Studies on the Inhibition Mechanism of Linalyl Alcohol against the Spoilage Microorganism Brochothrix thermosphacta
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Test Bacteria
2.1.1. Reagents
2.1.2. Bacterial Strains and Culture Conditions
2.2. Research on Bacteriostatic Activity
2.2.1. Determination of Minimum Inhibitory Concentration (MIC) of Linalyl Alcohol
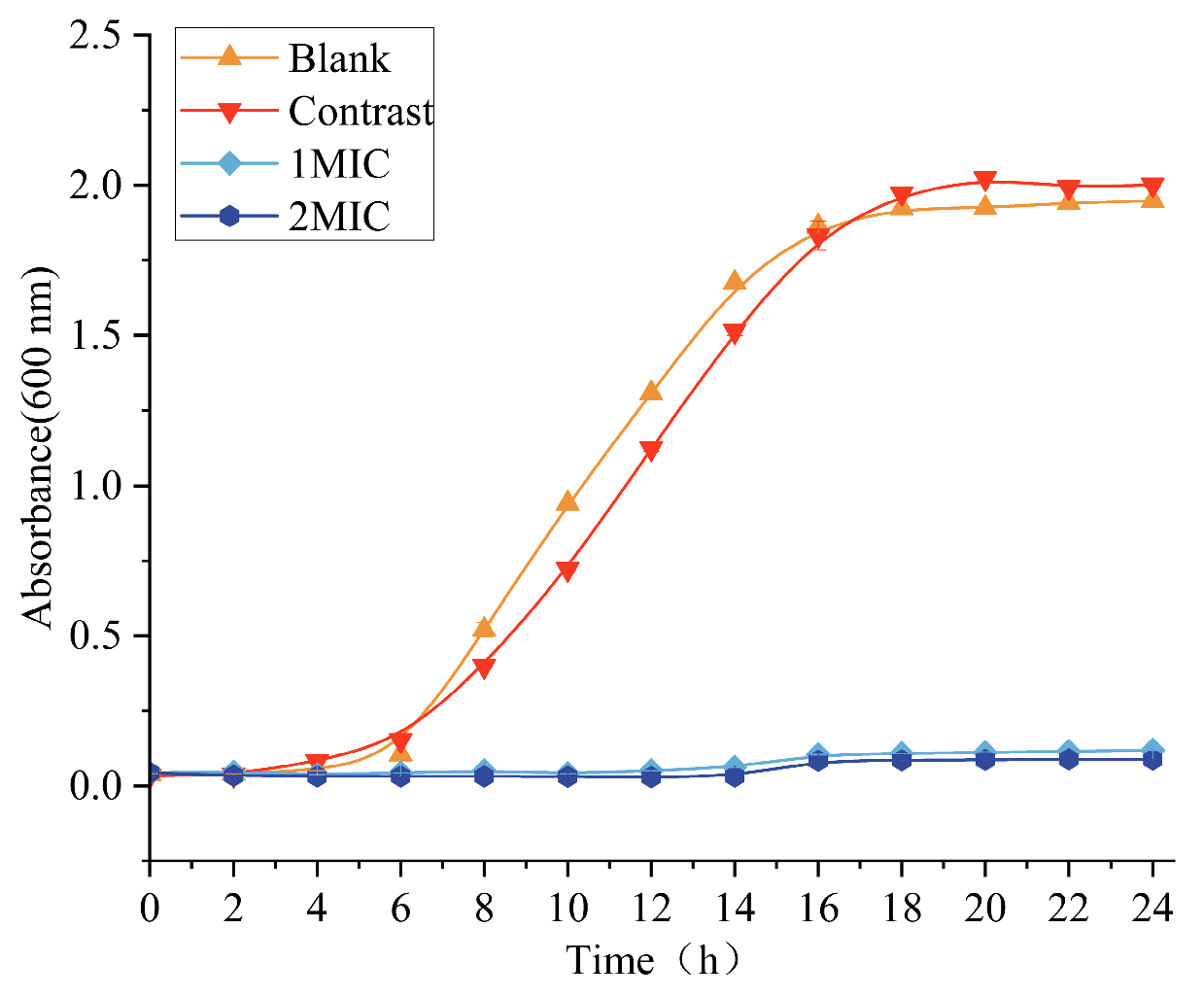
2.2.2. Growth Curve
2.3. Mechanism of Action of Linalyl Alcohol on B. thermosphacta Cell Membranes
2.3.1. Surface Hydrophobicity
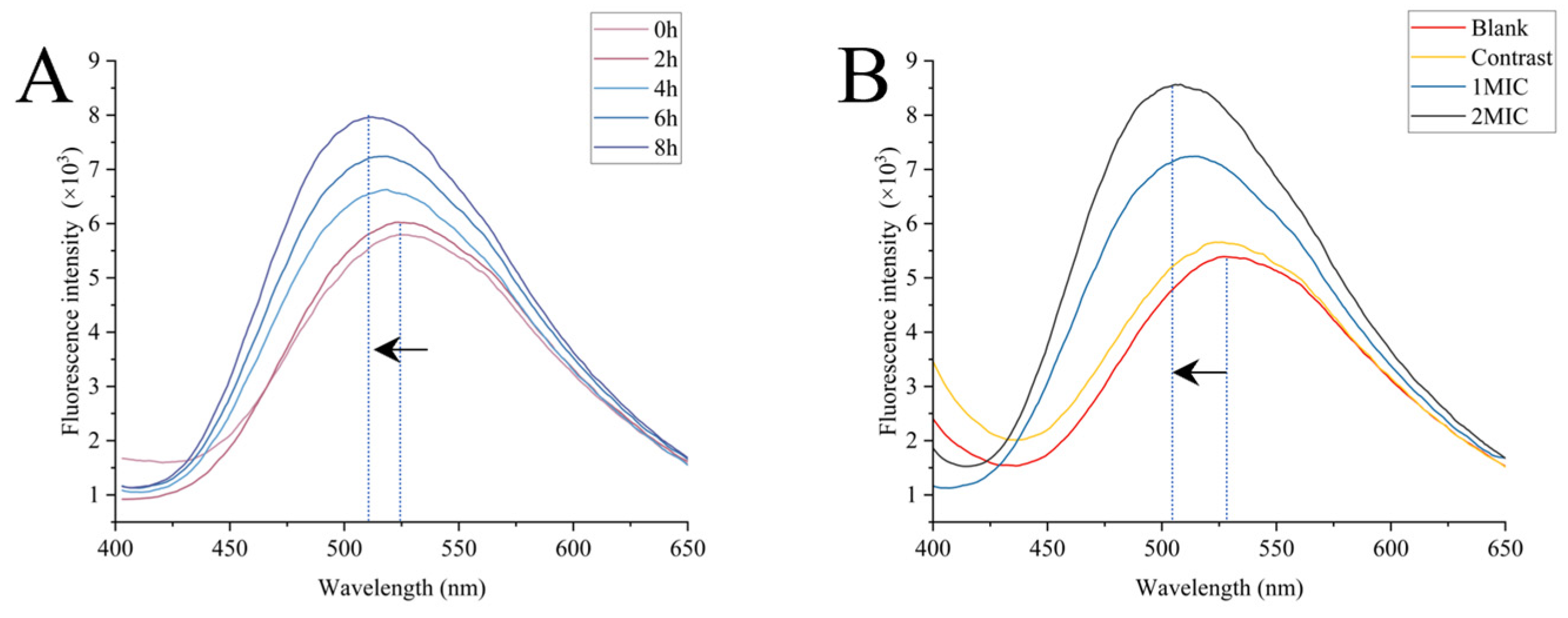
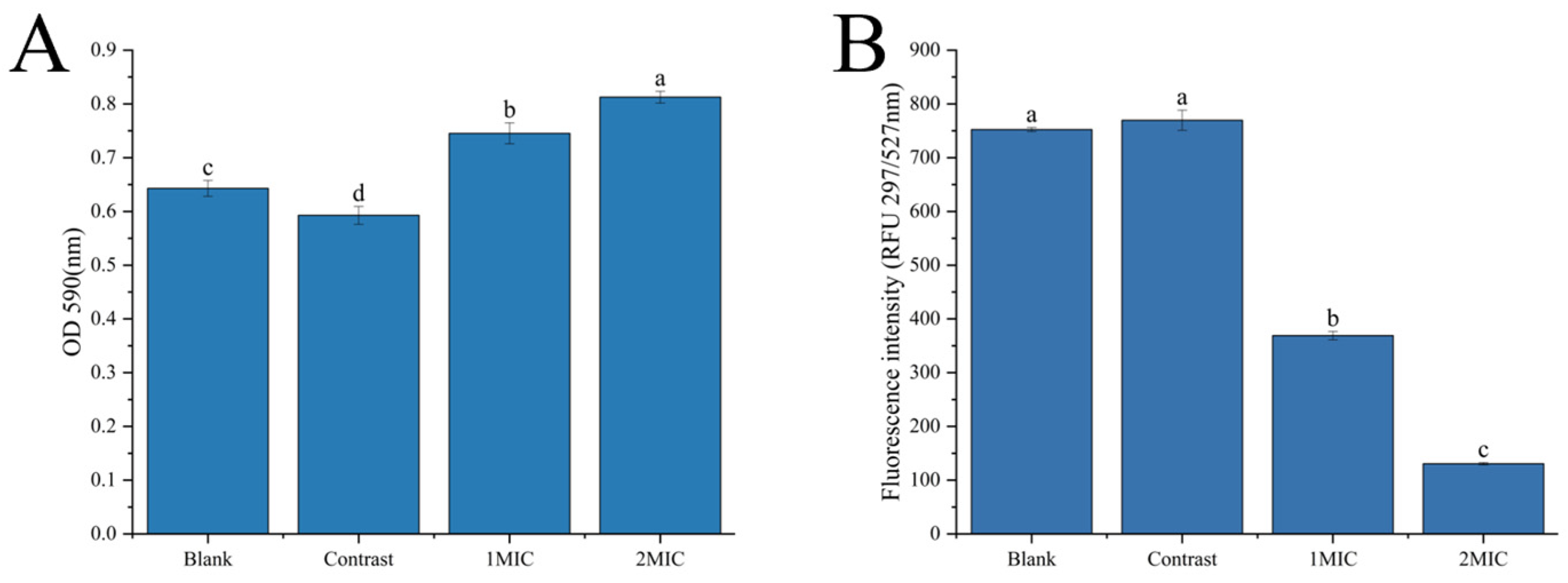
2.3.2. Crystal Violet and Fluorescein Diacetate Staining
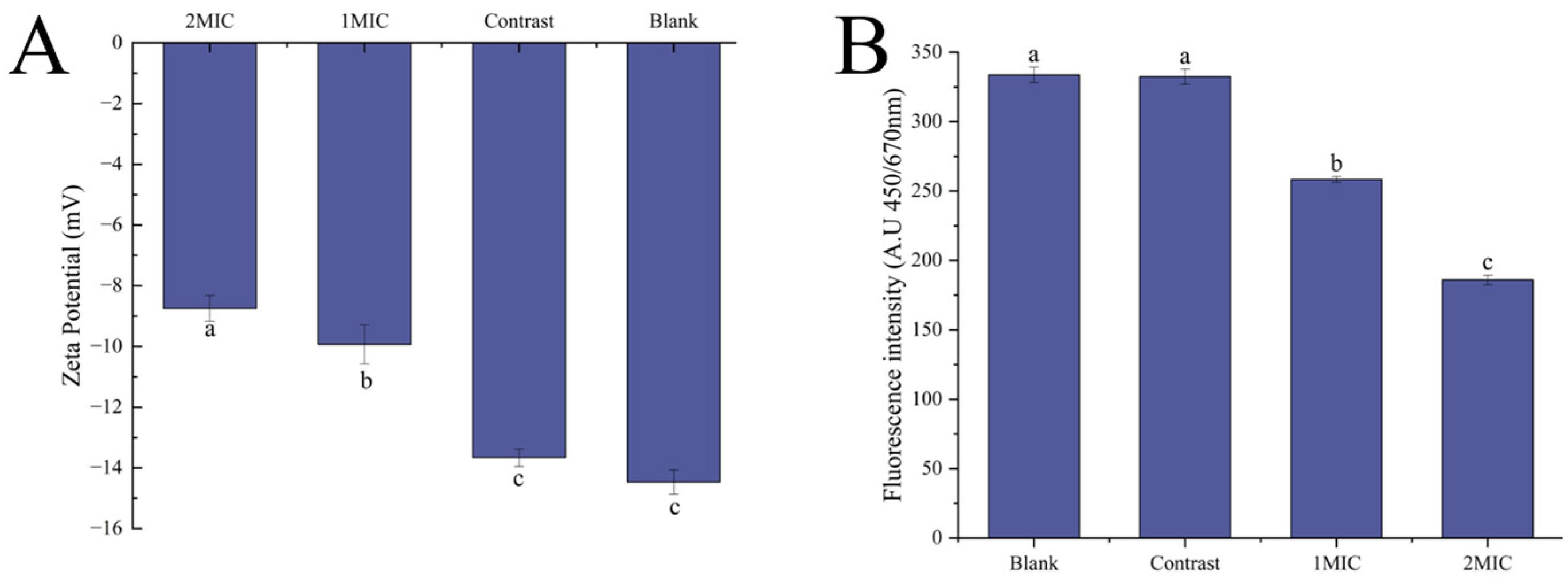
2.3.3. Zeta Potential and Membrane Depolarization Tests
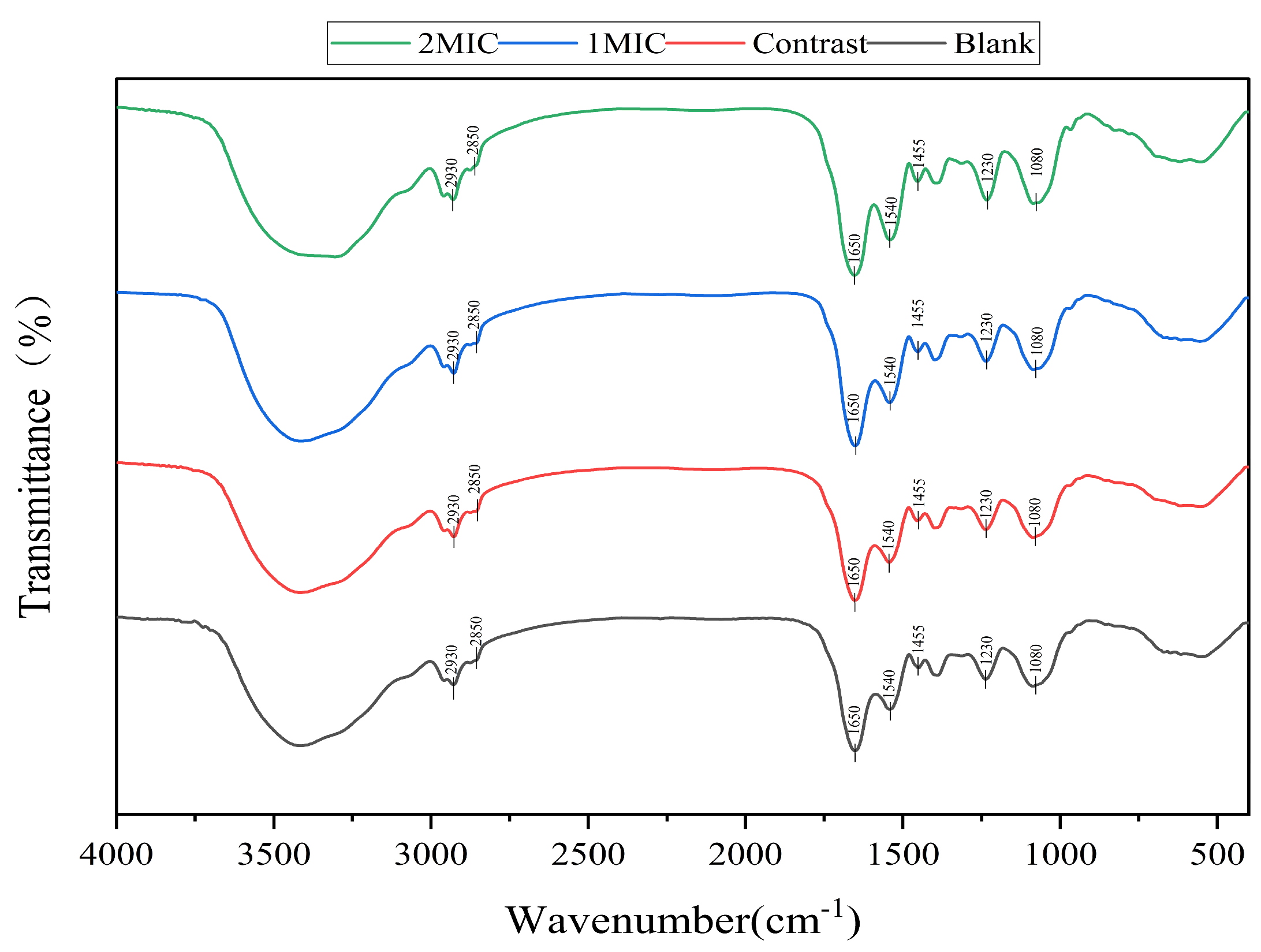
2.3.4. Determination of Chemical Configuration
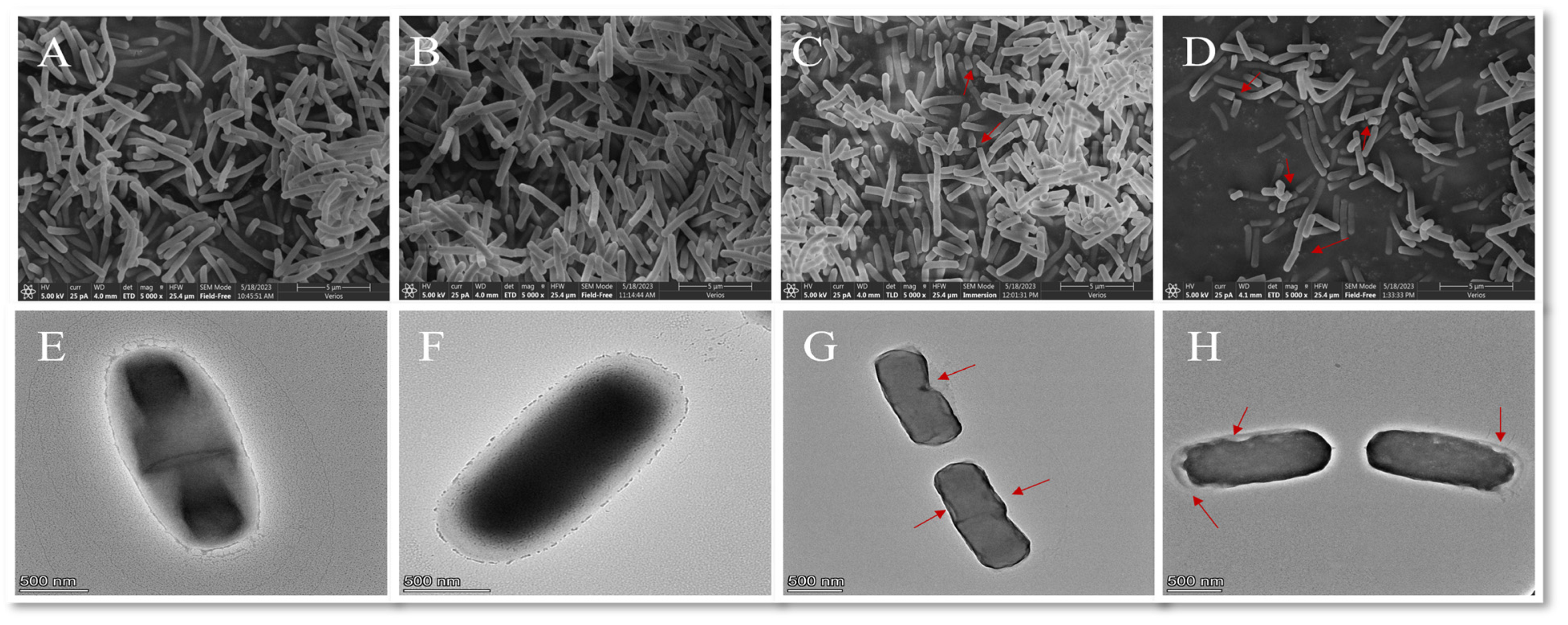
2.3.5. Electron Microscope Analysis
2.4. Effect on Intracellular Substances
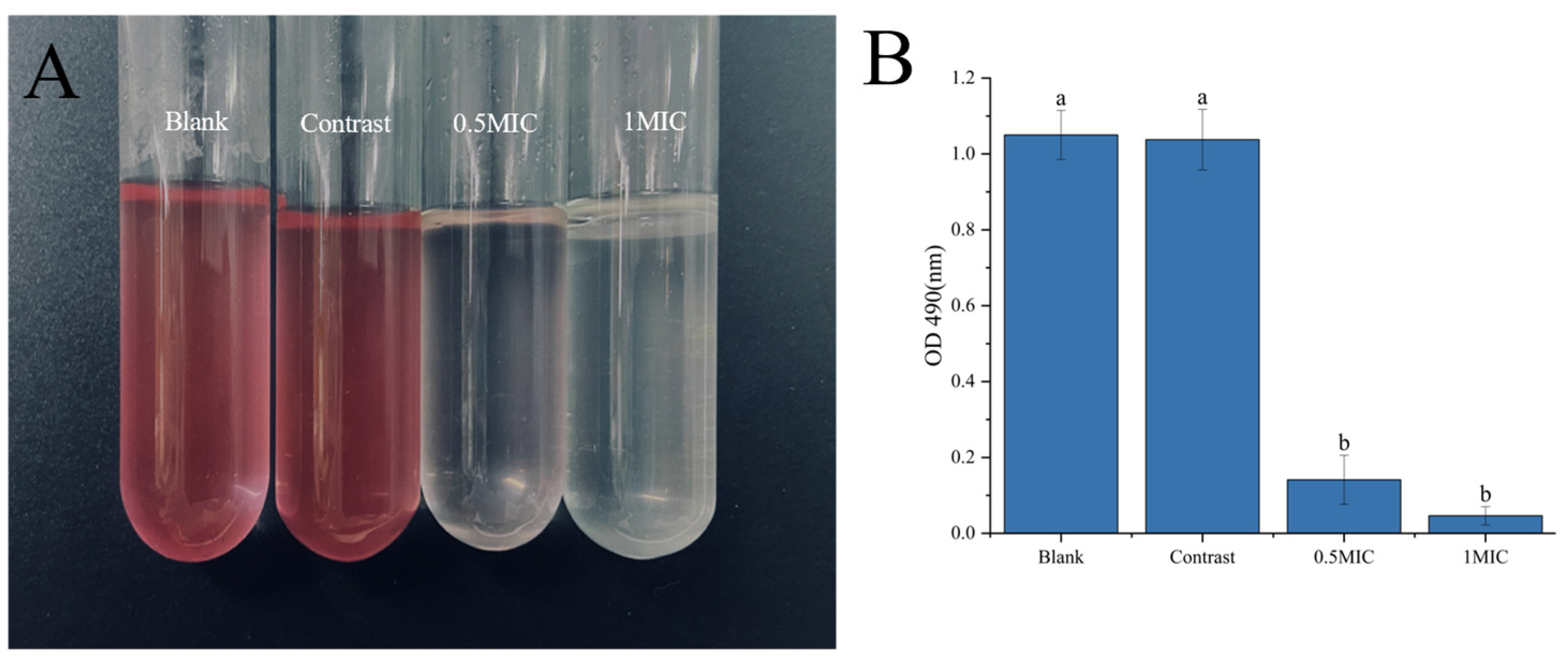
2.4.1. Respiratory Chain Dehydration
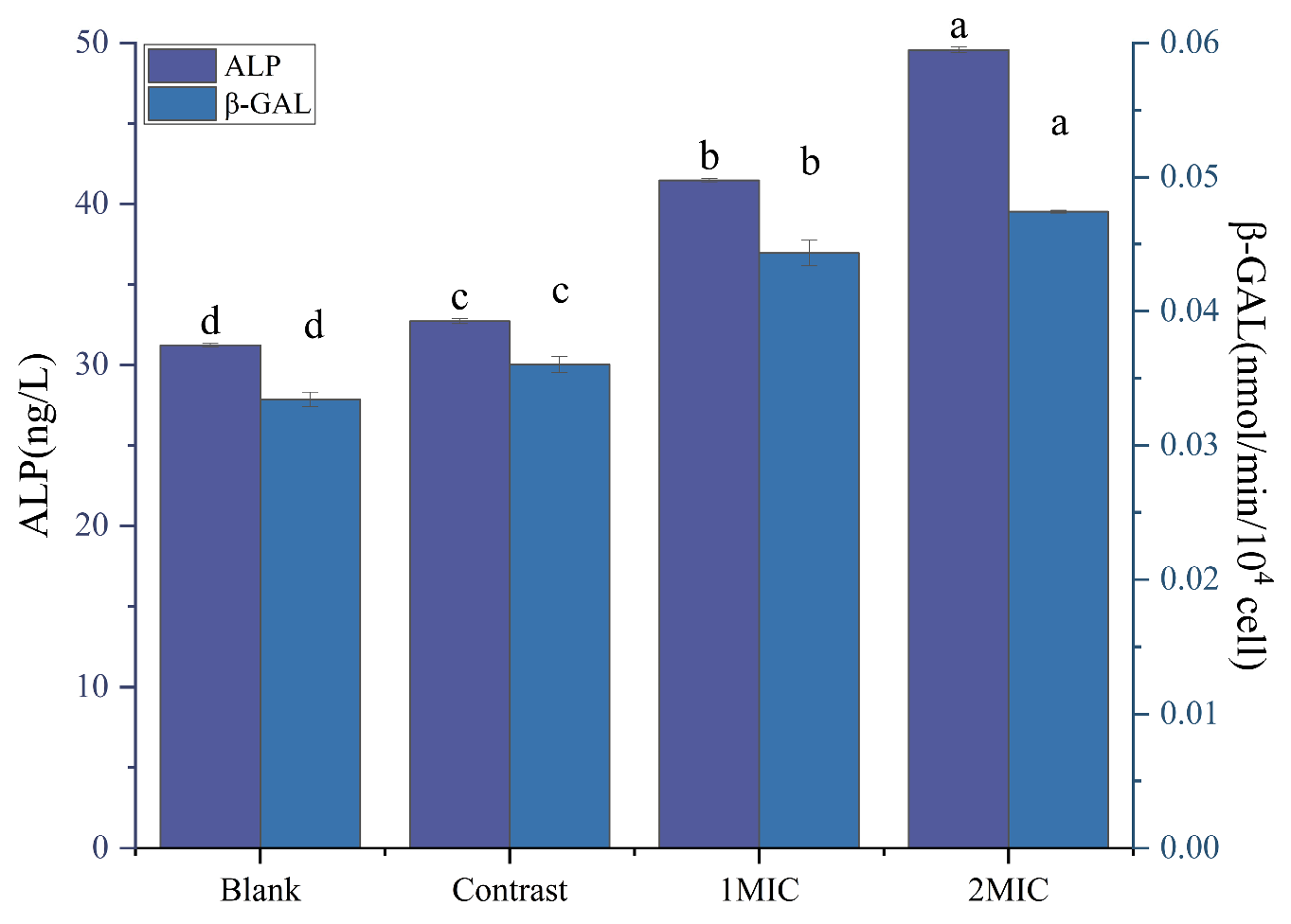
2.4.2. β-Galactosidase and Alkaline Phosphatase
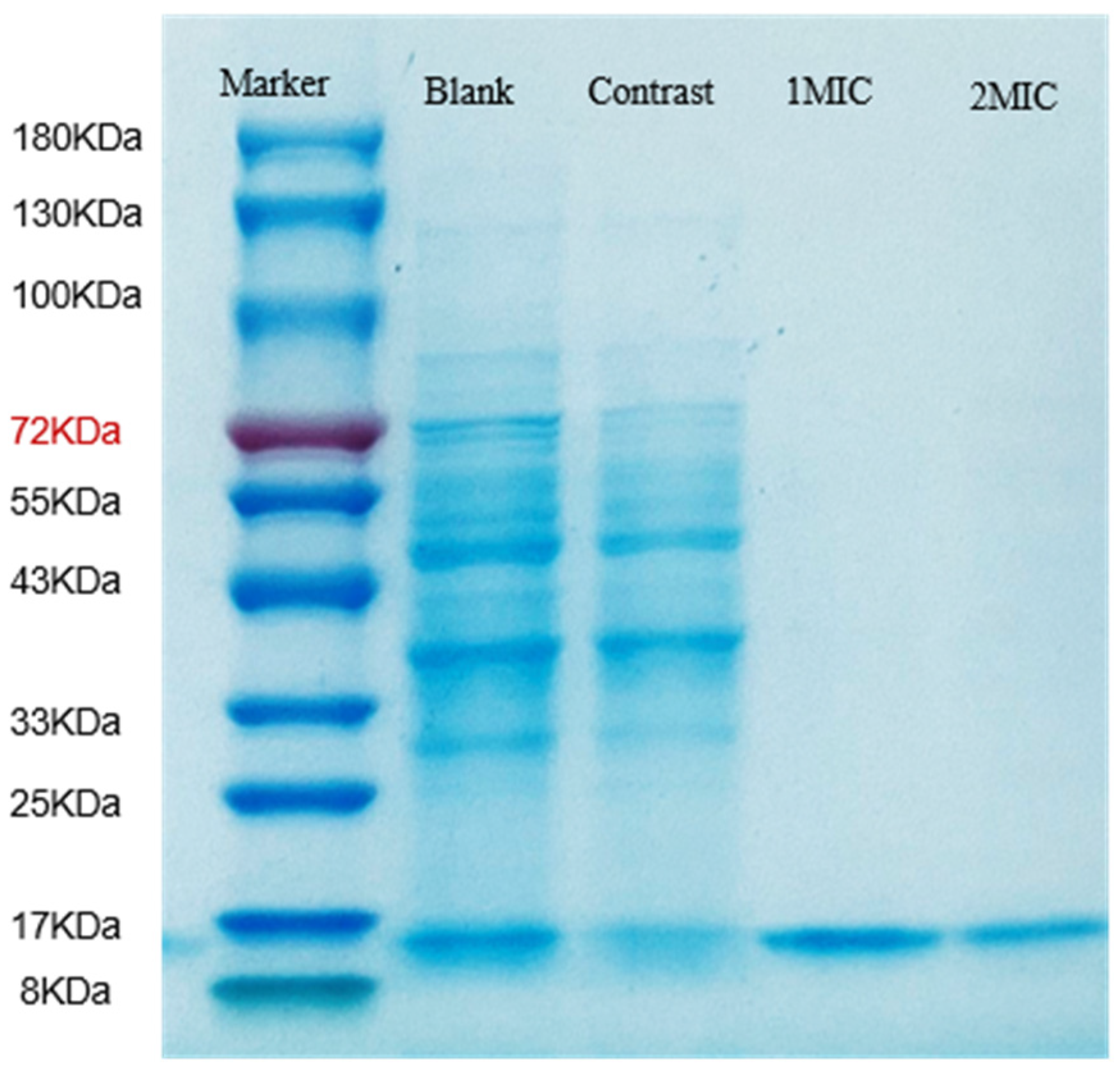
2.4.3. SDS-PAGE of Proteins
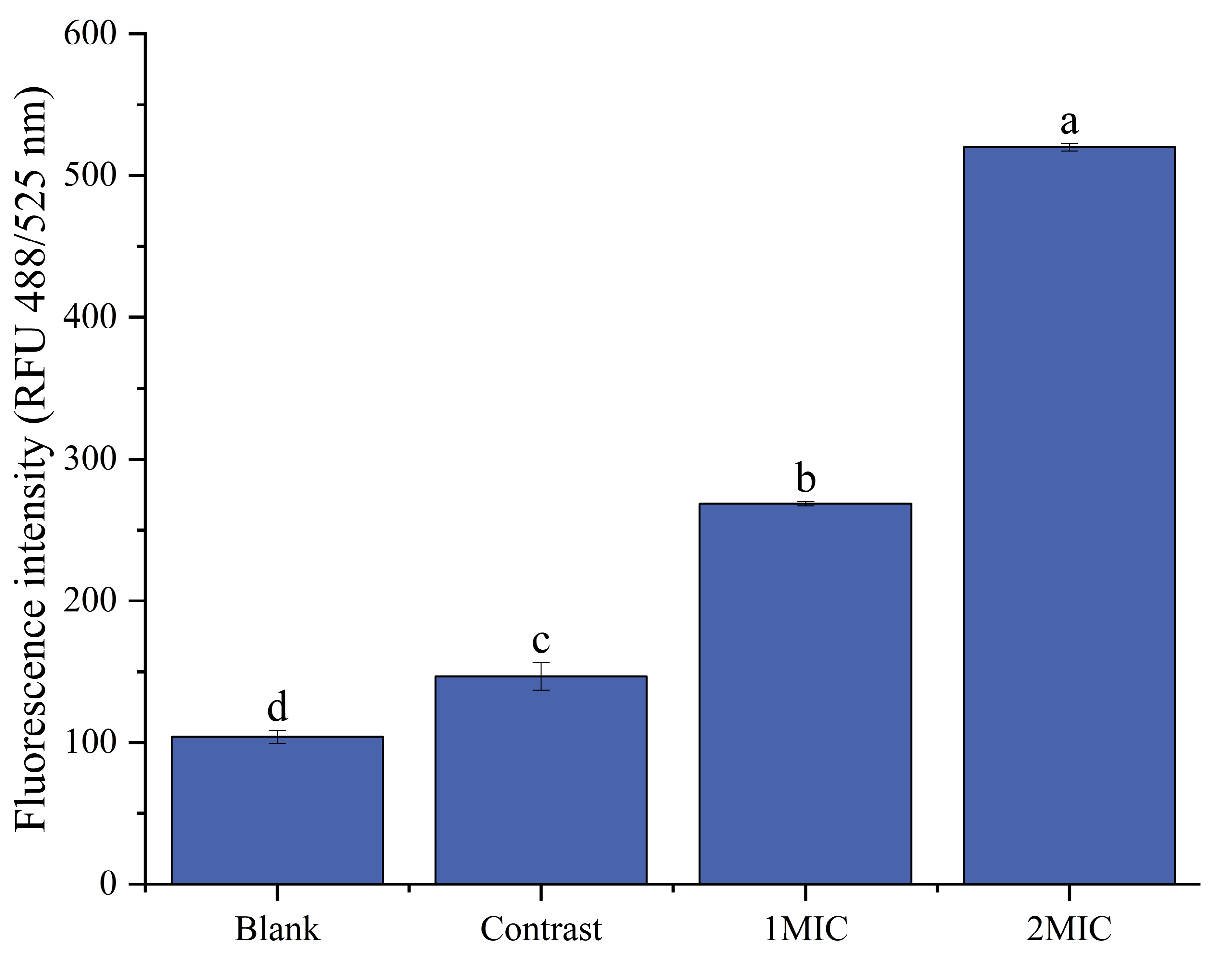
2.4.4. Detection of the Intracellular Reactive Oxygen Species Level
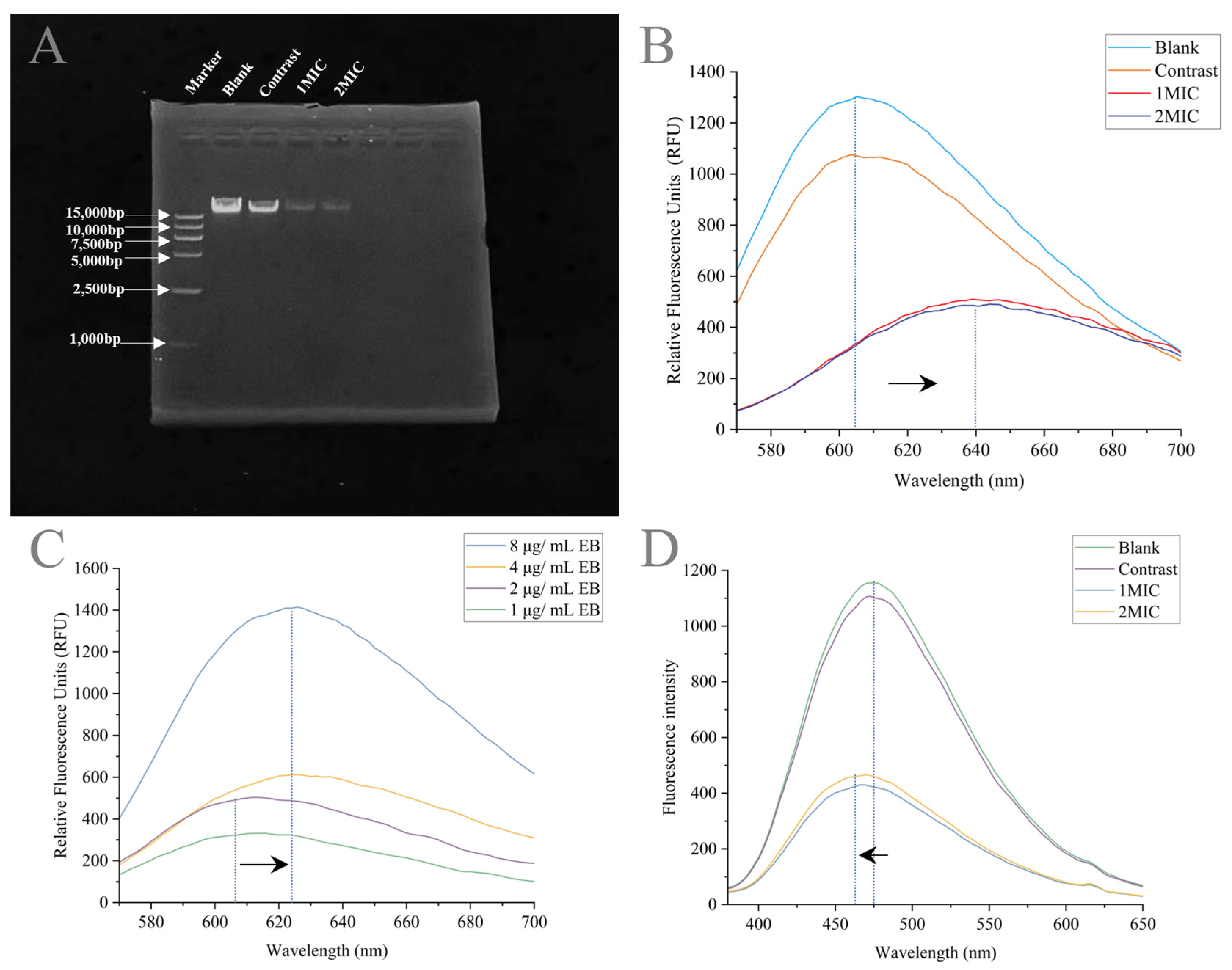
2.4.5. Linalyl Alcohol–DNA binding Assay
2.4.6. Spectral Measurement
2.5. Statistical Analyses
3. Results and Discussion
3.1. Minimum Inhibitory Concentration of Linalyl Alcohol
3.2. Effect of Linalyl Alcohol on the Growth Curve of B. thermosphacta
3.3. Mechanism of Linalyl Alcohol Cell Membrane Inhibition against B. Thermosphacta
3.3.1. Surface Hydrophobicity Analysis
3.3.2. Membrane Damage
Analysis of Crystal Violet and Fluorescein Diacetate Staining
Zeta Potential and Membrane Depolarization
Analysis of Cell Membrane Composition
