Potential Regulatory Gene Network Associated with the Ameliorative Effect of Oat Antibacterial Peptides on Rat Colitis
Abstract
:1. Introduction
2. Main Materials and Methods
2.1. Materials
2.2. Preparation of Oat Antimicrobial Peptides
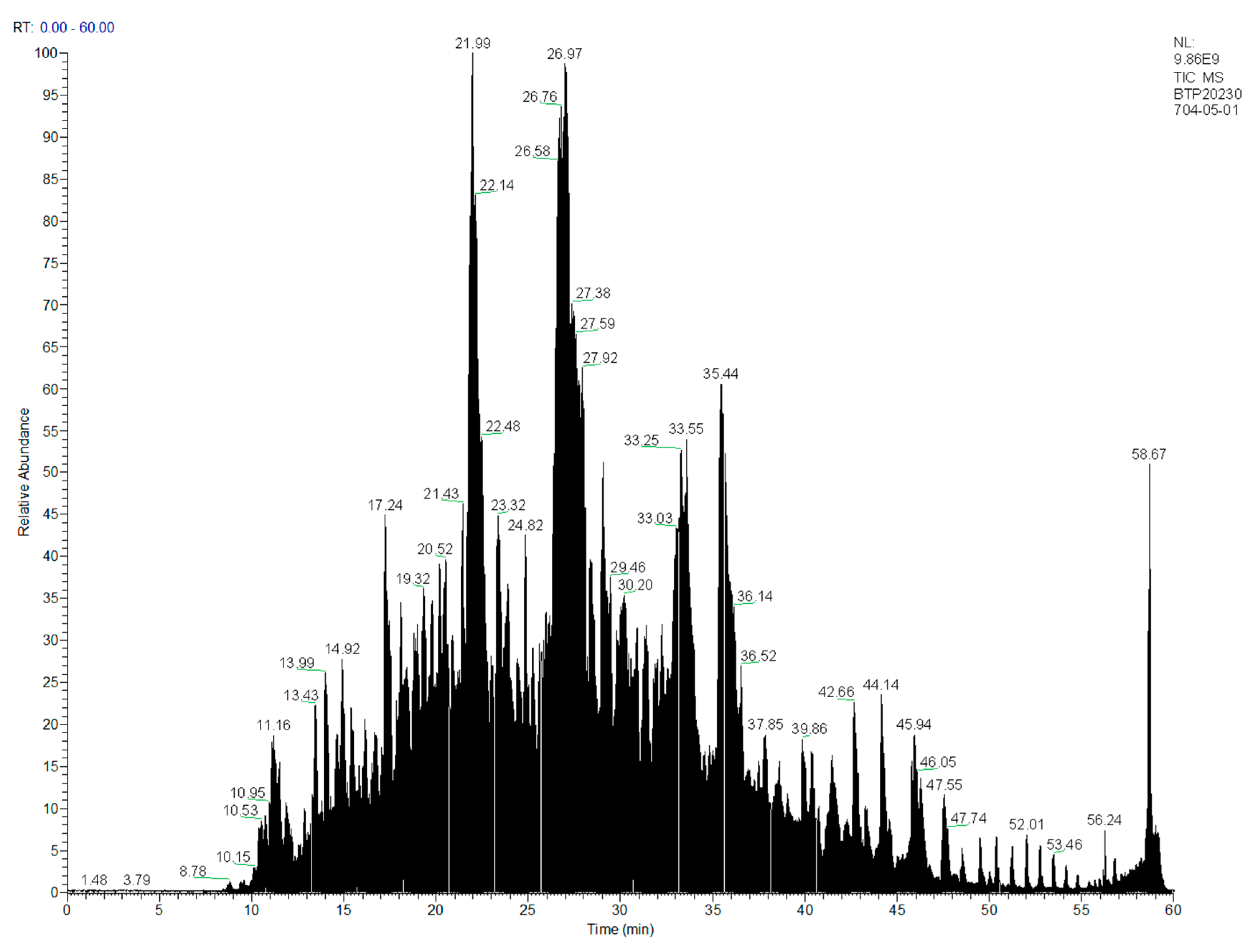
2.3. Oat Antimicrobial Peptides Sequence Analysis Based on LC-MS/MS
2.4. Determination of Anti-Inflammatory Activity of Antimicrobial Peptides In Vitro
2.5. DSS-Induced Colitis Model and Preventive Effects of Colitis Assessment
2.6. Metabolomics and Transcriptome Combined Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results and Discussion
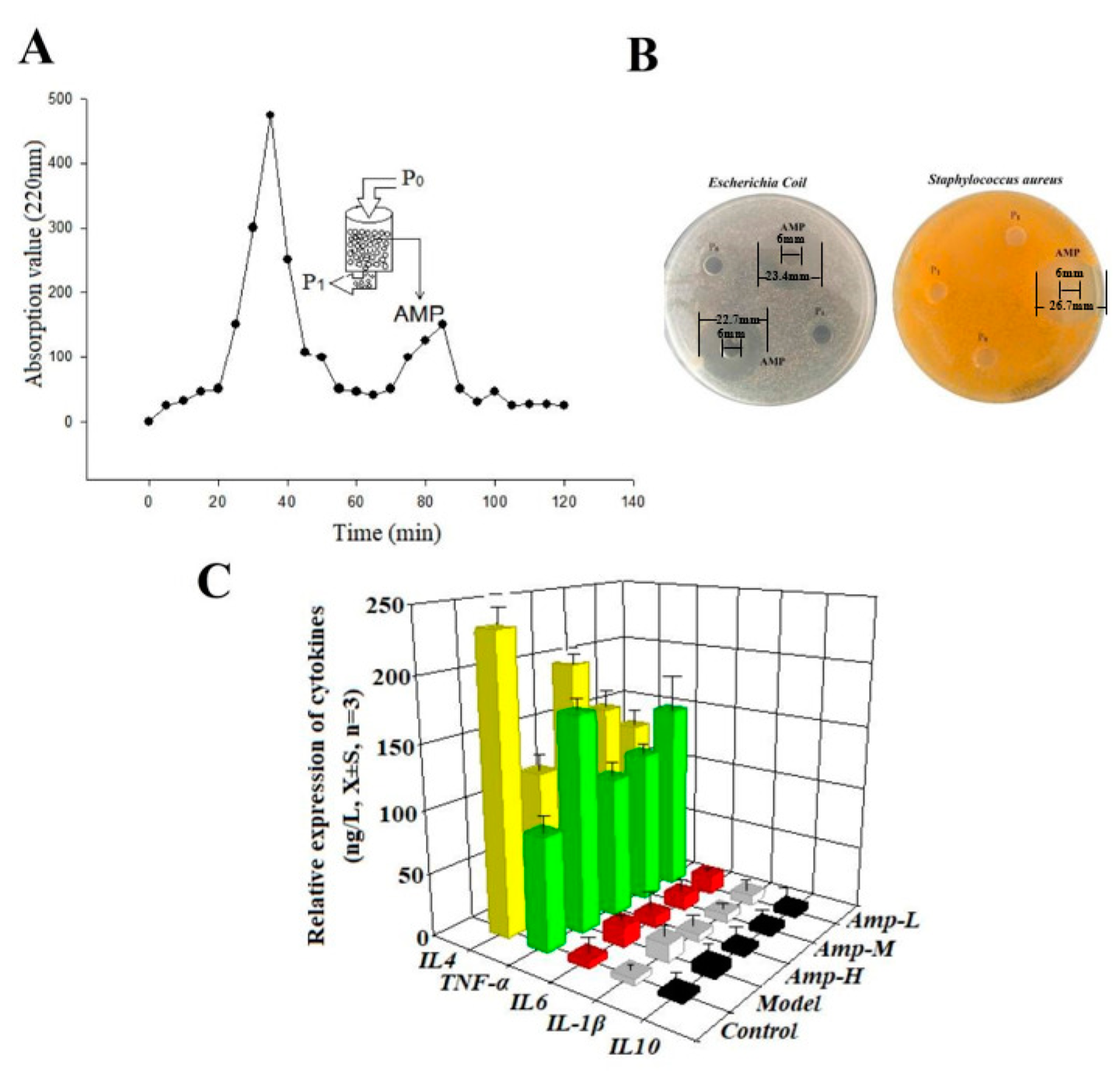
3.1. Enrichment of Antimicrobial Peptides and Determination of Anti-Inflammatory Activity
3.2. Determination of Primary Structure of Oat Antimicrobial Peptides
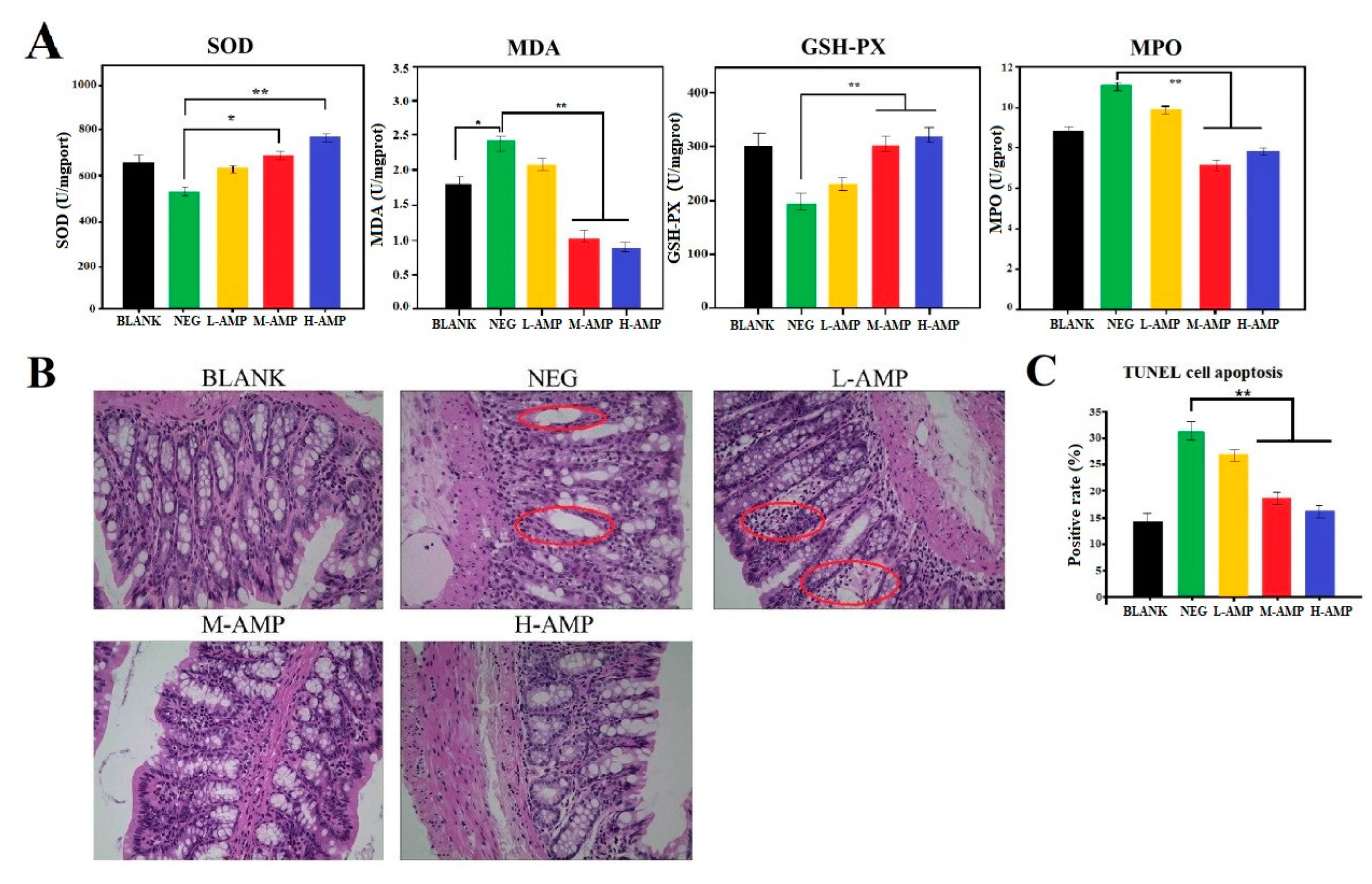
3.3. Function Evaluation of AMP for Preventing Colitis in Rats
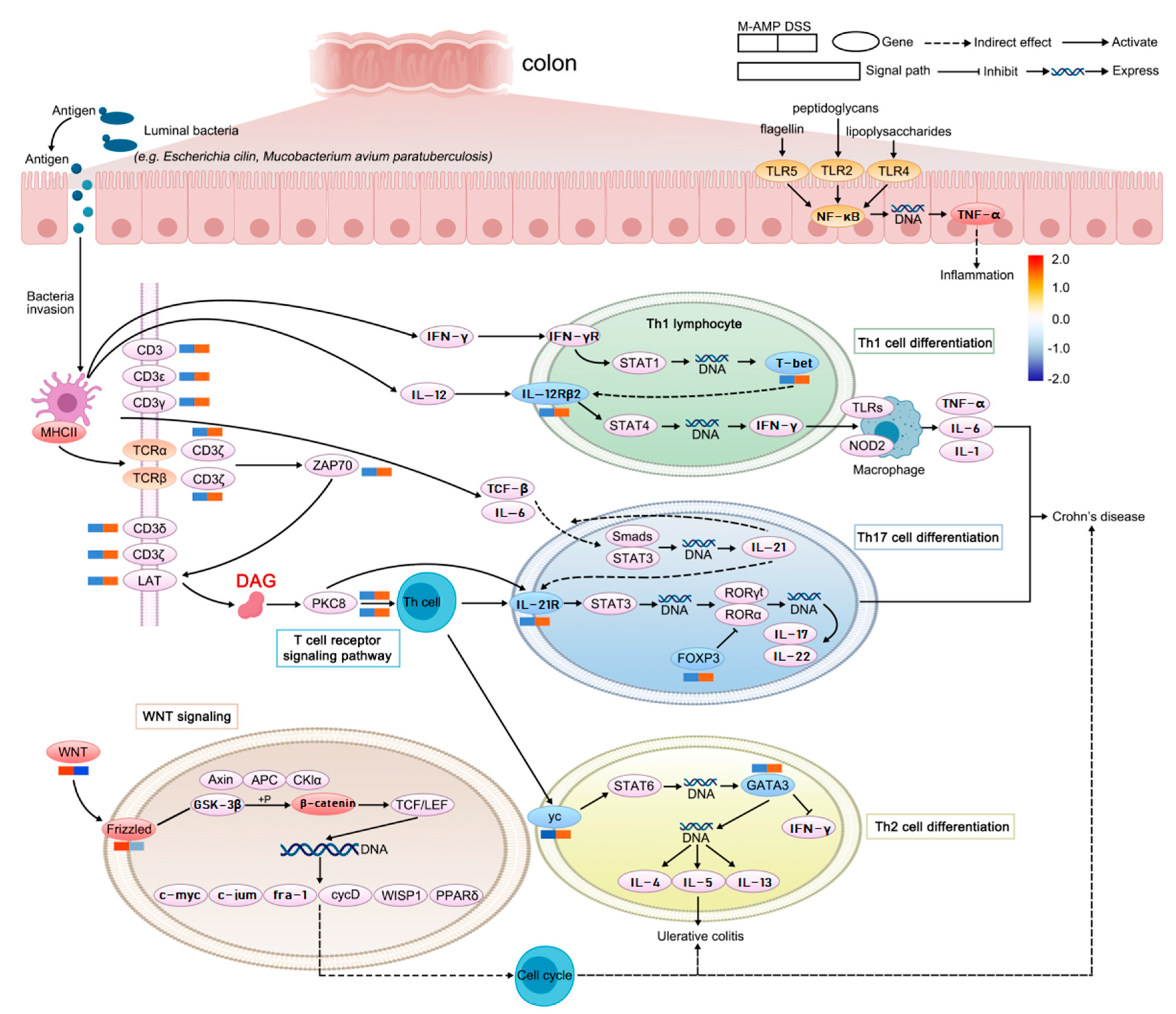
3.4. Mechanism of Action of AMP to Prevent Colitis
3.4.1. Expression Trend of Metabolites during Anti-Colitis Action of Antimicrobial Peptides
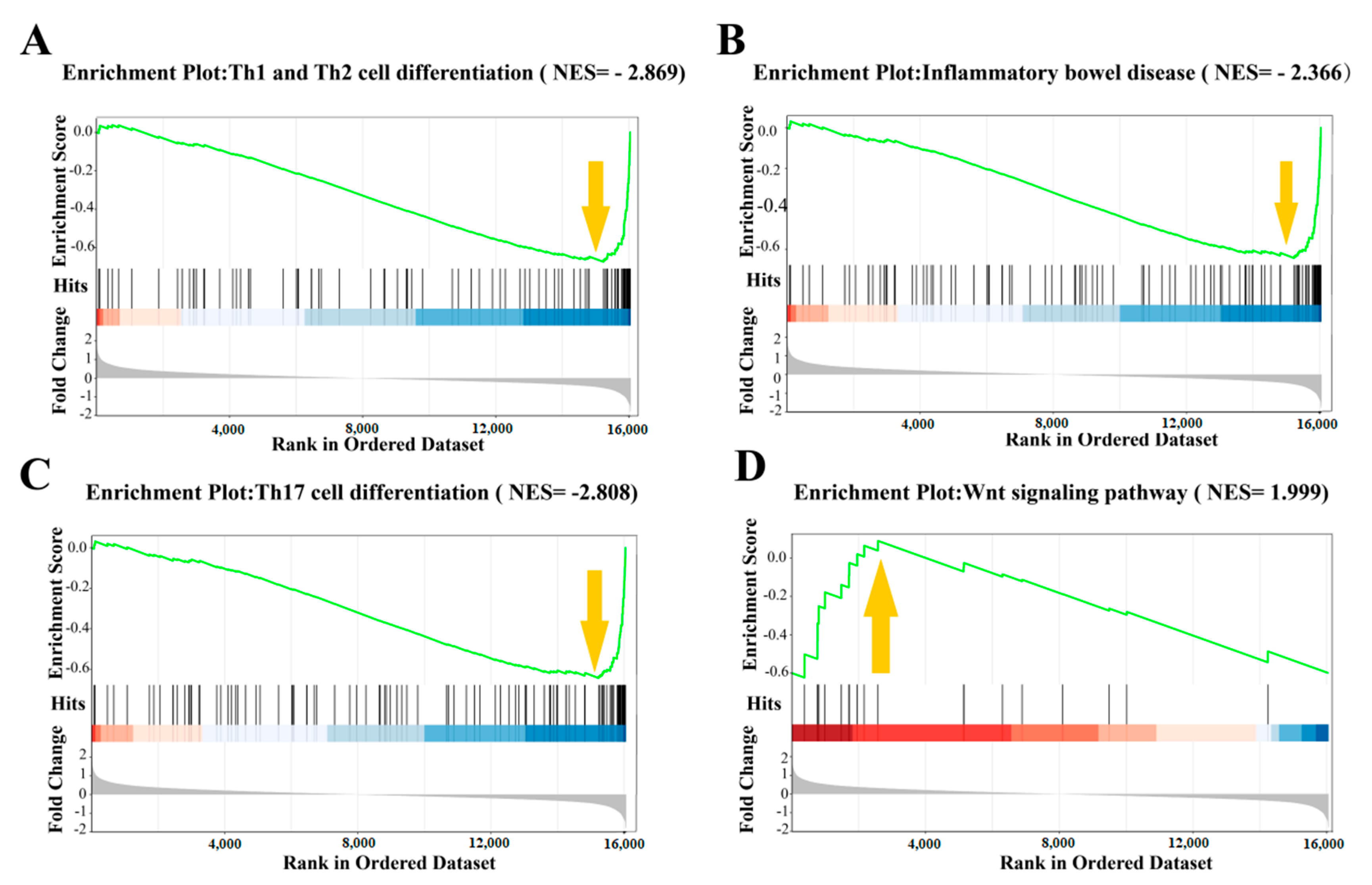
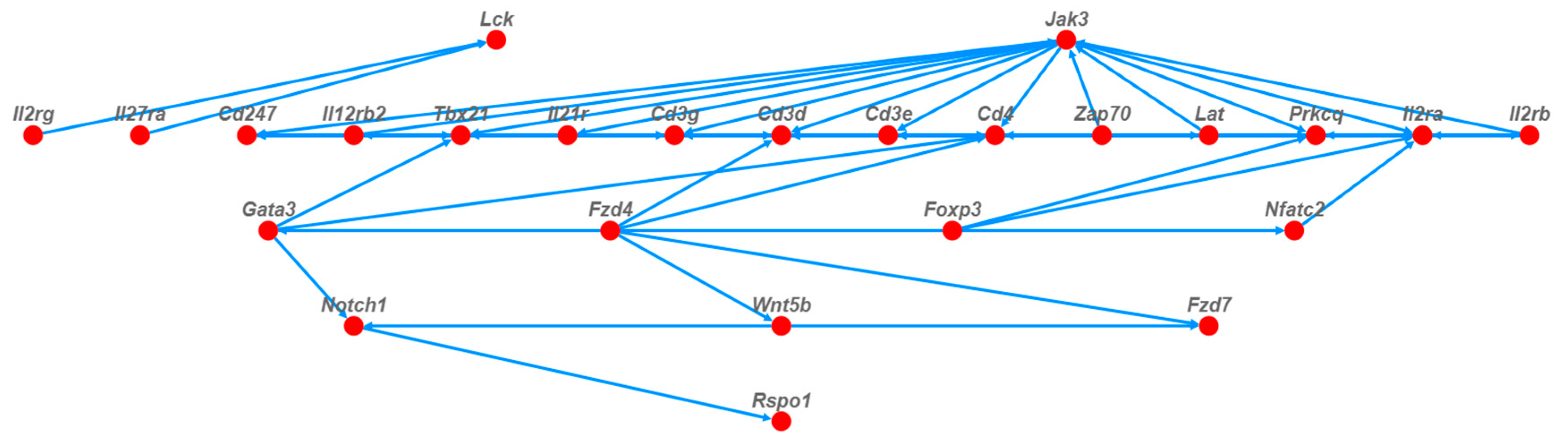
3.4.2. Trend of Gene Expression during Anti-Colitis Action of Antimicrobial Peptides
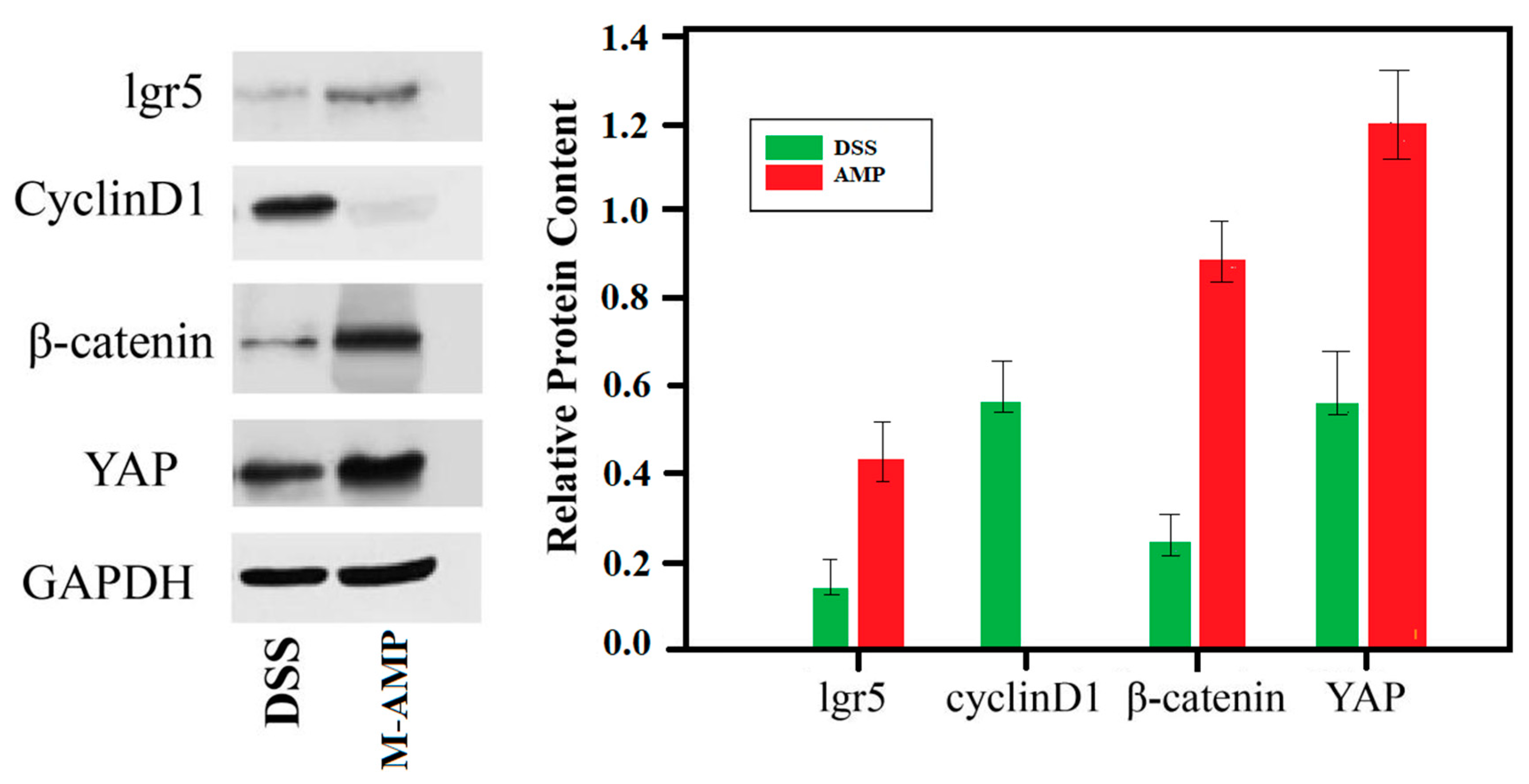
