Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
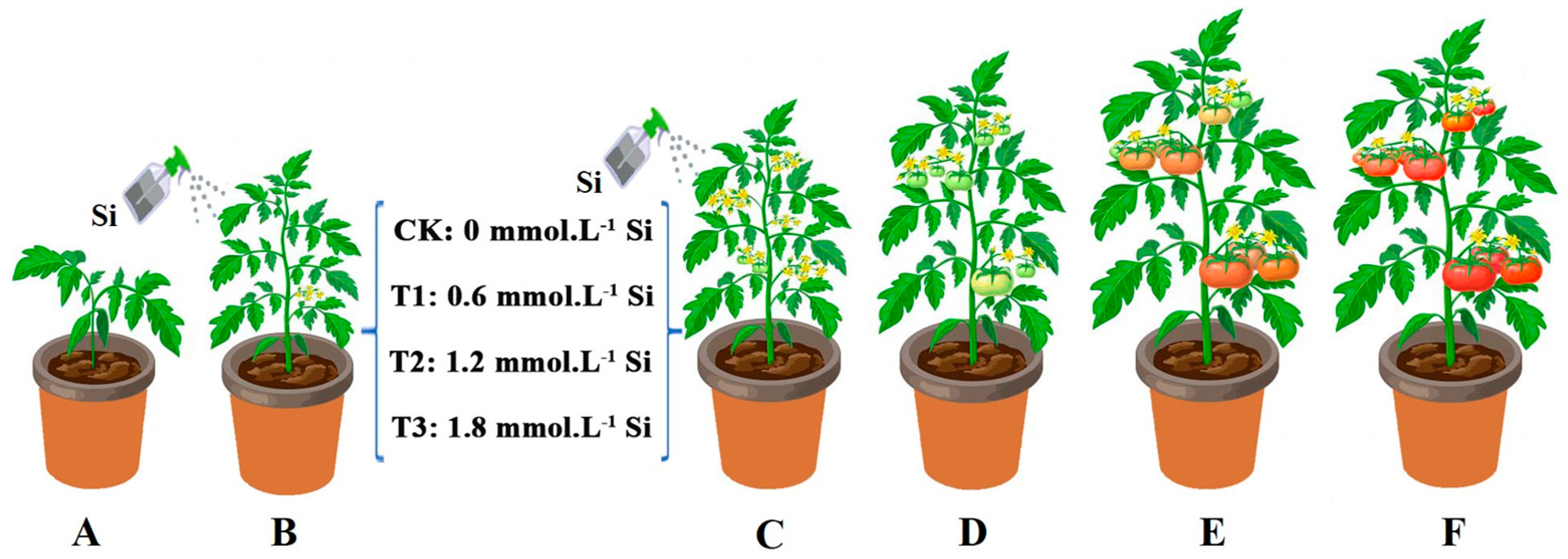
2.2. Experimental Design
2.3. Experimental Methods
2.3.1. Indicators of Appearance Quality
2.3.2. Indicators of Nutritional Quality
2.3.3. Determination of Carotenoid Components
2.3.4. Expression of Key Genes in Relation to Carotenoid Metabolic Pathways
2.3.5. Determination of Amino Acid Components
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Levels of Si on the Appearance Quality of Tomato Fruits
3.2. Effects of Different Levels of Si on the Nutritional Quality and Safety Quality of Tomato Fruits
3.3. Effects of Different Levels of Si on the Color Parameters and Carotenoid Metabolism of Tomato Fruits
3.3.1. Effect of Different Levels of Si on the Color Parameters of Tomato Fruits
3.3.2. Effects of Different Levels of Si on the Carotenoid Components of Tomato Fruits
3.3.3. Effects of Different Levels of Si on the Key Gene Expression of Carotenoid Metabolism Pathway in Tomato Fruits
3.4. Effects of Different Levels of Si on the Amino Acid of Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, K.M.; Senthil-Nathan, S.; Pavithra, G.; Asahel, A.; Malarvizhi, P.; Murugan, P.; Deva-Andrews, A.; Sivanesh, H.; Stanley-Raja, V.; Ramasubramanian, R. The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture 2022, 13, 6. [Google Scholar] [CrossRef]
- Sarker, K.K.; Akanda, M.; Biswas, S.K.; Roy, D.K.; Khatun, A.; Goffar, M.A. Field performance of alternate wetting and drying furrow irrigation on tomato crop growth, yield, water use efficiency, quality and profitability. J. Integr. Agric. 2016, 15, 2380–2392. [Google Scholar] [CrossRef]
- Causse, M.; Buret, M.; Robini, K.; Verschave, P. Inheritance of nutritional and sensory quality traits in fresh market tomato and relation to consumer preferences. Food Sci. 2003, 68, 2342–2350. [Google Scholar] [CrossRef]
- Wang, F.; Kang, S.; Du, T.; Li, F.; Qiu, R. Determination of comprehensive quality index for tomato and its response to different irrigation treatments. Agric. Water Manag. 2011, 98, 1228–1238. [Google Scholar] [CrossRef]
- Krumbein, A.; Schwarz, D. Grafting: A possibility to enhance health-promoting and flavour compounds in tomato fruits of shaded plants? Sci. Hortic. 2013, 149, 97–107. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Aljabary, A.M.A.O.; Halshoy, H.S.; Hama, J.R.; Rashid, H.A.; Rashid, H.W. Soil-borne microbes, natural stimulants, and post-harvest treatments alter quality and phytochemicals of tomato fruit. Int. J. Veg. Sci. 2023, 1–13. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J. Response of tomato fruit quality depends on period of LED supplementary light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef]