Morphological Changes in B. thermosphacta
3.3.3. Study of the Effect of Intracellular Substances
Respiratory Chain Dehydrogenase
β-Galactosidase (β-GAL) and Alkaline Phosphatase (ALP)
SDS-PAGE of Proteins
Oxidative Damage to Cell Membranes
Effects of Linalyl Alcohol on Genomic DNA of B. thermosphacta
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barakat, R.K.; Griffiths, M.W.; Harris, L.J. Isolation and Characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from Cooked, Modified Atmosphere Packaged, Refrigerated, Poultry Meat. Int. J. Food Microbiol. 2000, 62, 83–94. [Google Scholar] [CrossRef]
- Borch, E.; Kant-Muermans, M.-L.; Blixt, Y. Bacterial Spoilage of Meat and Cured Meat Products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and Psychrotrophic Bacteria from Meat and Their Spoilage Potential In Vitro and in Beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Pennacchia, C.; Ercolini, D.; Villani, F. Development of a Real-Time PCR Assay for the Specific Detection of Brochothrix Thermosphacta in Fresh and Spoiled Raw Meat. Int. J. Food Microbiol. 2009, 134, 230–236. [Google Scholar] [CrossRef]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdague, J.L. Characterisation of Volatile Compounds Produced by Bacteria Isolated from the Spoilage Flora of Cold-Smoked Salmon. Int. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef]
- Jaffres, E.; Lalanne, V.; Mace, S.; Cornet, J.; Cardinal, M.; Serot, T.; Dousset, X.; Joffraud, J.-J. Sensory Characteristics of Spoilage and Volatile Compounds Associated with Bacteria Isolated from Cooked and Peeled Tropical Shrimps Using SPME-GC-MS Analysis. Int. J. Food Microbiol. 