3.4.3. Western Blot Verification Experiment
3.4.4. The Preventive Mechanism of Oat Antimicrobial Peptides on Colitis was Analyzed in Combination with Metabolome and Transcriptome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moon, W.; Loftus, E.V., Jr. Review article: Recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 43, 863–883. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moghaddam, M.M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Ko, S.J.; Kang, N.H.; Kim, M.K.; Park, J.; Park, E.; Park, G.H.; Kang, T.W.; Na, D.E.; Park, J.B.; Yi, Y.E.; et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb. Pathog. 2019, 127, 70–78. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Wu, Z.; Hoover, D.M.; Yang, D.; Boulègue, C.; Santamaria, F.; Oppenheim, J.J.; Lubkowski, J.; Lu, W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human β-defensin 3. Proc. Natl. Acad. Sci. USA 2003, 100, 8880–8885. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J. Leukoc. Biol. 2001, 167, 3329–3338. [Google Scholar]
- Brunetti, J.; Roscia, G.; Lampronti, I.; Gambari, R.; Quercini, L.; Falciani, C.; Bracci, L.; Pini, A. Immunomodulatory and Anti-inflammatory Activity in vitro and in vivo of a Novel Antimicrobial Candidate. J. Biol. Chem. 2016, 291, 25742–25748. [Google Scholar] [CrossRef]
