Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Equipment
2.2. Synthesis of Hapten and Antigen
2.2.1. Synthesis of Hapten AFB1-CMO
2.2.2. Synthesis of Antigen
2.3. Synthesis of Monoclonal Antibody
2.4. Establishment of the ic-ELISA
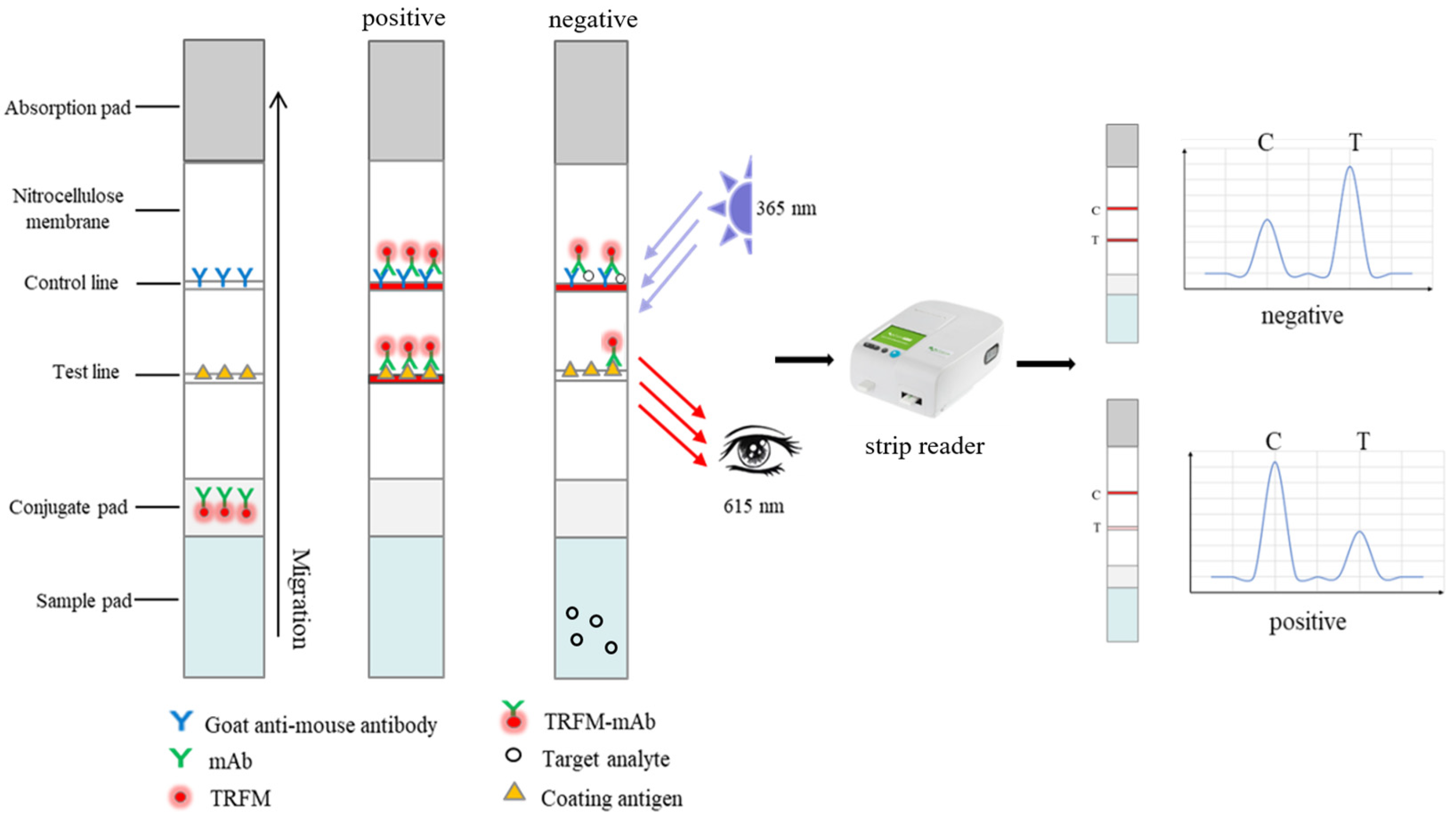
2.5. Preparation of TRFICA and Procedure
2.6. Validation of the ic-ELISA and Time-Resolved Fluorescent Immunochromatographic Assay (TRFICA)
3. Results and Discussion
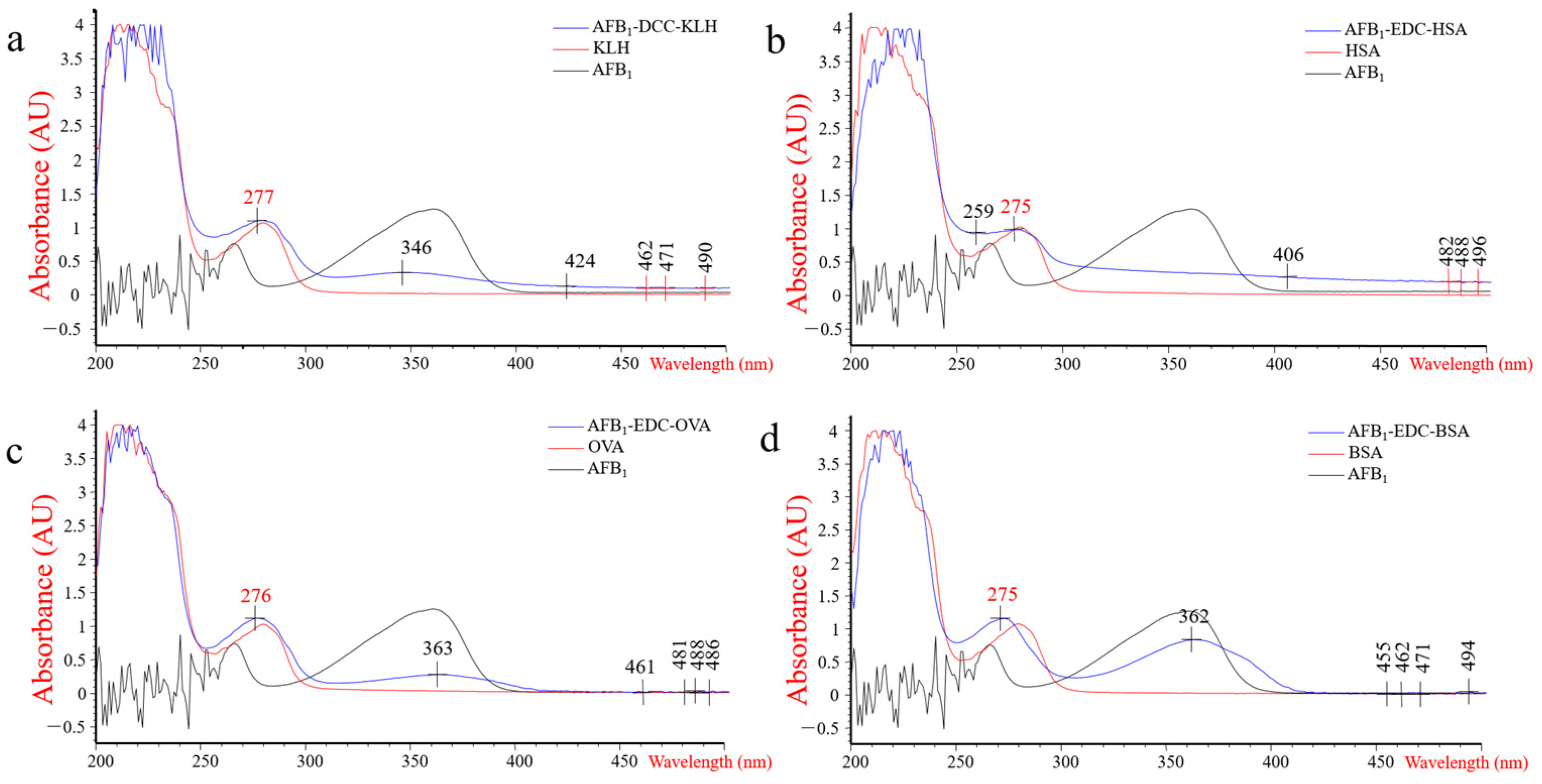
3.1. Characterization of Haptens and Antigens
3.2. Identification of Antiserum and Monoclonal Antibody
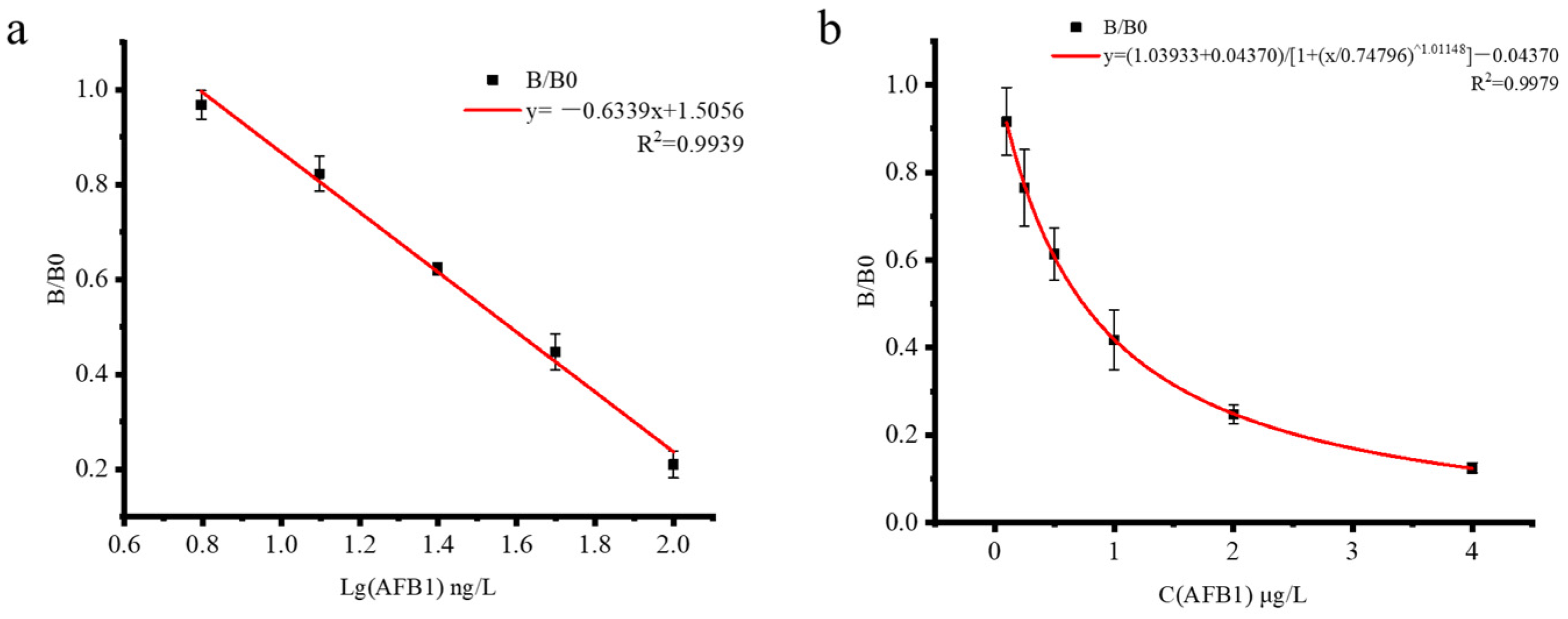
3.3. ic-ELISA Performance Test
3.4. Time-Resolved Fluorescent Immunochromatographic Assay (TRFICA)
3.5. Method Validation
3.6. Comparison of Published Detection Methods for Major AFB1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFTs | aflatoxins | DMF | N′N-Dimethylformamide |
| AFB1 | aflatoxin B1 | BSA | bovine serum albumin |
| AFB2 | aflatoxin B2 | DCC | N′N-dicyclohexylcarbodiimide |
| AFG1 | aflatoxin G1 | KLH | keyhole limpet |
| AFG2 | aflatoxin G2 | EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| mAb | monoclonal antibody | DMSO | dimethyl sulfoxide |
| ic-ELISA | indirect competitive enzyme-linked immunosorbent assay | TRFICA | time-resolved fluorescence immunoassay assay |