2011, 147, 195–202. [Google Scholar] [CrossRef]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The Antimicrobial Effects and Synergistic Antibacterial Mechanism of the Combination of Epsilon-Polylysine and Nisin against Bacillus Subtilis. Food Control 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Chen, Z.; He, B.; Zhou, J.; He, D.; Deng, J.; Zeng, R. Chemical Compositions and Antibacterial Activities of Essential Oils Extracted from Alpinia Guilinensis against Selected Foodborne Pathogens. Ind. Crop. Prod. 2016, 83, 607–613. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, X.; Li, X.; Wang, W. Effect of Chitosan Coating Combined with Sulfur Dioxide Fumigation on the Storage Quality of Fresh Areca Nut: Co-Application of Chitosan and Sulfur Dioxide on Fresh Areca Nut Storage. J. Food Process. Preserv. 2017, 41, e12974. [Google Scholar] [CrossRef]
- Alviano, W.S.; Mendonca-Filho, R.R.; Alviano, D.S.; Bizzo, H.R.; Souto-Padron, T.; Rodrigues, M.L.; Bolognese, A.M.; Alviano, C.S.; Souza, M.M.G. Antimicrobial Activity of Croton Cajucara Benth Linalool-Rich Essential Oil on Artificial Biofilms and Planktonic Microorganisms. Oral. Microbiol. Immunol. 2005, 20, 101–105. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind. Crop. Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Islam, M.T.; Martorell, M.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Anti-Schistosoma Mansoni Effects of Essential Oils and Their Components. Phytother. Res. 2020, 34, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.-K. Antimicrobial Effect of Linalool and Alpha-Terpineol against Periodontopathic and Cariogenic Bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Eliu Castell-Rodriguez, A.; Becerril-Millan, R.; Aurelio Rodriguez-Monroy, M.; Margarita Canales-Martinez, M. Wound Healing Activity of the Essential Oil of Bursera Morelensis, in Mice. Molecules 2020, 25, 1795. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Hancianu, M.; Costache, I.-I.; Miron, A. Linalool: A Review on a Key Odorant Molecule with Valuable Biological Properties. Flavour Frag. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Lapczynski, A.; Letizia, C.S.; Api, A.M. Fragrance Material Review on D-Linalool. Food Chem. Toxicol. 2008, 46 (Suppl. S11), S193–S194. [Google Scholar] [CrossRef]