- Heinbockel, L.; Weindl, G.; Martinez-De-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19-2.5. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef]
- Giakountis, A.; Moulos, P.; Zarkou, V.; Oikonomou, C.; Harokopos, V.; Hatzigeorgiou, A.G.; Reczko, M.; Hatzis, P. A Positive Regulatory Loop between a Wnt-Regulated Non-coding RNA and ASCL2 Controls Intestinal Stem Cell Fate. Cell Rep. 2016, 15, 2588–2596. [Google Scholar] [CrossRef]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; Born, M.v.D.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal Crypt Homeostasis Results from Neutral Competition between Symmetrically Dividing Lgr5 Stem Cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef]
- Sturm, A.; Dignass, A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, M.; Han, Z.; Wang, Y.; Yang, P.; Xu, C.; Wang, Q.; Du, L.; Li, Q.; Zhao, H.; et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016, 7, e2387. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Oudenaarden, A.; Clevers, H. Identifying the Stem Cell of the Intestinal Crypt: Strategies and Pitfalls. Cell Stem Cell 2012, 11, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Bordoni, A.; Brodkorb, A.; Capozzi, F.; Velickovic, T.C.; Corredig, M.; Cotter, P.D.; De Noni, I.; Gaudichon, C.; Golding, M.; et al. An International Network for Improving Health Properties of Food by Sharing our Knowledge on the Digestive Process. Food Dig. 2011, 2, 23–25. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Liu, S.; Dai, A.; Chi, X.; Zhang, D. Non-targeted metabolomics and microbial analyses of the impact of oat antimicrobial peptides on rats with dextran sulfate sodium-induced enteritis. Front. Nutr. 2023, 9, 1095483. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.; Park, H.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2016, 116, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. The Study on the Mechanism of Ulcerative Enteritis in Mice and the Effect of Curcumin for Ulcerative Enteritis Based on the SIRT1/MTOR Signaling Pathway. Doctoral Dissertation, Qing Dao University, Qingdao, China, 2019. [Google Scholar] [CrossRef]
- Pierre, J.F.; Peters, B.M.; La Torre, D.; Sidebottom, A.M.; Tao, Y.; Zhu, X.; Cham, C.M.; Wang, L.; Kambal, A.; Harris, K.G.; et al. Peptide YY: A Paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 2023, 381, 502–508. [Google Scholar] [CrossRef]
- Song, W.G. Isolation, Identification, Structure-Activity Relationship and Pilot Scale Preparation of Cottonseed Protein Antimicrobial Peptides; Jiangnan University: Wuxi, China, 2020. [Google Scholar]
- Anonymous. Optimization of extraction conditions and purification of antimicrobial peptides from capsicum seeds. Food Sci. 2019, 24, 258–264. (In Chinese) [Google Scholar]
- Epand, R.M. Host Defense Peptides and Their Potential as Therapeutic Agents; Plant Antimicrobial Peptides; Springer: Berlin/Heidelberg, Germany, 2016; Chapter 5; pp. 111–136. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.P.A.; Thevissen, K. Antifungal Plant Defensins: Mechanisms of Action and Production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef]
- Rashidian, G.; Moghaddam, M.M.; Mirnejad, R.; Azad, Z.M. Supplementation of zebrafish (danio rerio) diet using a short anti-microbial peptide: Evaluation of growth performance, immunomodulatory function, antioxidant activity, and disease re-sistance. Fish Shellfish. Immunol. 2021, 119, 42–50. [Google Scholar] [CrossRef]
- Li, F.-L.; Fu, V.; Liu, G.; Tang, T.; Konradi, A.W.; Peng, X.; Kemper, E.; Cravatt, B.F.; Franklin, J.M.; Wu, Z.; et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat. Chem. Biol. 2022, 18, 1076–1086. [Google Scholar] [CrossRef]
- Deng, F.E.H. Wnt/β-Catenin Pathway Activated by YAP Mediates Self-Renewal, Repair and Tumorigenesis of Intestinal Epithelial Cells after DSS Injury. Doctoral Dissertation, Southern Medical University, Guangzhou, China, 2018. [Google Scholar]
