Metabolomics Reveals the Response Mechanisms of Potato Tubers to Light Exposure and Wounding during Storage and Cooking Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Botanical Materials and Scientific Methodology
2.3. Sample Preparation and Extraction for Widely Targeted Metabolomics
2.4. Preparation and Extraction of Anthocyanin, Flavonoid, Carotenoid, and SGA
2.5. UPLC-MS/MS Parameters
2.6. Data on Metabolite Analysis
3. Results
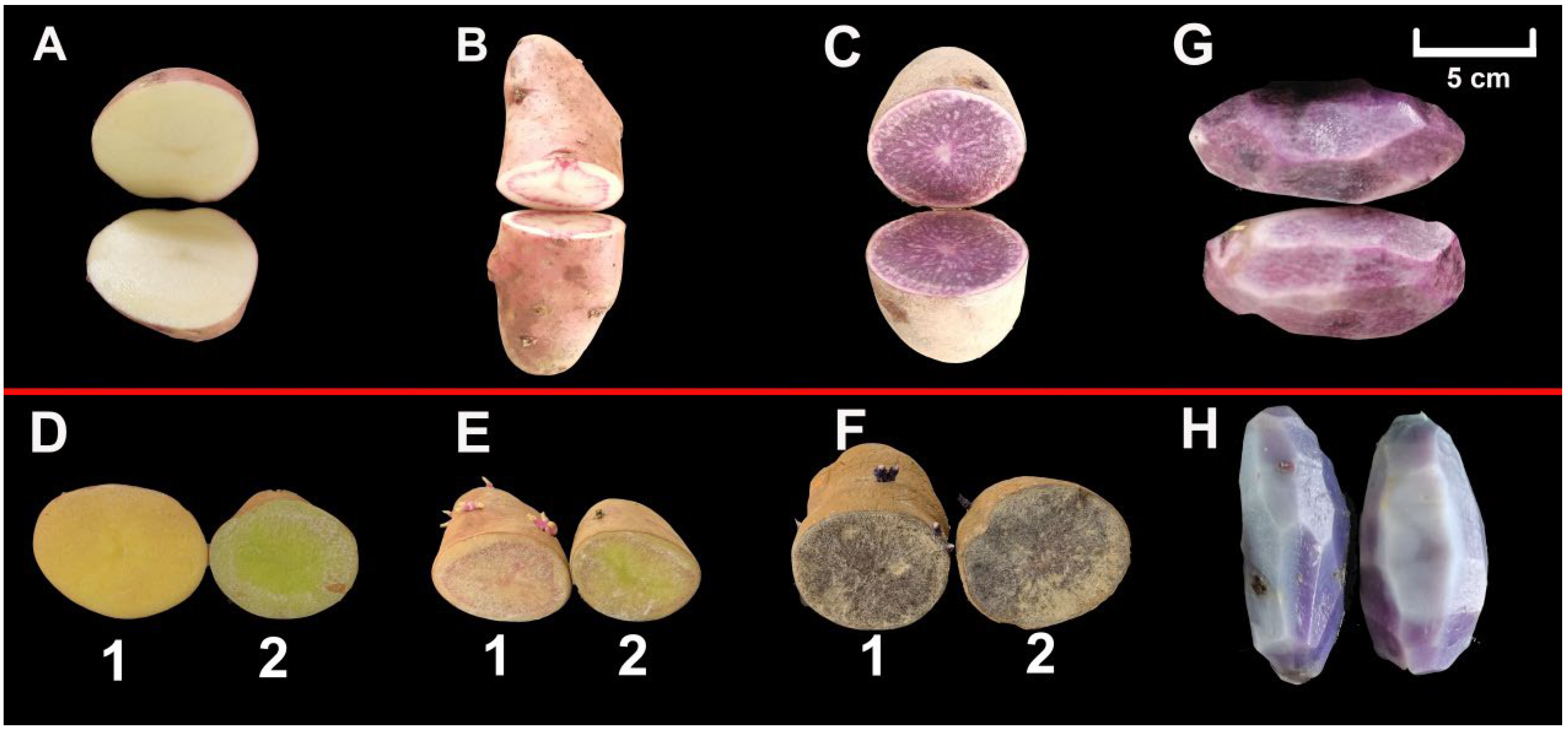
3.1. Morphology of Potato Tuber
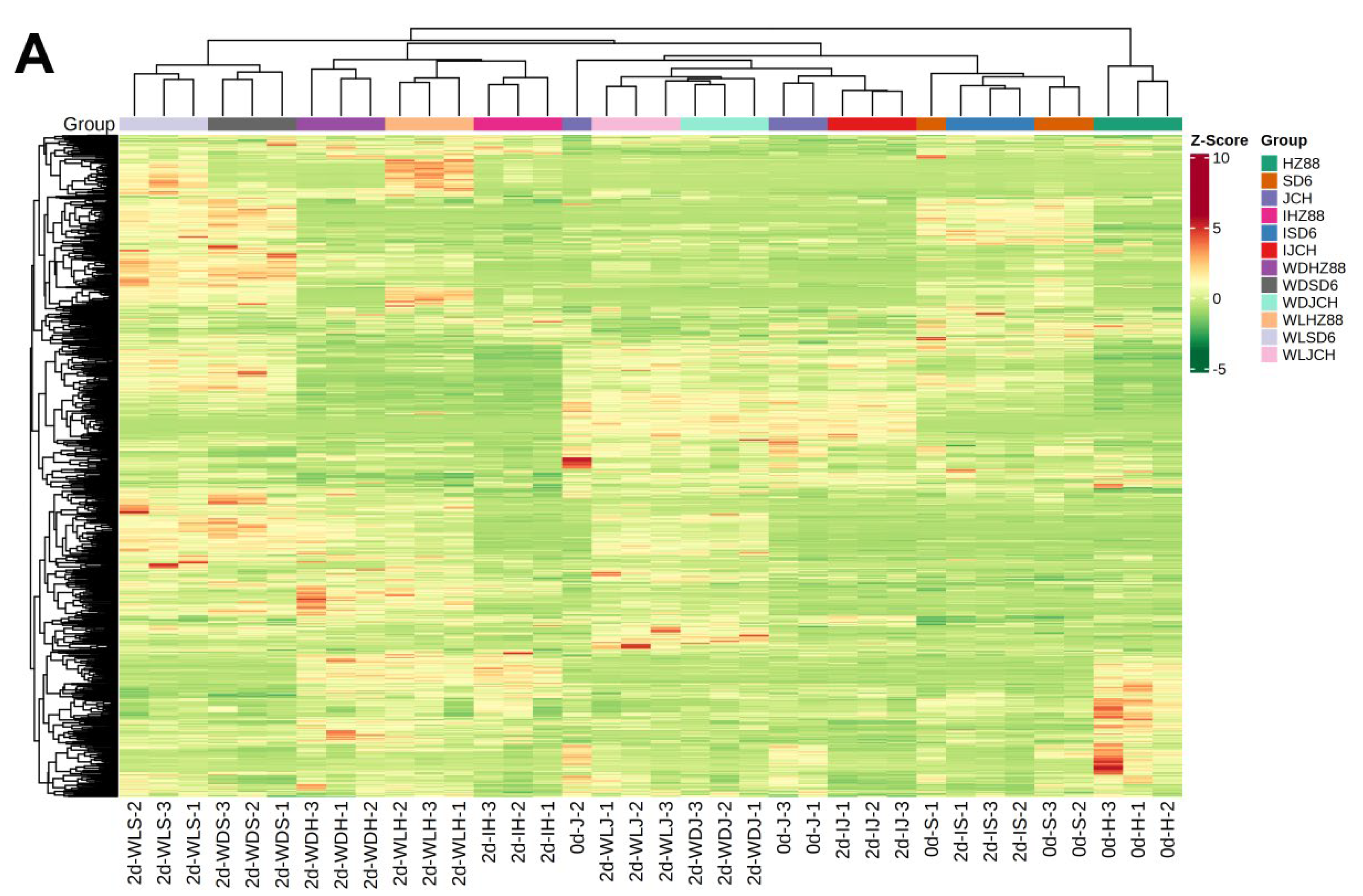
3.2. Metabolic Profiling in Potato Tubers
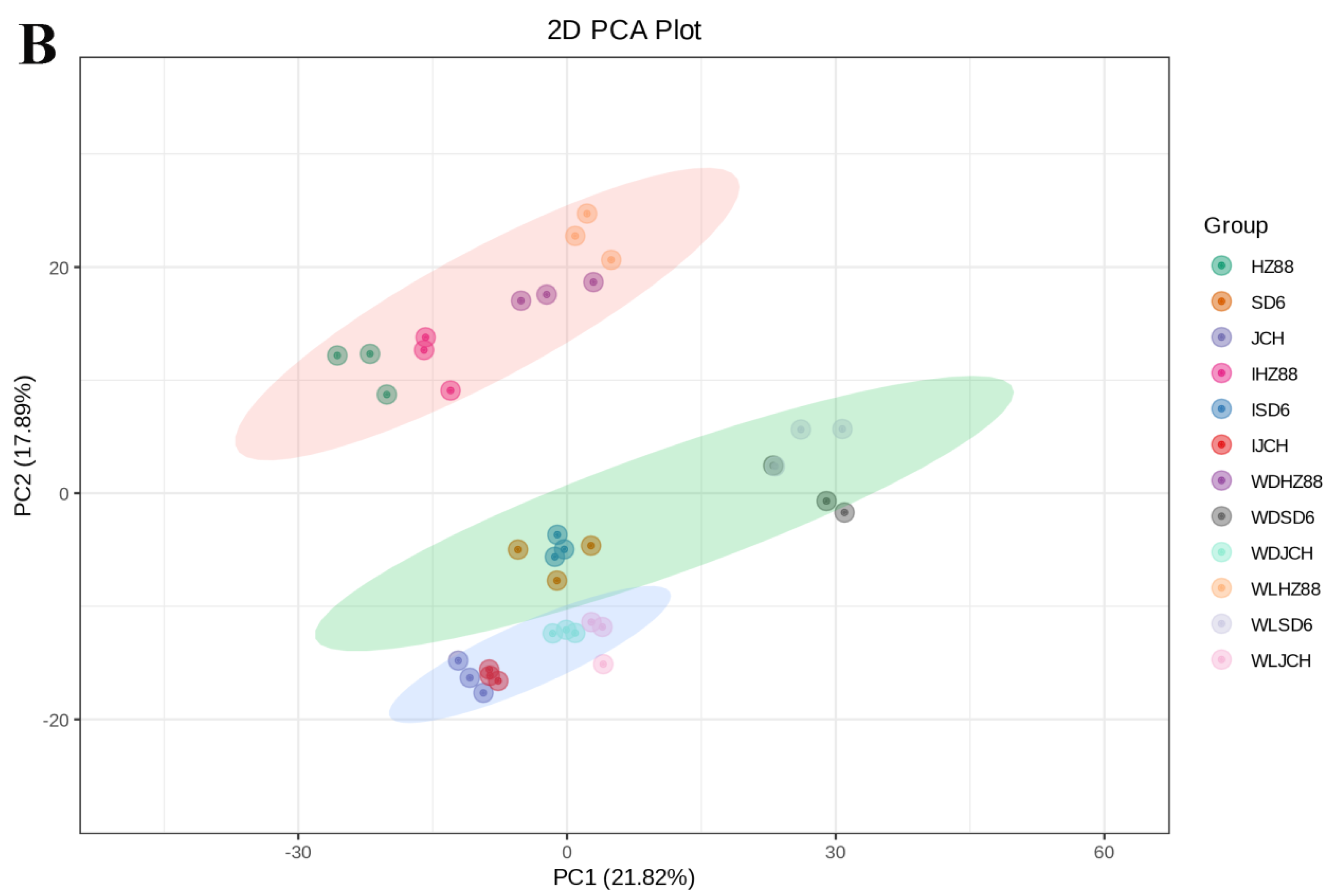
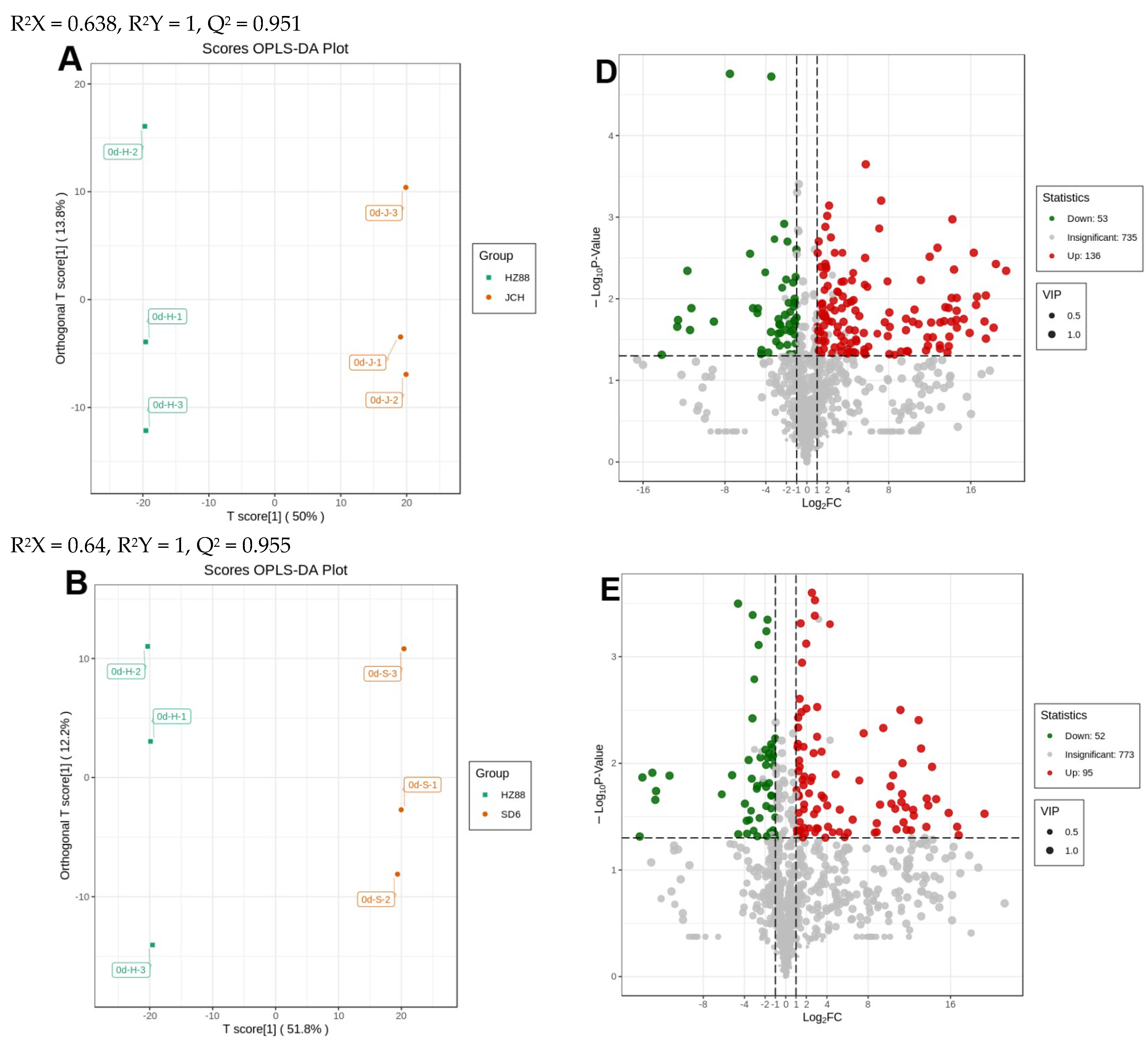
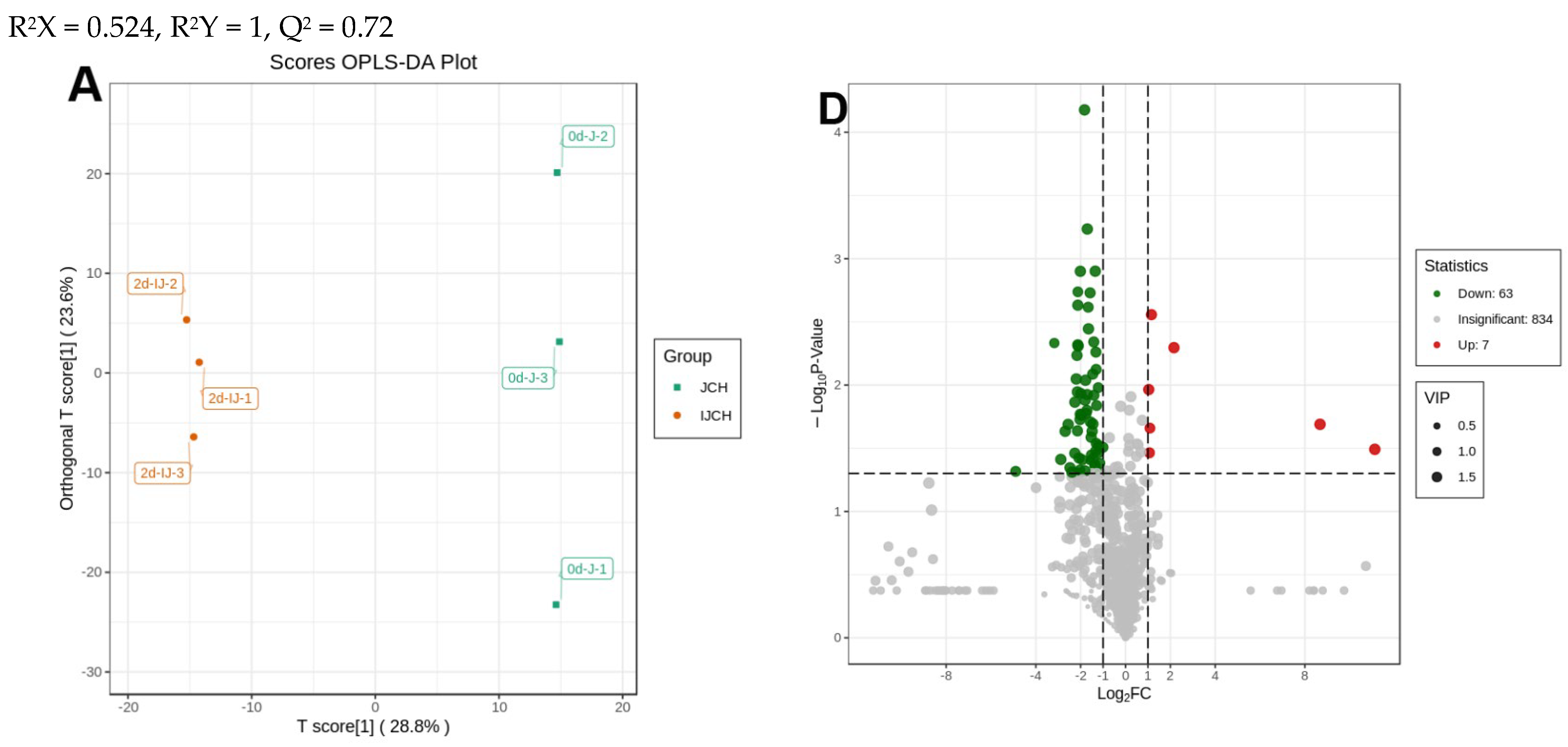
3.3. Distinguishable Features among the Various Metabolite Profiles
3.4. Alkaloid Metabolic Profiling in Potato Tubers
3.5. The Effect of Cooking Process on Anthocyanin, Carotenoid, and Flavonoid Contents
4. Discussion
4.1. Metabolites Identified in Potato Tubers
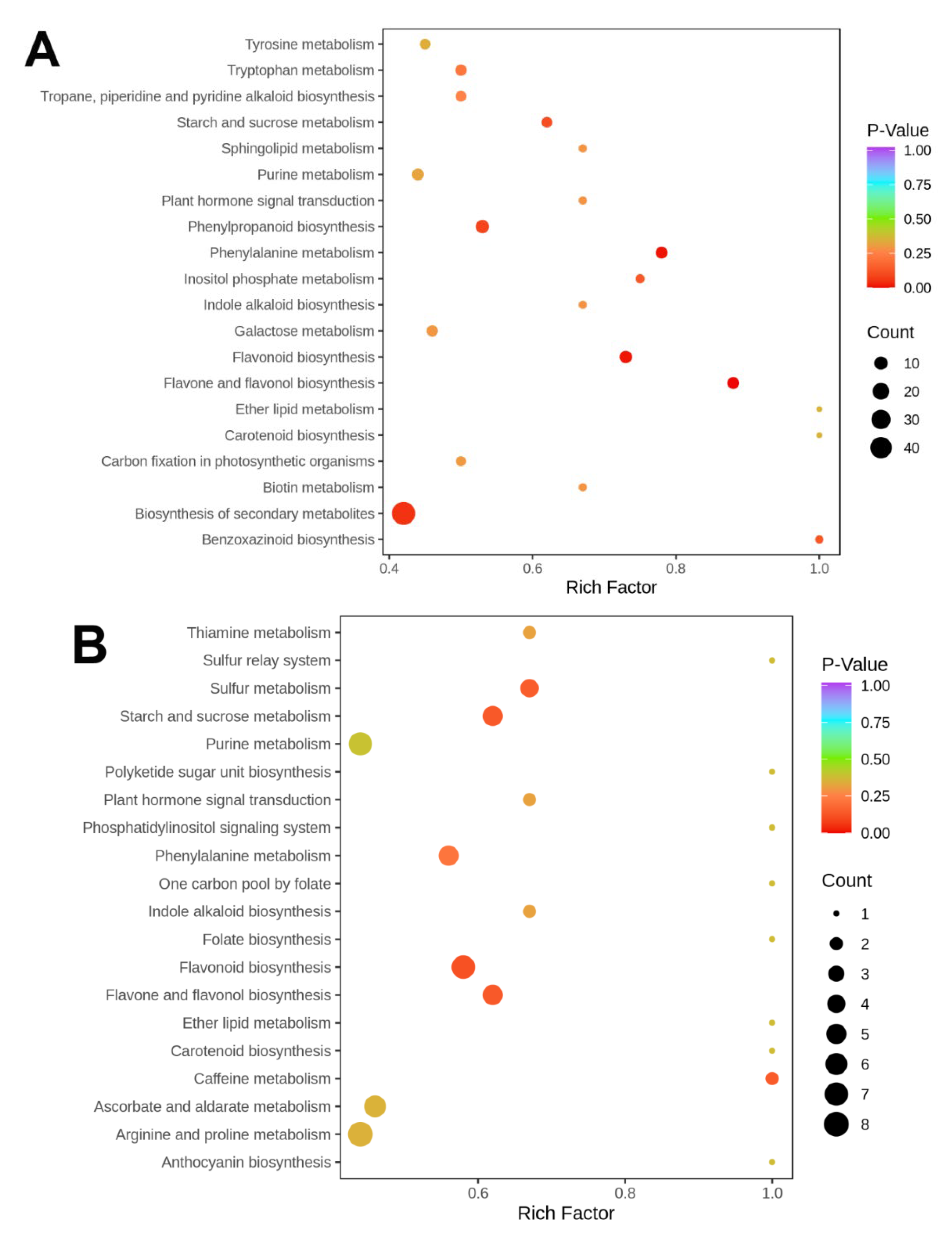
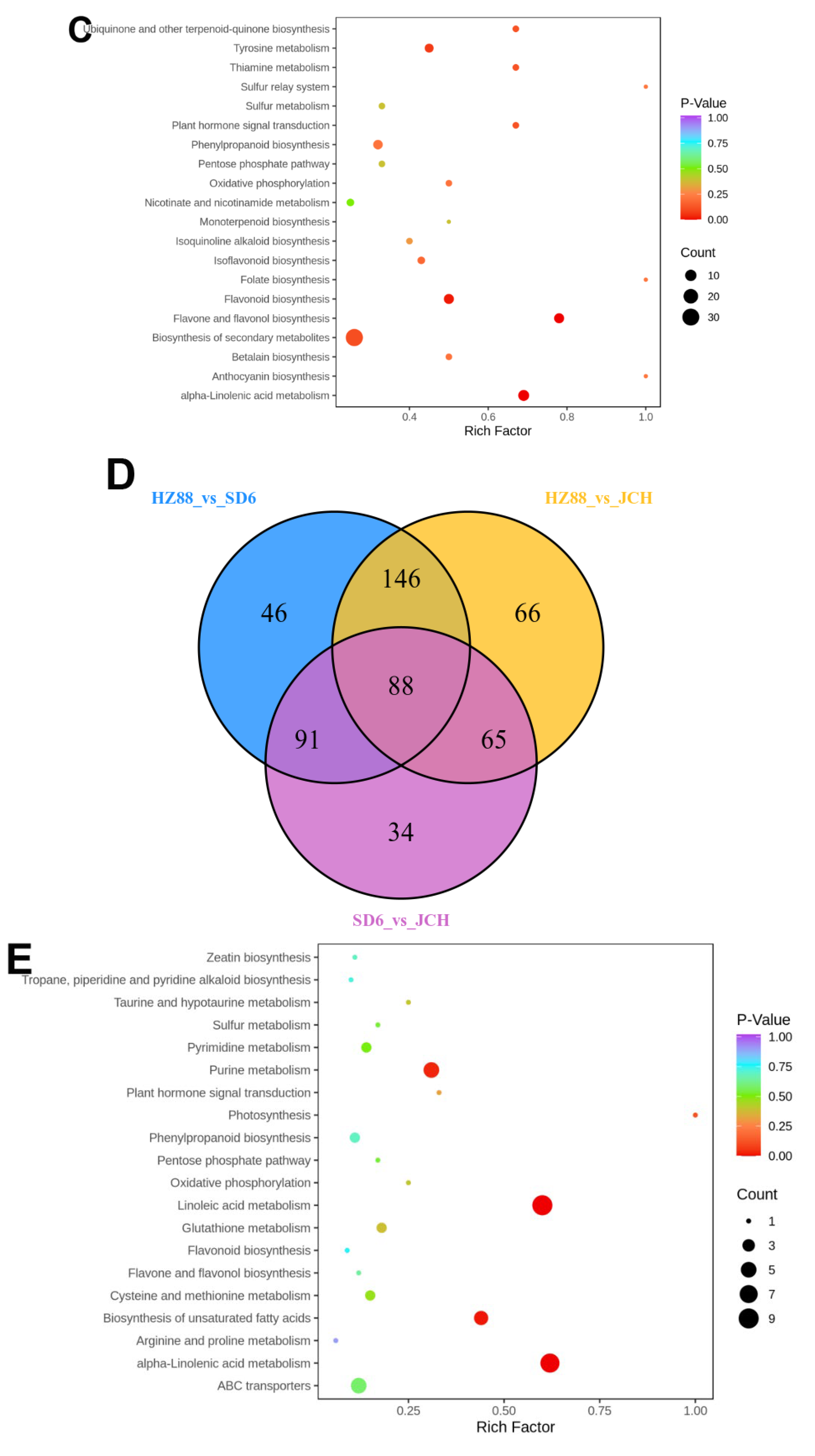
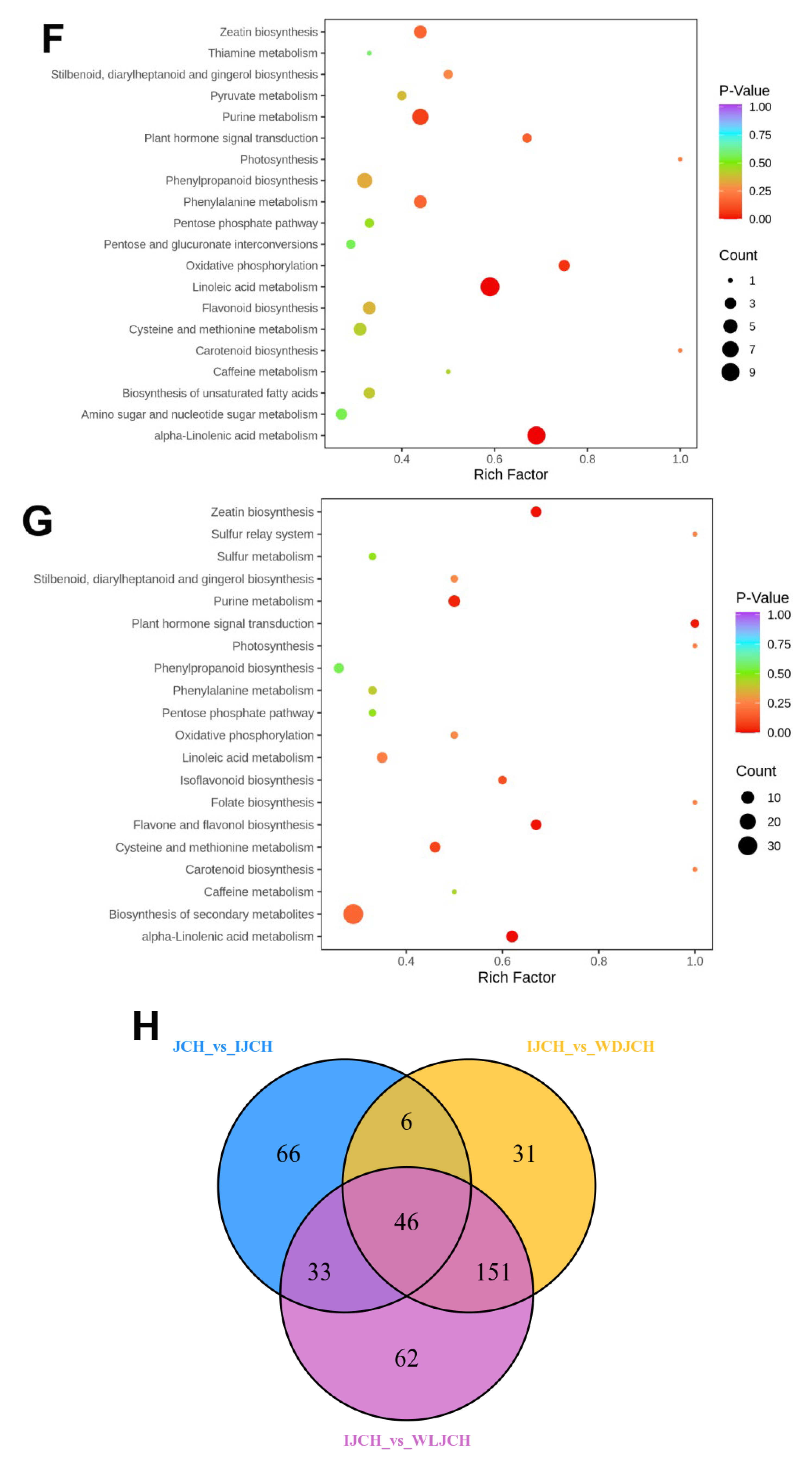