- Batista, P.A.; de Paula Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; da Silva Brum, L.F.; Santos, A.R.S.D. Evidence for the Involvement of Ionotropic Glutamatergic Receptors on the Antinociceptive Effect of (-)-Linalool in Mice. Neurosci. Lett. 2008, 440, 299–303. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-Inflammatory Effects of Linalool in RAW 264.7 Macrophages and Lipopolysaccharide-Induced Lung Injury Model. J. Surg. Res. 2013, 180, E47–E54. [Google Scholar] [CrossRef]
- Miyashita, M.; Sadzuka, Y. Effect of Linalool as a Component of Humulus Lupulus on Doxorubicin-Induced Antitumor Activity. Food Chem. Toxicol. 2013, 53, 174–179. [Google Scholar] [CrossRef]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of Inhaled Linalool in Anxiety, Social Interaction and Aggressive Behavior in Mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- de Oliveira Souza Senra, T.; Zeringota, V.; de Oliveira Monteiro, C.M.; Calmon, F.; Maturano, R.; Gomes, G.A.; Faza, A.; de Carvalho, M.G.; Daemon, E. Assessment of the Acaricidal Activity of Carvacrol, (E)-Cinnamaldehyde, Trans-Anethole, and Linalool on Larvae of Rhipicephalus Microplus and Dermacentor Nitens (Acari: Ixodidae). Parasitol. Res. 2013, 112, 1461–1466. [Google Scholar] [CrossRef]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella Putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Pseudomonas Aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Su, R.; Guo, P.; Zhang, Z.; Wang, J.; Guo, X.; Guo, D.; Wang, Y.; Lu, X.; Shi, C. Antibacterial Activity and Mechanism of Linalool against Shigella Sonnei and Its Application in Lettuce. Foods 2022, 11, 3160. [Google Scholar] [CrossRef]
