Influence of Nitrogen-Modified Atmosphere Storage on Lipid Oxidation of Peanuts: From a Lipidomic Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Storage Condition
2.2. Determination of Peanut-Lipid Oxidation Properties
2.2.1. Extraction of Peanut Lipid
2.2.2. Measurement of Acid Value
2.2.3. Measurement of Carbonyl Value
2.2.4. Measurement of 2-Thiobarbituric Acid Value
2.2.5. Measurement of the Content of Vitamin E
2.3. Detection and Analysis of Metabolites
2.3.1. Extraction of Metabolites
2.3.2. LC-MS Analysis
2.3.3. Identification of Metabolites
2.4. Statistical Analysis
3. Results and Discussion
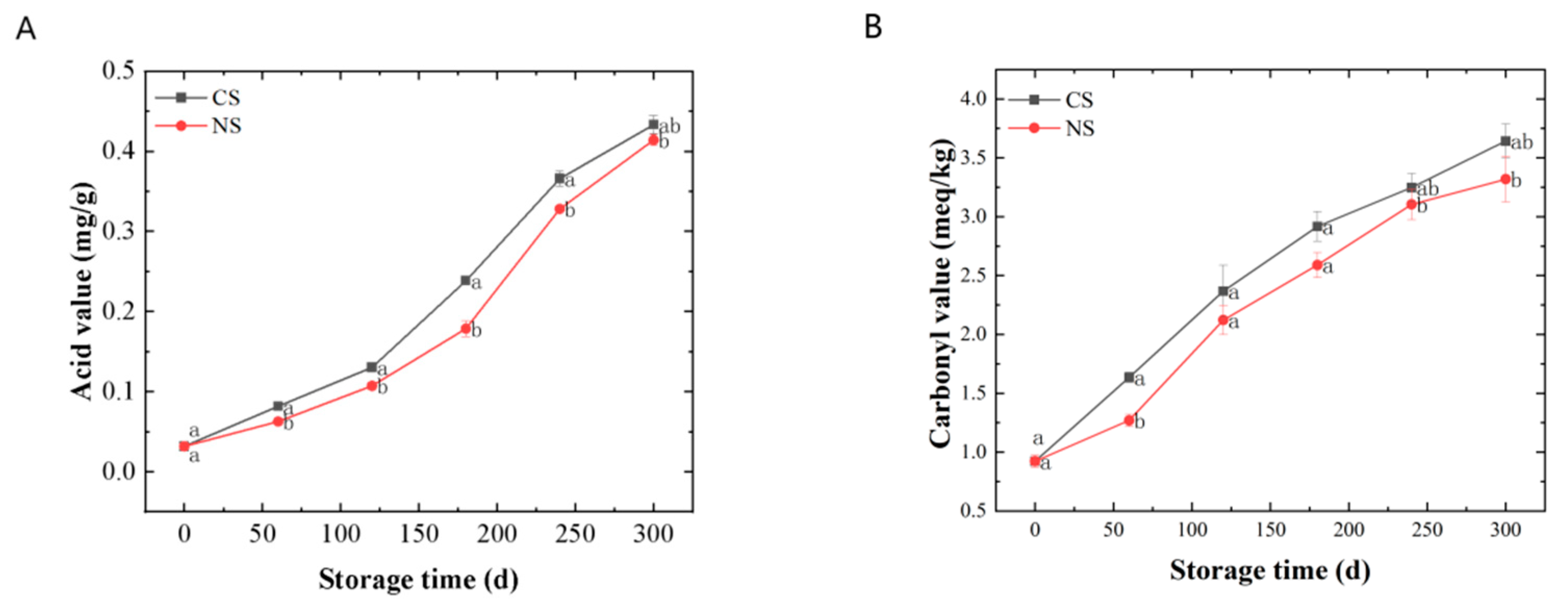
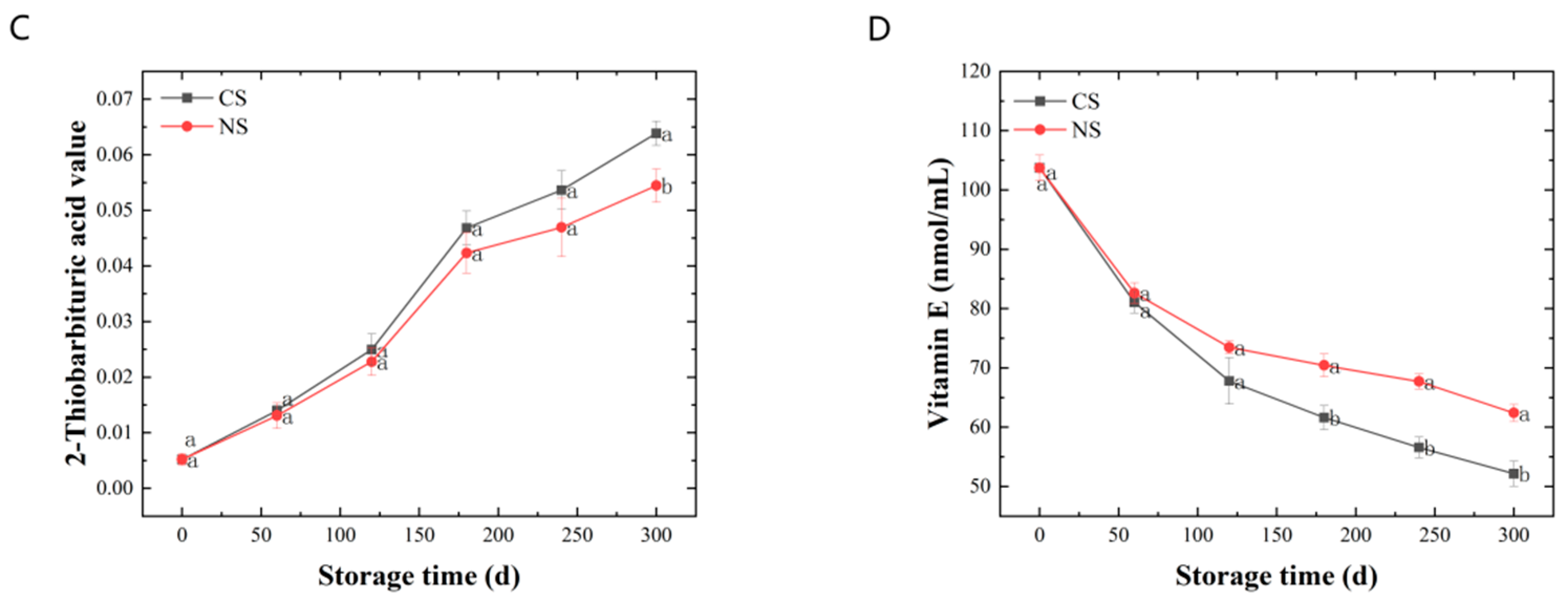
3.1. Quality Changes of Lipid Extracted from Peanuts during Storage
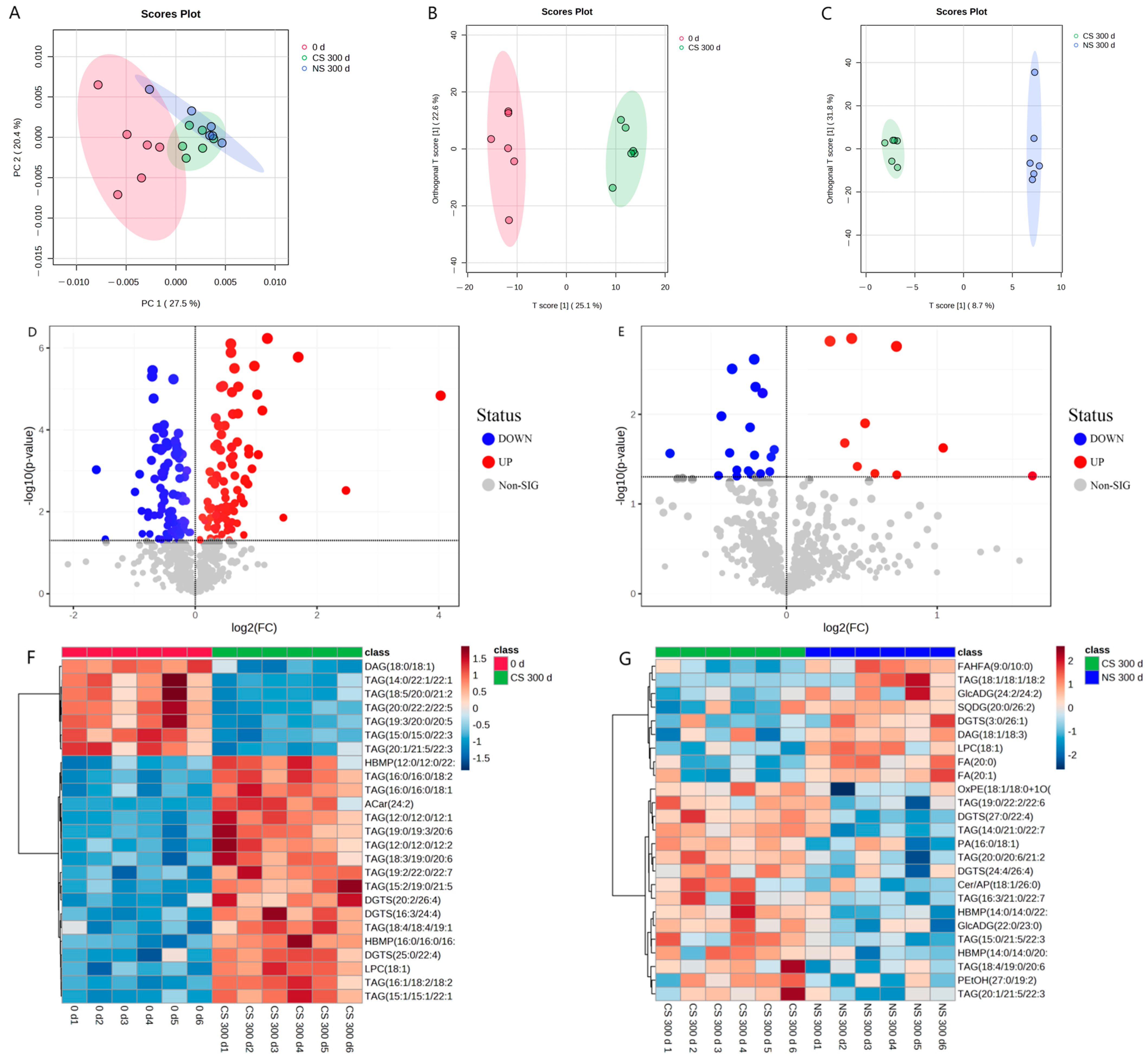
3.2. Overview of Lipidomic Profiles for Lipid Extracted from Peanuts
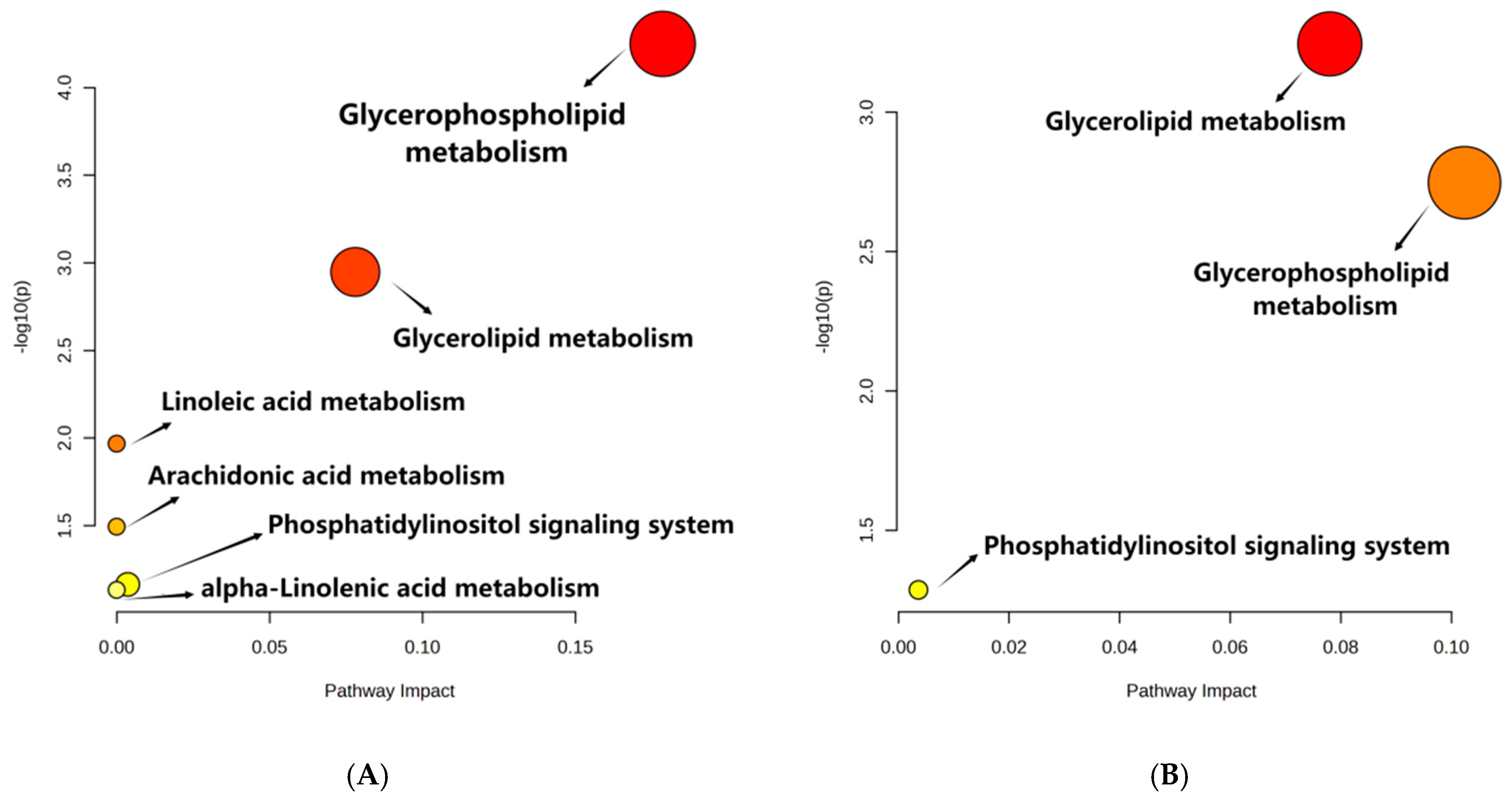
3.3. Metabolic Pathway Analysis
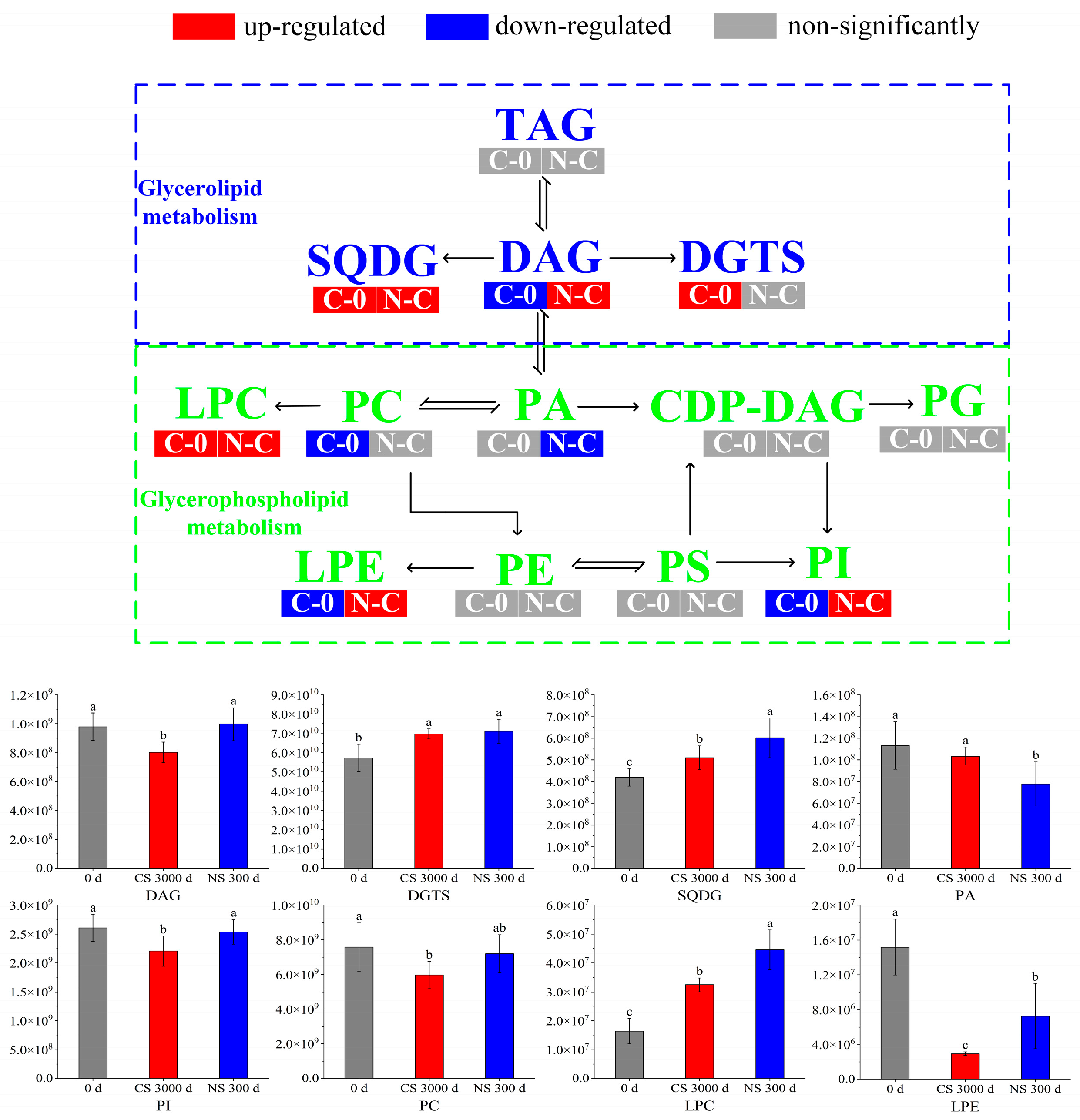
3.4. Glycerolipid Metabolism
3.5. Glycerophospholipid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, Y. Effect of lipoxygenase-3 on storage characteristics of peanut seeds. J. Stored Prod. Res. 2020, 87, 101589. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, R.; Xu, Y.; Zhong, K.; Bu, Q.; Gao, H. A comparison of lipid contents in different types of peanut cultivars using UPLC-Q-TOF-MS-Based lipidomic study. Foods 2021, 11, 4. [Google Scholar] [CrossRef]