- Volcheck, G.W. How the Immune System Works. Ann. Allergy Asthma Immunol. 2001, 86, 350. [Google Scholar] [CrossRef]
- Koohsari, H.; Tamaoka, M.; Campbell, H.R.; Martin, J.G. The role of gamma delta T cells in airway epithelia injury and bronchial responsiveness after chlorine gas exposure in mice. Reopir. Res. 2007, 369, 1627–1640. [Google Scholar]
- Lee, J.W.; Wang, P.; Kattah, M.G.; Youssef, S.; Steinman, L.; DeFea, K.; Straus, D.S. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J. Immuno. 2008, 181, 6536–6545. [Google Scholar] [CrossRef]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 Cells Upregulate Polymeric Ig Receptor and Intestinal IgA and Contribute to Intes-tinal Homeostasis. J. Immunol. 2012, 189, 4197–4198. [Google Scholar] [CrossRef]
- O’Connor, W., Jr.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell–mediated intestinal inflammation. Nat. Immunol. 2009, 10, 603–609. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4_ T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Stinchcombe, J.C.; Asano, Y.; Kaufman, C.J.G.; Böhlig, K.; Peddie, C.J.; Collinson, L.M.; Nadler, A.; Griffiths, G.M. Ectocytosis renders T cell receptor signaling self-limiting at the immune synapse. Science 2023, 380, 818–823. [Google Scholar] [CrossRef]