- Meunier, J.; Cornu, S.; Keller, C.; Barboni, D. The role of silicon in the supply of terrestrial ecosystem services. Environ. Chem. Lett. 2022, 20, 2109–2121. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Guneri, M.; Ashraf, M. Mitigation effects of silicon on tomato plants bearing fruit grown at high boron levels. J. Plant Nutr. 2011, 34, 1985–1994. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz. J. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Gong, H.; Zhao, H.L.; Li, H.L.; Hu, Y.H.; Wang, Y.C. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Integr. Agric. 2018, 17, 2151–2159. [Google Scholar] [CrossRef]
- Alam, A.; Hariyanto, B.; Ullah, H.; Salin, K.R.; Datta, A. Effects of silicon on growth, yield and fruit quality of cantaloupe under drought stress. Silicon 2021, 13, 3153–3162. [Google Scholar] [CrossRef]
- Morato De Moraes, D.H.; Mesquita, M.; Magalhães Bueno, A.; Alves Flores, R.; Elias De Oliveira, H.F.; Raimundo De Lima, F.S.; de Mello Prado, R.; Battisti, R. Combined effects of induced water deficit and foliar application of silicon on the gas exchange of tomatoes for processing. Agronomy 2020, 10, 1715. [Google Scholar] [CrossRef]
- Qin, L.; Kang, W.; Qi, Y.; Zhang, Z.; Wang, N. The influence of silicon application on growth and photosynthesis response of salt stressed grapevines (Vitis vinifera L.). Acta Physiol. Plant. 2016, 38, 68. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Han, Y.; Wen, J.; Peng, Z.; Zhang, D.; Hou, M. Effects of silicon amendment on the occurrence of rice insect pests and diseases in a field test. J. Integr. Agric. 2018, 17, 2172–2181. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Jarosz, Z. The effect of silicon application and type of medium on yielding and chemical composition of tomato. Acta Sci. Pol. Hortorum Cultus 2014, 13, 171–183. [Google Scholar]
- Jarosz, Z. The effect of silicon application and type of substrate on yield and chemical composition of leaves and fruit of cucumber. J. Elementol. 2013, 18, 403–414. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiol. Biochem. 2021, 166, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Pal, B.; Badole, S.; Hazra, G.C.; Mandal, B. Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Commun. Soil Sci. Plant Anal. 2016, 47, 284–290. [Google Scholar] [CrossRef]
- Kovács, S.; Kutasy, E.; Csajbók, J. The multiple role of silicon nutrition in alleviating environmental stresses in sustainable crop production. Plants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Christian, M.M.; Shimelis, H.; Laing, M.D.; Tsilo, T.J. The effect of silicon fertilizers on agronomic performance of bread wheat under drought stress and non-stress conditions. J. Agron. Crop Sci. 2023, 209, 827–840. [Google Scholar] [CrossRef]
- Meng, X.; Luo, S.; Dawuda, M.M.; Gao, X.; Wang, S.; Xie, J.; Tang, Z.; Liu, Z.; Wu, Y.; Jin, L. Exogenous silicon enhances the systemic defense of cucumber leaves and roots against CA-induced autotoxicity stress by regulating the ascorbate-glutathione cycle and photosystem II. Ecotoxicol. Environ. Saf. 2021, 227, 112879. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Hu, Y.; Han, W.; Yin, J.; Li, H.; Gong, H. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Labbé, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, P.; Khoshgoftarmanesh, A.H.; Shirvani, M.; Sharifnabi, B.; Nili, N. Effect of silicon nutrition on oxidative stress induced by Phytophthora melonis infection in cucumber. Plant Dis. 2011, 95, 455–460. [Google Scholar] [CrossRef]