- Opio, P.; Photchanachai, S. Modified atmosphere influences Aflatoxin B1 contamination and quality of peanut (Arachis hypogaea L.) Kernels Cv. Khon Kaen 84-8. J. Stored Prod. Res. 2018, 78, 67–73. [Google Scholar] [CrossRef]
- Wu, Q.; Li, C.; Zhang, D.; Tian, Q.; Tao, X.; Luo, Z.; Fu, X.; Zhang, Y. Nitrogen modified atmosphere packaging maintains the bioactive compounds and antioxidant capacity of postharvest fresh edible peanuts. Postharvest Biol. Technol. 2022, 190, 111957. [Google Scholar] [CrossRef]
- Martín, M.P.; Grosso, A.L.; Nepote, V.; Grosso, N.R. Sensory and chemical stabilities of high-oleic and normal-oleic peanuts in shell during long-term storage. J. Food Sci. 2018, 83, 2362–2368. [Google Scholar] [CrossRef]
- Gim, S.Y.; Jung, J.; Kwon, Y.; Kim, M.J.; Kim, G.; Lee, J. Effects of chitosan and collagen containing α-tocopherol on the oxidative stability in bulk oil and oil-in-water emulsion. Food Sci. Biotechnol. 2018, 27, 947–956. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in oak (Quercus sp.): Potential application to reduce oxidative rancidity in foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Chen, F. Effect of storage temperature on lipid oxidation and changes in nutrient contents in peanuts. Food Sci. Nutr. 2019, 7, 2280–2290. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Van Litsenburg, M.J.; Kodde, J.; Hall, R.D.; De Vos, R.C.H.; Mumm, R. Analyses of metabolic activity in peanuts under hermetic storage at different relative humidity levels. Food Chem. 2022, 373, 131020. [Google Scholar] [CrossRef]