4.2. Metabolite Variations within Cultivars and Treatment Approaches
4.3. Alkaloid Metabolites
4.4. The Effect of Cooking on Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burlingame, B.; Mouillé, B.; Charrondière, R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- Brown, C.R.; Culley, D.; Pacifi, B.; Yang, C.P.; Wrolstad, R. Variation of Anthocyanin and Carotenoid Contents and Associated Antioxidant Values in Potato Breeding Lines. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 2005, 130, 174–180. [Google Scholar] [CrossRef]
- Yl, A.; Jl, B.; Xz, B.; Han, G.C.; Yl, D.; Kp, E.; Sz, A.; Gz, B. Genome-wide Analysis of MYB Gene Family in Potato Provides Insights into Tissue-specific Regulation of Anthocyanin Biosynthesis. Hortic. Plant J. 2021, 7, 129–141. [Google Scholar] [CrossRef]
- Friedman, M.; Dao, L. Distribution of glycoalkaloids in potato plants and commercial potato products. J. Agric. Food Chem. 1992, 40, 419–423. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- González Moreno, A.; de Cózar, A.; Prieto, P.; Domínguez, E.; Heredia, A. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nat. Commun. 2022, 13, 1786. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Murawska, Z.; Strzelczyk-Żyta, D.; Wasilewicz-Flis, I.; Marczewski, W. Phytotoxic potential of cultivated and wild potato species (Solanum sp.): Role of glycoalkaloids, phenolics and flavonoids in phytotoxicity against mustard (Sinapis alba L.). Acta Physiol. Plant. 2019, 41, 55. [Google Scholar] [CrossRef]
- Lulai, E.C. Skin-set, wound healing, and related defects. In Potato Biology and Biotechnology; Elsevier Science BV: Amsterdam, The Netherlands, 2007; pp. 471–500. [Google Scholar] [CrossRef]
- Nie, X.; Li, C.; Zhang, G.; Shao, Z.; Wang, X.; Shi, H.; Guo, H. Light exposure and wounding: Synergistic effects on steroidal glycoalkaloid accumulation in potato tubers during storage. Int. J. Food Sci. Technol. 2019, 54, 2939–2948. [Google Scholar] [CrossRef]
- Yda, B.; Mh, A.; Feng, F.A.; Xf, B.; Yuan, Z.C.; Feng, Z.A. The distribution and changes of glycoalkaloids in potato tubers under different storage time based on MALDI-TOF mass spectrometry imaging. Talanta 2020, 221, 121453. [Google Scholar] [CrossRef]