- Li, Y.-N.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Cai, J.-P.; Hu, Y.-S. Mechanisms Underlying the Inhibitory Effects of Linalool on Aspergillus Flavus Spore Germination. Appl. Microbiol. Biotechnol. 2022, 106, 6625–6640. [Google Scholar] [CrossRef]
- Liang, T.; Huo, G.; Chen, L.; Ding, L.; Wu, J.; Zhang, J.; Wang, R. Antibacterial Activity and Metabolomic Analysis of Linalool against Bovine Mastitis Pathogen Streptococcus Agalactiae. Life Sci. 2023, 313, 121299. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus Aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Huang, J.; Qian, C.; Xu, H.; Huang, Y. Antibacterial Activity of Artemisia Asiatica Essential Oil against Some Common Respiratory Infection Causing Bacterial Strains and Its Mechanism of Action in Haemophilus Influenzae. Microb. Pathog. 2018, 114, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Thennarasu, S.; Tan, A.; Penumatchu, R.; Shelburne, C.E.; Heyl, D.L.; Ramamoorthy, A. Antimicrobial and Membrane Disrupting Activities of a Peptide Derived from the Human Cathelicidin Antimicrobial Peptide LL37. Biophys. J. 2010, 98, 248–257. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and Anti-Biofilm Activities of Peppermint Essential Oil against Staphylococcus Aureus. LWT-Food Sci. Technol. 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, J.; Li, J.; Luo, Q.; Dong, X.; Tang, G.; Zhang, J.; Du, X.; Pu, Q.; He, L.; et al. Antibacterial Activity and Mechanism of Sanguinarine against Staphylococcus Aureus by Interfering with the Permeability of the Cell Wall and Membrane and Inducing Bacterial ROS Production. Front. Vet. Sci. 2023, 10, 1121082. [Google Scholar] [CrossRef]
- Bai, S.; Wang, J.; Yang, K.; Zhou, C.; Xu, Y.; Song, J.; Gu, Y.; Chen, Z.; Wang, M.; Shoen, C.; et al. A Polymeric Approach toward Resistance-Resistant Antimicrobial Agent with Dual-Selective Mechanisms of Action. Sci. Adv. 2021, 7, eabc9917. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.A.; Siegele, D.A.; Lockless, S.W. Use of a Fluorescence-Based Assay to Measure Escherichia Coli Membrane Potential Changes in High Throughput. Antimicrob. Agents Chemother. 2020, 64, e00910-20. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Simón, A.; Encina, A.E.; García-Angulo, P.; Álvarez, J.M.; Acebes, J.L. FTIR Spectroscopy Monitoring of Cell Wall Modifications during the Habituation of Bean (Phaseolus vulgaris L.) Callus Cultures to Dichlobenil. Plant Sci. 2004, 167, 1273–1281. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Hao, G.; Zhao, L.; Lu, X.; Wang, H.; Wang, L.; Zhang, J.; Ge, W. Antimicrobial Activity and Mechanism of Isothiocyanate from Moringa Oleifera Seeds against Bacillus cereus and Cronobacter sakazakii and Its Application in Goat Milk. Food Control 2022, 139, 109067. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-W.; Zhang, H.; Yang, Q.; Wang, H.; Zhang, G. Preparation, Characterization and Antibacterial Activity of Octenyl Succinic Anhydride Modified Inulin. Int. J. Biol. Macromol. 2015, 78, 79–86. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, T.; Wei, C.; Lan, W.; Zhao, Y.; Pan, Y.; Wu, V.C.H. Antibacterial Effect and Mechanism of Anthocyanin Rich Chinese Wild Blueberry Extract on Various Foodborne Pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, T.; Liang, S.; Ma, M.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Antibacterial Activity and Action Mechanism of Microencapsulated Dodecyl Gallate with Methyl-β-Cyclodextrin. Food Control 2020, 109, 106953. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Sun, Y.; Abdel-Samie, M.A.-S.; Lin, L. Antibacterial Activity and Mechanism of Chuzhou Chrysanthemum Essential Oil. J. Funct. Foods 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Thibane, V.S.; Ells, R.; Hugo, A.; Albertyn, J.; van Rensburg, W.J.J.; Van Wyk, P.W.J.; Kock, J.L.F.; Pohl, C.H. Polyunsaturated Fatty Acids Cause Apoptosis in C. Albicans and C. Dubliniensis Biofilms. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.-M.; Li, T.; Liang, H.; He, K.-K.; Zheng, Y.-M.; Tang, M.; Ke, C.; Song, L.-Y. Antibacterial Activities and Mechanisms of Vine Tea Extract and 2R, 3R-Dihydromyricetin on Escherichia Coli. LWT-Food Sci. Technol. 2021, 146, 111393. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Cheserek, M.J.; Su, G.; Le, G. Antibacterial Activity and Dual Mechanisms of Peptide Analog Derived from Cell-Penetrating Peptide against Salmonella Typhimurium and Streptococcus Pyogenes. Appl. Microbiol. Biotechnol. 2013, 97, 1711–1723. [Google Scholar] [CrossRef]
- Azimi, S.; Thomas, J.; Cleland, S.E.; Curtis, J.E.; Goldberg, J.B.; Diggle, S.P. O-Specific Antigen-Dependent Surface Hydrophobicity Mediates Aggregate Assembly Type in Pseudomonas Aeruginosa. mBio 2021, 12, e00860-21. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Zhang, D.; Wu, M.; Xue, Z.; Liu, Z.; Yang, S.; Li, H.; Gong, G. Evaluation of the Membrane Damage Mechanism of Protocatechualdehyde against Yersinia Enterocolitica and Simulation of Growth Inhibition in Pork (Vol 363, 130340, 2021). Food Chem. 2022, 373, 131203. [Google Scholar] [CrossRef]
- Tang, T.; Zhong, W.; Yang, L.; He, M.; Jiang, S.; Yin, D.; Guo, J.; Gao, Z. In Vitro and in Vivo Anti-Oomycetes Activities and Mechanisms of Linalool against Saprolegnia Ferax. Aquaculture 2024, 578, 740031. [Google Scholar] [CrossRef]
- Gurbanov, R.; Bilgin, M.; Severcan, F. Restoring Effect of Selenium on the Molecular Content, Structure and Fluidity of Diabetic Rat Kidney Brush Border Cell Membrane. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dalai, S.; Pakrashi, S.; Chakravarty, S.; Hussain, S.; Chandrasekaran, N.; Mukherjee, A. Studies on Interfacial Interactions of TiO2 Nanoparticles with Bacterial Cells under Light and Dark Conditions. Bull. Mat. Sci. 2014, 37, 371–381. [Google Scholar] [CrossRef]