- Darko, C.; Kumar Mallikarjunan, P.; Kaya-Celiker, H.; Frimpong, E.A.; Dizisi, K. Effects of packaging and pre-storage treatments on aflatoxin production in peanut storage under controlled conditions. J. Food Sci. Technol. 2018, 55, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Sabrina, S.; Gianpaola, P.; Antonio, M.; Miriam, H.; Giovanni, V. N2 controlled atmosphere reduces postharvest mycotoxins risk and pests attack on cereal grains. Phytoparasitica 2020, 48, 555–565. [Google Scholar] [CrossRef]
- Kumar, H.; Vijay, V.K.; Subbarao, P.M.V.; Chandra, R. Studies on the application of bio-carbon dioxide as controlled atmosphere on pest management in wheat grain storage. J. Stored Prod. Res. 2022, 95, 101911. [Google Scholar] [CrossRef]
- Sun, S.; Li, B.; Yang, T.; Luo, F.; Zhao, J.; Cao, J.; Lin, Q. Preservation mechanism of high concentration carbon dioxide controlled atmosphere for paddy rice storage based on quality analyses and molecular modeling tools. J. Cereal Sci. 2019, 85, 279–285. [Google Scholar] [CrossRef]
- Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pavlović, S.; Borković-Mitić, S.; Grigorov, I.; Stanković, S.; et al. The effects of a meldonium pre-treatment on the course of the faecal-induced sepsis in rats. Int. J. Mol. Sci. 2021, 22, 9698. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; Xing, R.; Yu, N.; Chen, Y. Integration of lipidomics and metabolomics for the authentication of camellia oil by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry coupled with chemometrics. Food Chem. 2022, 373, 131534. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.C.T.; Calingacion, M.; Garson, M.J.; Fitzgerald, M.A. Lipidomics reveals associations between rice quality traits. Metabolomics 2020, 16, 54. [Google Scholar] [CrossRef]