| OVA | ovalbumin | CMO | O-carboxymethoxylamine hemihydrochloride |
| IC50 | half-maximal inhibitory concentration | HRP-IgG | Horseradish peroxidase-labeled goat anti-mouse immunoglobulin G |
| LOD | limit of detection | HAT | hypoxanthine aminopterin thymidine |
| LOQ | limit of quantitation | TMB | 3,3,5,5-tetramethylbenzidine |
| LFIA | lateral flow immunoassay | PVP | polyvinyl pyrrolidone |
| TRFMs | time-resolved fluorescent microspheres | HT | hypoxanthine thymidine |
| GNP-SA | gold nanoparticle-based strip assay | HSA | albumin human serum |
| CR | cross-reactivity | PEG | polyethylene glycol |
| T line | detection line | ZEN | zearalenone |
| C line | control line | OTA | ochratoxin A |
| CV | coefficient of variation | T-2 | T-2 toxin |
| SD | standard deviation | DON | Deoxynivalenol |
| R2 | correlation coefficient | T/C | the ratio of T to C lines |
References
- Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Available online: https://www.tandfonline.com/doi/epdf/10.1080/10408398.2019.1658570?needAccess=true (accessed on 4 January 2024).
- Altyar, A.E.; Kensara, O.A.; Sayed, A.A.; Aleya, L.; Almutairi, M.H.; Zaazouee, M.S.; Elshanbary, A.A.; El-Demerdash, F.M.; Abdel-Daim, M.M. Acute Aflatoxin B1-Induced Hepatic and Cardiac Oxidative Damage in Rats: Ameliorative Effects of Morin. Heliyon 2023, 9, e21837. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Abdallah, F.M.; Chen, X.; Rajkovic, A. Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro. Toxins 2023, 15, 653. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Abdallah, M.F.; Landschoot, S.; Audenaert, K.; De Saeger, S.; Chen, X.; Rajkovic, A. Aspergillus Flavus and Fusarium Verticillioides and Their Main Mycotoxins: Global Distribution and Scenarios of Interactions in Maize. Toxins 2023, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Sineque, A.; Macuamule, C.; Dos Anjos, F. Aflatoxin B1 Contamination in Chicken Livers and Gizzards from Industrial and Small Abattoirs, Measured by ELISA Technique in Maputo, Mozambique. Int. J. Environ. Res. Public Health 2017, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, C.; Dai, Y.; Chu, L.; Geng, S.; Zheng, S.; Kang, X. Determination of Aflatoxin B1 by Novel Nanofiber-Packed Solid-Phase Extraction Coupled with a High Performance Liquid Chromatography-Fluorescence Detector. Anal. Methods 2023, 15, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Junsai, T.; Poapolathep, S.; Sutjarit, S.; Giorgi, M.; Zhang, Z.; Logrieco, A.F.; Li, P.; Poapolathep, A. Determination of Multiple Mycotoxins and Their Natural Occurrence in Edible Vegetable Oils Using Liquid Chromatography–Tandem Mass Spectrometry. Foods 2021, 10, 2795. [Google Scholar] [CrossRef]