- Bélanger, R.R.; Benhamou, N.; Menzies, J.G. Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 2003, 93, 402–412. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, X.; Ding, C.; Xia, M.; Xue, R.; Sun, Z.; Chen, D.; Zhu-Salzman, K.; Zeng, R.; Song, Y. Priming of rice defense against a sap-sucking insect pest brown planthopper by silicon. J. Pest Sci. 2022, 95, 1371–1385. [Google Scholar] [CrossRef]
- Hou, M.; Han, Y. Silicon-mediated rice plant resistance to the Asiatic rice borer (Lepidoptera: Crambidae): Effects of silicon amendment and rice varietal resistance. J. Econ. Entomol. 2010, 103, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Sampaio, M.V.; Rodrigues, M.P.; Korndörfer, A.P.; Oliveira, R.S.; Ferreira, S.E.; Korndörfer, G.H. Induction of resistance by silicon in wheat plants to alate and apterous morphs of Sitobion avenae (Hemiptera: Aphididae). Environ. Entomol. 2014, 43, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, X.; Liu, C.; Fang, J. Anatomical berry characteristics during the development of grape berries with different shapes. Hortic. Plant J. 2021, 7, 295–306. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, H.; Wu, Y.; Yu, J.; Ali, B.; Zhang, J.; Tang, Z.; Xie, J.; Lyu, J.; Liao, W. Application of 5-aminolevulinic acid promotes ripening and accumulation of primary and secondary metabolites in postharvest tomato fruit. Front. Nutr. 2022, 9, 1036843. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.; Jiang, J.; Yang, H.; Li, J. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Cao, F.; Guan, C.; Dai, H.; Li, X.; Zhang, Z. Soluble solids content is positively correlated with phosphorus content in ripening strawberry fruits. Sci. Hortic. 2015, 195, 183–187. [Google Scholar] [CrossRef]
- Cao, B.; Wang, L.; Gao, S.; Xia, J.; Xu, K. Silicon-mediated changes in radial hydraulic conductivity and cell wall stability are involved in silicon-induced drought resistance in tomato. Protoplasma 2017, 254, 2295–2304. [Google Scholar] [CrossRef]
- Arya, S.P.; Mahajan, M.; Jain, P. Non-spectrophotometric methods for the determination of Vitamin C. Anal. Chim. Acta 2000, 417, 1–14. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, M.S.; Pai, S.R.; Pawar, N.V.; Oulkar, D.; Dixit, G.B. Free amino acid profiling in grain Amaranth using LC–MS/MS. Food Chem. 2012, 134, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Tan, G.; Zhou, W.; Wang, G. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alkarim, E.; Bayoumi, Y.; Metwally, E.; Rakha, M. Silicon supplements affect yield and fruit quality of cucumber (Cucumis sativus L.) grown in net houses. Afr. J. Agric. Res. 2017, 12, 2518–2523. [Google Scholar] [CrossRef]