- Qu, C.; Li, Z.; Yang, Q.; Wang, X.; Wang, D. Effect of drying methods on peanut quality during storage. J. Oleo Sci. 2022, 71, 57–66. [Google Scholar] [CrossRef]
- Putrawan, I.D.G.A.; Indarto, A.; Octavia, Y. Thermal stabilization of polyvinyl chloride by calcium and zinc carboxylates derived from byproduct of palm oil refining. Heliyon 2022, 8, e10079. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Zhu, J.; Hu, G. Comparison of high-pressure, freeze-thaw cycles and germination-parboiling treatments on lipids digestibility and rancidity of brown rice. Sci. Rep. 2022, 12, 15667. [Google Scholar] [CrossRef]
- Qu, C.; Xia, Y.; Yang, Q.; Li, W.; Hu, M.; Lu, P. Novel insights into rice deterioration for nitrogen controlled atmosphere and re-aeration storage based on no-targeted metabolomics. LWT Food Sci. Technol. 2023, 178, 114631. [Google Scholar] [CrossRef]
- Sayyari, Z.; Farahmandfar, R. Stabilization of sunflower oil with pussy willow (Salix aegyptiaca) extract and essential oil. Food Sci. Nutr. 2017, 5, 266–272. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Bhardwaj, A.; Bhandari, K.; Kumar, S.; Prasad, P.V.; Jha, U.; Siddique, K.H.M.; Nayyar, H. Nitric oxide secures reproductive efficiency in heat-stressed lentil (Lens culinaris Medik.) plants by enhancing the photosynthetic ability to improve yield traits. Physiol. Mol. Biol. Plants 2021, 27, 2549–2566. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Wu, W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT—Food Sci. Technol. 2020, 120, 108943. [Google Scholar] [CrossRef]
