Extraction from Grain Byproducts. ACS Food Sci. Technol. 2022, 2, 630–637. [Google Scholar] [CrossRef]
- Hassan, A.B.; Hoersten, D.; Mohamed Ahmed, I.A. Effect of radio frequency heat treatment on protein profile and functional properties of maize grain. Food Chem. 2019, 271, 142–147. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Xu, M.; Zhang, X.; Cao, S.; Hu, Y.; Luan, G. Physicochemical properties and protein structure of extruded corn gluten meal: Implication of temperature. Food Chem. 2023, 399, 133985. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Zhang, L.; Dai, L.; Yang, S.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Influence of calcium ions on the stability, microstructure and in vitro digestion fate of zein-propylene glycol alginate-tea saponin ternary complex particles for the delivery of resveratrol. Food Hydrocoll. 2020, 106, 105886. [Google Scholar] [CrossRef]
- Su, K.; Liu, L.; Pan, X.; Chen, S.; Zhang, X.; Cheng, W.; Xu, B. Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein. Foods 2023, 12, 1792. [Google Scholar] [CrossRef] [PubMed]
- Bohr, H.; Bohr, J. Microwave-enhanced folding and denaturation of globular proteins. Phys. Rev. E 2000, 61, 4310–4314. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, Y.; Zhang, Y.; Sun, Y.; Jin, T. Extrusion Modification: Effect of Extrusion on the Functional Properties and Structure of Rice Protein. Processes 2022, 10, 1871. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Teng, F.; Zhang, S.; Qi, B.; Wu, C.; Tian, T.; Wang, Z.; Li, Y. Application of ultrasound treatment for modulating the structural, functional and rheological properties of black bean protein isolates. Int. J. Food Sci. Technol. 2020, 55, 1637–1647. [Google Scholar] [CrossRef]
- Wang, X.; Gu, L.; Su, Y.; Li, J.; Yang, Y.; Chang, C. Microwave technology as a new strategy to induce structural transition and foaming properties improvement of egg white powder. Food Hydrocoll. 2020, 101, 105530. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods 2022, 11, 1373. [Google Scholar] [CrossRef]
- Fan, D.-M.; Hu, B.; Lin, L.-F.; Huang, L.-L.; Wang, M.-F.; Zhao, J.-X.; Zhang, H. Rice protein radicals: Growth and stability under microwave treatment. RSC Adv. 2016, 6, 97825–97831. [Google Scholar] [CrossRef]
- Viseu, M.I.; Carvalho, T.I.; Costa, S.M.B. Conformational Transitions in β-Lactoglobulin Induced by Cationic Amphiphiles: Equilibrium Studies. Biophys. J. 2004, 86, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, J. Effects of the non-covalent interactions between polyphenols and proteins on the formations of the heterocyclic amines in dry heated soybean protein isolate. Food Chem. 2022, 373, 131557. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Q.; Zheng, Y.; Wang, J.; Tian, Y.; Zheng, B.; Guo, Z. Effect of two-step microwave heating on the gelation properties of golden threadfin bream (Nemipterus virgatus) myosin. Food Chem. 2020, 328, 127104. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.M.; Watzky, M.A.; Finke, R.G. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim. Et Biophys. Acta (BBA)—Proteins Proteom. 2009, 1794, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 1: Effect of pH and heat treatment time on particle size and binding capacity. Food Hydrocoll. 2017, 73, 67–73. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, Y.; Teng, Y.; Zhao, M. Influence of glycosylation of deamidated wheat gliadin on its interaction mechanism with resveratrol. Food Chem. 2017, 221, 431–438. [Google Scholar] [CrossRef]