- Shen, D.-D.; Hua, Y.-P.; Huang, J.-Y.; Yu, S.-T.; Wu, T.-B.; Zhang, Y.; Chen, H.-L.; Yue, C.-P. Multiomic Analysis Reveals Core Regulatory Mechanisms underlying Steroidal Glycoalkaloid Metabolism in Potato Tubers. J. Agric. Food Chem. 2021, 70, 415–426. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Holm, D.G.; Broeckling, C.D.; Prenni, J.E.; Heuberger, A.L. Metabolomics and Ionomics of Potato Tuber Reveals an Influence of Cultivar and Market Class on Human Nutrients and Bioactive Compounds. Front. Nutr. 2018, 5, 36. [Google Scholar] [CrossRef]
- Nie, X.; Guo, H. An ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry method for the detection of steroidal glycoalkaloids in potato samples. Anal. Methods 2017, 9, 6613–6621. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.; White, J.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Booksx, I.; SAS Institute. SASSTAT 9.22 User’s Guide: The GLIMMIX Procedure (Book Excerpt); SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Hu, L. Implement Scientific Research Design and Statistical Analysis Correctly; The People’s Military Medical Press: Beijing, China, 2011. [Google Scholar]
- Franco, D.; Pateiro, M.; Amado, I.R.; López Pedrouso, M.; Zapata, C.; Vázquez, J.A.; Lorenzo, J.M. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 1891–1902. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Wei, L. Naringenin as a Potential Immunomodulator in Therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Millet grain phenolics and their role in disease risk reduction and health promotion: A review. J. Funct. Foods 2013, 5, 570–581. [Google Scholar] [CrossRef]
- Hassanien, M. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010, 123, 1019–1026. [Google Scholar] [CrossRef]
- Petersson, E.V.; Arif, U.; Schulzova, V.; Krtková, V.; HajLová, J.; Meijer, J.; Andersson, H.C.; Jonsson, L.; Sitbon, F. Glycoalkaloid and calystegine levels in table potato cultivars subjected to wounding, light, and heat treatments. J. Agric. Food Chem. 2013, 61, 5893–5902. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Westerberg, E.; Arif, U.; Huchelmann, A.; Guasca, A.O.; Beste, L.; Dalman, K.