- Szot, M.; Karpęcka-Gałka, E.; Dróżdż, R.; Frączek, B. Can nutrients and dietary supplements potentially improve cognitive performance also in esports? Healthcare 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, W.; Yang, Q.; Xia, Y.; Lu, P.; Hu, M. Metabolic mechanism of nitrogen modified atmosphere storage on delaying quality deterioration of rice grains. Food Chem. X 2022, 16, 100519. [Google Scholar] [CrossRef]
- Li, W.; Miyashita, A.; Sekimizu, K. Peanut triacylglycerols activate innate immunity both in insects and mammals. Sci. Rep. 2022, 12, 7464. [Google Scholar] [CrossRef]
- Zheng, P.; Shen, M.; Liu, R.; Cai, X.; Lin, J.; Wang, L.; Chen, Y.; Chen, G.; Cao, S.; Qin, Y. Revealing further insights into astringent seeds of Chinese fir by integrated metabolomic and lipidomic analyses. Int. J. Mol. Sci. 2023, 24, 15103. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhao, L.; Zhang, Y.; Fang, X. Lipidomics reveals the molecular mechanisms underlying the changes in lipid profiles and lipid oxidation in rape bee pollen dried by different methods. Food Res. Int. 2022, 162, 112104. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, S.; Wang, Q.; Shang, B.; Liu, J.; Xing, X.; Hong, Y.; Liu, H.; Duan, X.; Sun, H. Lipidomics and volatilomics reveal the changes in lipids and their volatile oxidative degradation products of brown rice during accelerated aging. Food Chem. 2023, 421, 136157. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, L.; Wang, W.; Wang, Q.; Liu, J.; Wang, Y.; Liu, H.; Shang, B.; Duan, X.; Sun, H. Lipidomics reveals the changes in non-starch and starch lipids of rice (Oryza sativa L.) during storage. J. Food Compos. Anal. 2022, 105, 104205. [Google Scholar] [CrossRef]
- Oishi, Y.; Otaki, R.; Iijima, Y.; Kumagai, E.; Aoki, M.; Tsuzuki, M.; Fujiwara, S.; Sato, N. Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, chlorella kessleri. Commun. Biol. 2022, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Iqbal, S.; Wen, F.; Tong, M.; Liu, J. Phosphorus-induced lipid class alteration revealed by lipidomic and transcriptomic profiling in oleaginous microalga nannochloropsis sp. PJ12. Mar. Drugs 2019, 17, 519. [Google Scholar] [CrossRef]
- Körber, T.T.; Frantz, N.; Sitz, T.; Abdalla, M.A.; Mühling, K.H.; Rohn, S. Alterations of content and composition of individual sulfolipids, and change of fatty acids profile of galactolipids in lettuce plants (Lactuca sativa L.) grown under sulfur nutrition. Plants 2022, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Du, A.; Fan, Z.; Shi, L. Novel top-down high-resolution mass spectrometry-based metabolomics and lipidomics reveal molecular change mechanism in A2 milk after CSN2 gene mutation. Food Chem. 2022, 391, 133270. [Google Scholar] [CrossRef]
- Guo, A.; Yang, Y.; Wu, J.; Qin, N.; Hou, F.; Gao, Y.; Li, K.; Xing, G.; Li, S. Lipidomic and transcriptomic profiles of glycerophospholipid metabolism during hemerocallis citrina baroni flowering. BMC Plant Biol. 2023, 23, 50. [Google Scholar] [CrossRef]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef]