- Lou, X.; Yang, Q.; Sun, Y.; Pan, D.; Cao, J. The effect of microwave on the interaction of flavour compounds with G-actin from grass carp (Catenopharyngodon idella). J. Sci. Food Agric. 2017, 97, 3917–3922. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Liu, Z.; Shen, Q. Comparison of the characteristics of prolamins among foxtail millet varieties with different palatability: Structural, morphological, and physicochemical properties. Int. J. Biol. Macromol. 2021, 186, 194–205. [Google Scholar] [CrossRef]
- Meng, G.T.; Ching, K.M.; Ma, C.Y. Thermal aggregation of globulin from an indigenous Chinese legume, Phaseolus angularis (red bean). Food Chem. 2002, 79, 93–103. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Li, X.-Y.; Liu, B.-H.; Yin, Y.-Q.; Zhang, H.-L.; Zhang, Y.-H. Comprehensive application possibility: Construction hydrophilic, amphiphilic and hydrophobic system of modified zein by enzymatic or cysteine modification. Food Hydrocoll. 2023, 135, 108159. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Wang, Z.; Fan, F.; Chen, H.; Ma, W.; Du, M. Effects of high pressure homogenize treatment on the physicochemical and emulsifying properties of proteins from scallop (Chlamys farreri). Food Hydrocoll. 2019, 94, 537–545. [Google Scholar] [CrossRef]





| Sample | Total Protein Content (%) | Extraction Rate of Zein (%) |
|---|---|---|
| Control | 8.964 ± 0.123 c | 4.033 ± 0.08 b |
| 900 W | 8.792 ± 0.141 abc | 3.942 ± 0.05 ab |
| 1800 W | 8.806 ± 0.101 bc | 3.974 ± 0.04 ab |
| 2700 W | 8.596 ± 0.115 ab | 3.915 ± 0.04 a |
| 3600 W | 8.565 ± 0.126 a | 3.902 ± 0.02 a |
| Sample | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| Control | 31 ± 0.03 c | 14.7 ± 0.05 c | 28.5 ± 0.11 b | 24.8 ± 0.12 b |
| 900 W | 31.4 ± 0.04 d | 18.5 ± 0.09 e | 27.1 ± 0.08 a | 22.9 ± 0.09 a |
| 1800 W | 33.3 ± 0.02 e | 10.7 ± 0.02 a | 29.7 ± 0.06 c | 26.4 ± 0.10 e |
| 2700 W | 29.8 ± 0.06 a | 15 ± 0.03 d | 30.1 ± 0.05 e | 25 ± 0.04 c |
| 3600 W | 29.9 ± 0.03 b | 14.2 ± 0.03 b | 29.9 ± 0.04 d | 25.8 ± 0.03 d |
| Sample | Particle Size (nm) | PDI (%) |
|---|---|---|
| Control | 341.63 ±1.23 c | 16.46 ± 2.7 b |
| 900 W | 338.30 ± 1.6 b | 15.69 ± 1.74 b |
| 1800 W | 273.27 ± 1.92 a | 12.14 ± 0.79 a |
| 2700 W | 404.7 ± 2.15 e | 23.86 ± 0.65 c |
| 3600 W | 370.2 ± 2.3 d | 18.7 ± 1.46 b |
| Sample | Tp (°C) | ΔH (J/g) |
|---|---|---|
| Control | 96.88 ± 0.47 b | −75 ± 1.25 a |
| 900 W | 101.36 ± 0.35 d | −108.65 ± 0.95 c |
| 1800 W | 104.56 ± 0.24 c | −98.67 ± 1.4 b |
| 2700 W | 108.15 ± 0.57 e | −114.1 ± 0.2 d |
| 3600 W | 104.75 ± 0.32 a | −96.7 ± 1.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.; Zhou, Y.; Song, B.; Wu, Y.; Wang, Y.; Wang, Y.; Liu, Y.; Xu, X.; Zhao, C.; Liu, J. Effect of Microwave Intermittent Drying on the Structural and Functional Properties of Zein in Corn Kernels. Foods 2024, 13, 207. https://doi.org/10.3390/foods13020207
Mao S, Zhou Y, Song B, Wu Y, Wang Y, Wang Y, Liu Y, Xu X, Zhao C, Liu J. Effect of Microwave Intermittent Drying on the Structural and Functional Properties of Zein in Corn Kernels. Foods. 2024; 13(2):207. https://doi.org/10.3390/foods13020207
Chicago/Turabian StyleMao, Sining, Yuhan Zhou, Bin Song, Yuzhu Wu, Yu Wang, Yiran Wang, Yanjia Liu, Xiuying Xu, Chengbin Zhao, and Jingsheng Liu. 2024. "Effect of Microwave Intermittent Drying on the Structural and Functional Properties of Zein in Corn Kernels" Foods 13, no. 2: 207. https://doi.org/10.3390/foods13020207