- Kavanagh, O.; Elliott, C.T.; Campbell, K. Progress in the Development of Immunoanalytical Methods Incorporating Recombinant Antibodies to Small Molecular Weight Biotoxins. Anal. Bioanal. Chem. 2015, 407, 2749–2770. [Google Scholar] [CrossRef]
- Wu, M.; Xia, J.; Liu, T.; Xue, G.; Fang, X.; Lai, W.; Peng, J. Simultaneous Detection of OTA and AFB1 in Cereals Based on Bispecific Monoclonal Antibody Using Quantum Dot Nanobead Lateral Flow Immunoassay. Food Agric. Immunol. 2023, 34, 48–66. [Google Scholar] [CrossRef]
- Ren, Y.; Tian, R.; Wang, T.; Cao, J.; Li, J.; Deng, A. An Extremely Highly Sensitive ELISA in Pg mL−1 Level Based on a Newly Produced Monoclonal Antibody for the Detection of Ochratoxin A in Food Samples. Molecules 2023, 28, 5743. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Tang, X.; Zhang, W.; Zhang, Q.; Li, P. Time-Resolved Fluorescence Immunochromatography Assay (TRFICA) for Aflatoxin: Aiming at Increasing Strip Method Sensitivity. Front. Microbiol. 2020, 11, 676. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Wang, D.; Zhang, Q.; Li, P.; Ding, X. Rapid On-Site Sensing Aflatoxin B1 in Food and Feed via a Chromatographic Time-Resolved Fluoroimmunoassay. PLoS ONE 2015, 10, e0123266. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, P.; Zhang, Q.; Zhang, Z.; Zhang, W.; Jiang, J. Time-Resolved Fluorescence Immunochromatographic Assay Developed Using Two Idiotypic Nanobodies for Rapid, Quantitative, and Simultaneous Detection of Aflatoxin and Zearalenone in Maize and Its Products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Yang, B.; Pan, Y.; Wang, Y.; Chen, D.; Liu, Z.; Yang, W.; Tao, Y.; Yuan, Z. Development and Validation of a Sensitive Monoclonal Antibody-Based Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Determination of the Aflatoxin M1 Levels in Milk. Toxicon 2016, 113, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Sani, A.; Khezri, M.; Moradnia, H. Determination of Aflatoxin M1 in Milk by ELISA Technique in Mashad (Northeast of Iran). ISRN Toxicol. 2012, 2012, 121926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Peng, D.; Wang, Y.; Pan, Y.; Wan, D.; Zhang, X.; Yuan, Z. A Novel Hapten and Monoclonal-Based Enzyme-Linked Immunosorbent Assay for Sulfonamides in Edible Animal Tissues. Food Chem. 2014, 154, 52–62. [Google Scholar] [CrossRef] [PubMed]
- China. Hygienical Standard for Feeds. The General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, the Standardization Administration of China. 2017. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=9E5467EA1922E8342AF5F180319F34A0 (accessed on 15 December 2023).