- Zarza, X.; Shabala, L.; Fujita, M.; Shabala, S.; Haring, M.A.; Tiburcio, A.F.; Munnik, T. Extracellular spermine triggers a rapid intracellular phosphatidic acid response in arabidopsis, involving PLDδ activation and stimulating ion flux. Front. Plant Sci. 2019, 10, 601. [Google Scholar] [CrossRef]
- Jia, Y.; Tao, F.; Li, W. Lipid profiling demonstrates that suppressing arabidopsis phospholipase Dδ retards ABA-promoted leaf senescence by attenuating lipid degradation. PLoS ONE 2013, 8, e65687. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, H.; Yin, G.; Zhou, Y.; Lu, X.; Xin, X. Dynamic changes in membrane lipid metabolism and antioxidant defense during soybean (Glycine max L. Merr.) seed aging. Front. Plant Sci. 2022, 13, 908949. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Zhang, B.; Yu, W.; Xiao, Y.; Peng, F. Lipid metabolomic and transcriptomic analyses reveal that phosphatidylcholine enhanced the resistance of peach seedlings to salt stress through phosphatidic acid. J. Agric. Food Chem. 2023, 71, 8846–8858. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Nachiappan, V. Benzoquinone alters the lipid homeostasis in saccharomyces cerevisiae. Toxicol. Res. 2019, 8, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Lung, S.C.; Li, X.; Li, X.D.; Chye, M.L. Thermodynamic insights into an interaction between ACYL-CoA–BINDING PROTEIN2 and LYSOPHOSPHOLIPASE2 in arabidopsis. J. Biol. Chem. 2019, 294, 6214–6226. [Google Scholar] [CrossRef]