| Peptide | z | Observed (M + H) | Calc.mass (M + H) | Mass Error (ppm) | Score |
|---|---|---|---|---|---|
| T.VIDAPGHRDFIKN.M | 2 | 1481.795 | 1481.786 | 6.1 | 654.0 |
| T.VIDAPGHRDF.I | 2 | 1126.563 | 1126.564 | −1.2 | 598.4 |
| L.EDLRIPTAY.V | 2 | 1077.556 | 1077.558 | −1.0 | 588.0 |
| K.IGGIGTVPVGRVE.T | 2 | 1253.723 | 1253.721 | 1.0 | 579.3 |
| V.IDAPGHRDF.I | 2 | 1027.492 | 1027.496 | −3.4 | 514.7 |
| Metabolite Name | NEG | AMP | Regulated | Metabolite Name | NEG | AMP | Regulated |
|---|---|---|---|---|---|---|---|
| PC (18:0/18:4(6Z, 9Z, 15Z)) | 4189.29 | 65,640.01 | up | YM-53601 | 18,941.12 | 15,287.58 | down |
| PC (16:1(9Z)/20:3(5Z, 11Z)) | 3102.32 | 38,582.48 | up | DL-Leucine | 5665.91 | 4322.96 | down |
| PC (14:0/22:4(7Z, 10Z, 16Z)) | 9856.89 | 112,375.47 | up | 3,5,5-Trimethyl 1,2-cyclohexanedione | 118.79 | 84.13 | down |
| Cysteinylglycine | 14.49 | 75.22 | up | 10-Acetoxyligustroside | 376.28 | 252.54 | down |
| PE(15:0/22:1(13Z)) | 69,607.06 | 529,159.57 | up | Indole | 1058.07 | 679.63 | down |
| PC (18:0/20:4(8Z, 11Z, 17Z)) | 3716.69 | 16,123.48 | up | 2-Aminomethylpyrimidine (hydrochloride) | 959.22 | 615.64 | down |
| PC (18:3(6Z, 12Z)/20:1(11Z)) | 17,535.9 | 70,552.72 | up | Methionyl-Valine | 106.73 | 68.24 | down |