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Transcript profiling of two potato cultivars during glycoalkaloid-inducing treatments shows differential expression of genes in sterol and glycoalkaloid metabolism. Sci. Rep. 2017, 7, 43268. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Xu, J.; Yi, C. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef] [PubMed]
- Galliard, T.; Matthew, J.A. Lipids of potato tubers. II. Lipid-degrading enzymes in different varieties of potato tuber. J. Sci. Food Agric. 1973, 24, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wahrenburg, Z.; Benesch, E.; Lowe, C.; Jimenez, J.; Vulavala, V.; Lü, S.; Hammerschmidt, R.; Douches, D.; Yim, W.; Santos, P.; et al. Transcriptional regulation of wound suberin deposition in potato cultivars with differential wound healing capacity. Plant J. Cell Mol. Biol. 2021, 107, 77–99. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G. Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine. Phytochemistry 1993, 33, 323–328. [Google Scholar] [CrossRef]
- Ginzberg, I.; Tokuhisa, J.G.; Veilleux, R.E. Potato Steroidal Glycoalkaloids: Biosynthesis and Genetic Manipulation. Potato Res. 2009, 52, 1–15. [Google Scholar] [CrossRef]
- Friedman, M.; Mcdonald, G.M.; Filadelfikeszi, M.A. Potato Glycoalkaloids: Chemistry, Analysis, Safety, and Plant Physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Sucha, L.; Tomsik, P. The Steroidal Glycoalkaloids from Solanaceae: Toxic Effect, Antitumour Activity and Mechanism of Action. Planta Medica 2016, 82, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Krasowski, M.D.; McGehee, D.S.; Moss, J. Natural inhibitors of cholinesterases: Implications for adverse drug reactions. Can. J. Anaesth. 1997, 44, 525–534. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Zhang, Q.; Zhang, J.; Sun, X. α-Chaconine Affects the Apoptosis, Mechanical Barrier Function, and Antioxidant Ability of Mouse Small Intestinal Epithelial Cells. Front. Plant Sci. 2021, 12, 673774. [Google Scholar] [CrossRef] [PubMed]
- D’Amelia, V.; Sarais, G.; Fais, G.; Dessì, D.; Giannini, V.; Garramone, R.; Carputo, D.; Melito, S. Biochemical Characterization and Effects of Cooking Methods on Main Phytochemicals of Red and Purple Potato Tubers, a Natural Functional Food. Foods 