- Cesari, A.B.; Paulucci, N.S.; Biasutti, M.A.; Morales, G.M.; Dardanelli, M.S. Changes in the lipid composition of bradyrhizobium cell envelope reveal a rapid response to water deficit involving lysophosphatidylethanolamine synthesis from phosphatidylethanolamine in outer membrane. Res. Microbiol. 2018, 169, 303–312. [Google Scholar] [CrossRef]
- Jung, J.; Lee, Y.P.; Bae, S.W.; Ahn, G.H.; Ryu, S.B. Lysophosphatidylethanolamine delays fruit softening of persimmon (Diospyros kaki). Hortic. Environ. Biotechnol. 2019, 60, 491–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, W.; Zhang, H.; Lu, P.; Chen, P.; Chen, L.; Qu, C. Influence of Nitrogen-Modified Atmosphere Storage on Lipid Oxidation of Peanuts: From a Lipidomic Perspective. Foods 2024, 13, 277. https://doi.org/10.3390/foods13020277
Ma X, Li W, Zhang H, Lu P, Chen P, Chen L, Qu C. Influence of Nitrogen-Modified Atmosphere Storage on Lipid Oxidation of Peanuts: From a Lipidomic Perspective. Foods. 2024; 13(2):277. https://doi.org/10.3390/foods13020277
Chicago/Turabian StyleMa, Xia, Wenhao Li, Huayang Zhang, Peng Lu, Pengxiao Chen, Liang Chen, and Chenling Qu. 2024. "Influence of Nitrogen-Modified Atmosphere Storage on Lipid Oxidation of Peanuts: From a Lipidomic Perspective" Foods 13, no. 2: 277. https://doi.org/10.3390/foods13020277
APA StyleMa, X., Li, W., Zhang, H., Lu, P., Chen, P., Chen, L., & Qu, C. (2024). Influence of Nitrogen-Modified Atmosphere Storage on Lipid Oxidation of Peanuts: From a Lipidomic Perspective. Foods, 13(2), 277. https://doi.org/10.3390/foods13020277