| PC (18:3(9Z, 12Z, 15Z)/20:0) | 19,331.62 | 67,597.27 | up | N-Malonyltryptophan | 1906.65 | 1195.56 | down |
| Glucosylceramide (18:1/18:0) | 14,091.41 | 46,609.31 | up | L-Histidine | 1423.92 | 895.25 | down |
| PC (18:0/22:5(7Z, 16Z, 19Z)) | 2056.87 | 6252.93 | up | N-Undecanoylglycine | 4919.8 | 3024.44 | down |
| PS (17:1(9Z)/22:2(13Z, 16Z)) | 500.16 | 1435.46 | up | Lysyl-Glutamine | 307.4 | 187.89 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chi, X.; Zhang, D. Potential Regulatory Gene Network Associated with the Ameliorative Effect of Oat Antibacterial Peptides on Rat Colitis. Foods 2024, 13, 236. https://doi.org/10.3390/foods13020236
Wang H, Chi X, Zhang D. Potential Regulatory Gene Network Associated with the Ameliorative Effect of Oat Antibacterial Peptides on Rat Colitis. Foods. 2024; 13(2):236. https://doi.org/10.3390/foods13020236
Chicago/Turabian StyleWang, Helin, Xiaoxing Chi, and Dongjie Zhang. 2024. "Potential Regulatory Gene Network Associated with the Ameliorative Effect of Oat Antibacterial Peptides on Rat Colitis" Foods 13, no. 2: 236. https://doi.org/10.3390/foods13020236
APA StyleWang, H., Chi, X., & Zhang, D. (2024). Potential Regulatory Gene Network Associated with the Ameliorative Effect of Oat Antibacterial Peptides on Rat Colitis. Foods, 13(2), 236. https://doi.org/10.3390/foods13020236