- Liang, H.; He, K.; Li, T.; Cui, S.; Tang, M.; Kang, S.; Ma, W.; Song, L. Mechanism and Antibacterial Activity of Vine Tea Extract and Dihydromyricetin against Staphylococcus Aureus. Sci. Rep. 2020, 10, 21416. [Google Scholar] [CrossRef]
- Guven, R.G.; Kaplan, A.; Guven, K.; Matpan, F.; Dogru, M. Effects of Various Inhibitors on Beta-Galactosidase Purified from the Thermoacidophilic Alicyclobacillus Acidocaldarius Subsp Rittmannii Isolated from Antarctica. Biotechnol. Bioprocess. Eng. 2011, 16, 114–119. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial Activity and Mechanism of Litsea Cubeba Essential Oil against Methicillin-Resistant Staphylococcus Aureus (MRSA). Ind. Crop. Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
| Bacteria for Test | Control | 1% Ethanol | Linalyl Alcohol Concentration (mL/L) | |||||
|---|---|---|---|---|---|---|---|---|
| 0.375 | 0.75 | 1.5 | 3 | 6 | 12 | |||
| B. thermosphacta | +++ | +++ | +++ | +++ | +++ | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X.; Chen, W.; Sun, Z. Studies on the Inhibition Mechanism of Linalyl Alcohol against the Spoilage Microorganism Brochothrix thermosphacta. Foods 2024, 13, 244. https://doi.org/10.3390/foods13020244
Wang L, Liu X, Chen W, Sun Z. Studies on the Inhibition Mechanism of Linalyl Alcohol against the Spoilage Microorganism Brochothrix thermosphacta. Foods. 2024; 13(2):244. https://doi.org/10.3390/foods13020244
Chicago/Turabian StyleWang, Longteng, Xing Liu, Wenxue Chen, and Zhichang Sun. 2024. "Studies on the Inhibition Mechanism of Linalyl Alcohol against the Spoilage Microorganism Brochothrix thermosphacta" Foods 13, no. 2: 244. https://doi.org/10.3390/foods13020244
APA StyleWang, L., Liu, X., Chen, W., & Sun, Z. (2024). Studies on the Inhibition Mechanism of Linalyl Alcohol against the Spoilage Microorganism Brochothrix thermosphacta. Foods, 13(2), 244. https://doi.org/10.3390/foods13020244