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yang, S.; Sun, J.; Chen, G.; Wen, Y.; Yang, J.; Nie, X.; Liu, C. Metabolomics Reveals the Response Mechanisms of Potato Tubers to Light Exposure and Wounding during Storage and Cooking Processes. Foods 2024, 13, 308. https://doi.org/10.3390/foods13020308
Wang X, Yang S, Sun J, Chen G, Wen Y, Yang J, Nie X, Liu C. Metabolomics Reveals the Response Mechanisms of Potato Tubers to Light Exposure and Wounding during Storage and Cooking Processes. Foods. 2024; 13(2):308. https://doi.org/10.3390/foods13020308
Chicago/Turabian StyleWang, Xin, Shuiyan Yang, Jinghan Sun, Guoyan Chen, Yunman Wen, Jin Yang, Xuheng Nie, and Chao Liu. 2024. "Metabolomics Reveals the Response Mechanisms of Potato Tubers to Light Exposure and Wounding during Storage and Cooking Processes" Foods 13, no. 2: 308. https://doi.org/10.3390/foods13020308
APA StyleWang, X., Yang, S., Sun, J., Chen, G., Wen, Y., Yang, J., Nie, X., & Liu, C. (2024). Metabolomics Reveals the Response Mechanisms of Potato Tubers to Light Exposure and Wounding during Storage and Cooking Processes. Foods, 13(2), 308. https://doi.org/10.3390/foods13020308