- Abidi, W.; Akrimi, R.; Hajlaoui, H.; Rejeb, H.; Gogorcena, Y. Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars. Agriculture 2023, 13, 195. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Salama, D.M.; El-Tanahy, A.; El-Bassiony, A.M. Effects of prolonged restriction in water supply and spraying with potassium silicate on growth and productivity of potato. Plant Arch. 2019, 19, 2585–2595. [Google Scholar]
- Prakash, N.B.; Chandrashekar, N.; Mahendra, C.; Patil, S.U.; Thippeshappa, G.N.; Laane, H.M. Effect of foliar spray of soluble silicic acid on growth and yield parameters of wetland rice in hilly and coastal zone soils of Karnataka, South India. J. Plant Nutr. 2011, 34, 1883–1893. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H.; Yin, J. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, H.; Weerahewa, H.; Saparamadu, M. Effect of root or foliar application of soluble silicon on plant growth, fruit quality and anthracnose development of capsicum. Trop. Agric. Res. 2014, 26, 74–81. [Google Scholar] [CrossRef]
- González-Terán, G.E.; Gómez-Merino, F.C.; Trejo-Téllez, L.I. Effects of silicon and calcium application on growth, yield and fruit quality parameters of cucumber established in a sodic soil. Acta Sci. Pol. Hortorum Cultus 2020, 19, 149–158. [Google Scholar] [CrossRef]
- Isa, M.; Bai, S.; Yokoyama, T.; Ma, J.F.; Ishibashi, Y.; Yuasa, T.; Iwaya-Inoue, M. Silicon enhances growth independent of silica deposition in a low-silica rice mutant, lsi1. Plant Soil 2010, 331, 361–375. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Nada, M.M. Effect of foliar application with potassium silicate and glycine betaine on growth and early yield quality of strawberry plants. J. Plant Prod. 2020, 11, 1295–1302. [Google Scholar] [CrossRef]
- Wróbel, S. Effects of fertilization of potato cultivar Jelly with foliar fertilizers YaraVita Ziemniak and Actisil. Biul. Inst. Hod. I Aklim. Roślin 2012, 209, 295–306. [Google Scholar]
- Sayed, E.G.; Mahmoud, A.W.M.; El-Mogy, M.M.; Ali, M.A.; Fahmy, M.A.; Tawfic, G.A. The effective role of nano-silicon application in improving the productivity and quality of grafted tomato grown under salinity stress. Horticulturae 2022, 8, 293. [Google Scholar] [CrossRef]
- Stamatakis, A.; Papadantonakis, N.; Savvas, D.; Lydakis-Simantiris, N.; Kefalas, P. Effects of silicon and salinity on fruit yield and quality of tomato grown hydroponically. In Proceedings of the International Symposium on Managing Greenhouse Crops in Saline Environment, Pisa, Italy, 9–12 July 2003; Volume 609, pp. 141–147. [Google Scholar] [CrossRef]
- Talebi, S.; Majd, A.; Mirzai, M.; Jafari, S.; Abedini, M. The study of potassium silicate effects on qualitative and quantitative performance of potato (Solanum tuberosum L.). Biol. Forum 2015, 7, 1021–1026. [Google Scholar]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscler. Rep. 2009, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, K.; Li, H.; Li, Y.; Zhu, Y.; Su, D.; Zhang, Y.; Deng, H.; Wang, Y.; Liu, M. Gibberellins involved in fruit ripening and softening by mediating multiple hormonal signals in tomato. Hortic. Res. 2023, uhad275. [Google Scholar] [CrossRef]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.C.; Park, S.; Bae, J.; Ahn, M.; Lee, H.; Kwak, S. Down-regulation of sweetpotato lycopene β-cyclase gene enhances tolerance to abiotic stress in transgenic calli. Mol. Biol. Rep. 2014, 41, 8137–8148. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Recent trends in the analysis of amino acids in fruits and derived foodstuffs. Anal. Bioanal. Chem. 2013, 405, 7941–7956. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Luo, S.; Tang, Z.; Liu, Z.; Wei, S.; Liu, F.; Zhao, X.; Yu, J.; Zhong, Y. Comprehensive evaluation of amino acids and polyphenols in 69 varieties of green cabbage (Brassica oleracea L. var. capitata L.) based on multivariate statistical analysis. Molecules 2021, 26, 5355. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, X.; Sun, Z. Application of silicon fertilizer affects nutritional quality of rice. Chil. J. Agric. Res. 2017, 77, 163–170. [Google Scholar] [CrossRef]






| Gene Name | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| Actin | Solyc11g005330 | TGTCCCTATTTACGAGGGTTATGC | CAGTTAAATCACGACCAGCAAGAT |
| PSY1 | NM_001247883 | CTGGTACGGTTGGGTTGATGAGTG | CGGATAGACTGCCTGTGCTAATTC |
| PDS | NM_001247166 | CCCATGCCACGACCAGAAGATTG | TGCTGTAGACAAACCACCCAAACC |
| LCYB | NM_001247297 | GAGTCGTTGGAATCGGTGGTACAG | AGAAGCCATGCCAATAACGAGGTC |
| LCYE | NM_001247408 | TCCTGCTGGTCTTGCTCTTGC | TCTTTGAACTCGTCCTCCCATACAC |
| ZEP | NM_001309304 | AAGAGGTCGTGTTACATTGCTTGG | ATGATTGCAGCCATTCTAGCCAGC |
| Treatments | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| Phytoene content (ug·g−1) | 10.508 ± 0.077 a | 10.584 ± 0.126 a | 9.980 ± 0.146 b | 8.956 ± 0.223 c |
| Lycopene content (mg·g−1) | 6.822 ± 0.129 b | 6.835 ± 0.179 b | 7.703 ± 0.126 a | 6.895 ± 0.111 b |
| β-carotene content (mg·g−1) | 6.410 ± 0.012 b | 6.872 ± 0.089 a | 6.033 ± 0.031 c | 6.064 ± 0.079 c |
| Lutein content (mg·g−1) | 0.347 ± 0.003 c | 0.359 ± 0.003 c | 0.407 ± 0.004 a | 0.393 ± 0.005 b |
| Violanthin content (ug·g−1) | 28.451 ± 0.069 ab | 29.096 ± 0.692 a | 27.262 ± 0.085 bc | 26.147 ± 0.465 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jin, N.; Xie, Y.; Zhu, W.; Yang, Y.; Wang, J.; Lei, Y.; Liu, W.; Wang, S.; Jin, L.; et al. Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon. Foods 2024, 13, 223. https://doi.org/10.3390/foods13020223
Wang L, Jin N, Xie Y, Zhu W, Yang Y, Wang J, Lei Y, Liu W, Wang S, Jin L, et al. Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon. Foods. 2024; 13(2):223. https://doi.org/10.3390/foods13020223
Chicago/Turabian StyleWang, Li, Ning Jin, Yandong Xie, Wen Zhu, Ye Yang, Jiaying Wang, Yongzhong Lei, Wenkai Liu, Shuya Wang, Li Jin, and et al. 2024. "Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon" Foods 13, no. 2: 223. https://doi.org/10.3390/foods13020223
APA StyleWang, L., Jin, N., Xie, Y., Zhu, W., Yang, Y., Wang, J., Lei, Y., Liu, W., Wang, S., Jin, L., Yu, J., & Lyu, J. (2024). Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon. Foods, 13(2), 223. https://doi.org/10.3390/foods13020223