- Biancardi, A.; Dall’Asta, C. A Simple and Reliable Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Aflatoxin B1 in Feed. Food Addit. Contam. Part A 2014, 31, 1736–1743. [Google Scholar] [CrossRef]
- Kim, S.-H.; Cha, S.-H.; Karyn, B.; Park, S.-W.; Son, S.-W.; Kang, H.-G. Production of Group Specific Monoclonal Antibody to Aflatoxins and Its Application to Enzyme-Linked Immunosorbent Assay. Toxicol. Res. 2011, 27, 125–131. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Luo, J.; Qin, J.; Jiang, J.; Qin, L.; Zhao, Z.; Yang, S.; Yang, M. Development of a Sensitive Indirect Competitive Enzyme-Linked Immunosorbent Assay for High-Throughput Detection and Risk Assessment of Aflatoxin B1 in Animal-Derived Medicines. Toxicon 2021, 197, 99–105. [Google Scholar] [CrossRef]
- Wei, L.; Xu, D.; Yuan, B.; Pang, C.; Xu, H.; Nie, K.; Yang, Q.; Ozkan, S.A.; Zhang, Y.; Guo, Y.; et al. A Dynamic and Pseudo-Homogeneous MBs-icELISA for the Early Detection of Aflatoxin B1 in Food and Feed. Toxins 2023, 15, 660. [Google Scholar] [CrossRef]
- Moon, J.; Kim, G.; Lee, S. A Gold Nanoparticle and Aflatoxin B1-BSA Conjugates Based Lateral Flow Assay Method for the Analysis of Aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, J.; Huang, Z. Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip. Foods 2023, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a Rainbow Lateral Flow Immunoassay for the Simultaneous Detection of Four Mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.; Zhang, J.; Shen, X.; Xu, Z.; Li, X.; Lei, H. High Bioaffinity Controllable Assembly Nanocarrier UiO-66-NH2@Quantum Dot-Based Immunochromatographic Assay for Simultaneous Detection of Five Mycotoxins in Cereals and Feed. J. Agric. Food Chem. 2023, 71, 16797–16806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, P.; Zheng, B. A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods 2021, 10, 2109. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. Europium Nanospheres-Based Time-Resolved Fluorescence for Rapid and Ultrasensitive Determination of Total Aflatoxin in Feed. J. Agric. Food Chem. 2015, 63, 10313–10318. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zheng, P.; Zhao, S.; Liu, H.; Wang, Z.; Peng, T.; Wang, J.; Yao, K.; Wang, S.; Zeng, Y.; et al. Time-Resolved Fluorescent Immunochromatographic Assay-Based on Three Antibody Labels for the Simultaneous Detection of Aflatoxin B1 and Zearalenone in Chinese Herbal Medicines. Food Addit. Contam. Part A 2018, 35, 2434–2442. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Q.; Zhang, Z.; Ding, X.; Jiang, J.; Zhang, W.; Li, P. Rapid, on-Site and Quantitative Paper-Based Immunoassay Platform for Concurrent Determination of Pesticide Residues and Mycotoxins. Anal. Chim. Acta 2019, 1078, 142–150. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A Smartphone-Based Dual Detection Mode Device Integrated with Two Lateral Flow Immunoassays for Multiplex Mycotoxins in Cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]

| Mycotoxins | T/C | CV% |
|---|---|---|
| AFB1 | 0.2849 | 100 |
| ZEN | 2.163 | 13.2 |
| OTA | 2.066 | 13.8 |
| T-2 | 2.026 | 14.1 |
| DON | 2.303 | 12.4 |
| Method | Sample | Spiked (ng/kg) | LOD (ng/kg) | LOQ (ng/kg) | Intra-CV (%, n = 5) | Recovery (%) | Inter-CV (%, n = 15) |
|---|---|---|---|---|---|---|---|
| Ic-ELISA | Maize | 11 | 6.58 | 10.5 | 5.53 | 83 ± 3 | 3.34 |
| 22 | 4.59 | 94 ± 8 | 8.19 | ||||
| 44 | 5.82 | 75 ± 6 | 7.96 | ||||
| Feed | 15 | 5.54 | 8.36 | 5.12 | 84 ± 4 | 4.56 | |
| 30 | 5.76 | 80 ± 3 | 4.25 | ||||
| 60 | 5.10 | 72 ± 3 | 3.89 | ||||
| TRFICA | Maize | 250 | 62.7 | 102 | 7.79 | 94 ± 0.1 | 13.6 |
| 500 | 6.04 | 95 ± 0.09 | 9.05 | ||||
| 1000 | 3.18 | 93 ± 0.1 | 14.3 | ||||
| Feed | 500 | 121 | 201 | 7.10 | 90 ± 0.1 | 10.3 | |
| 1000 | 3.66 | 96 ± 0.1 | 11.2 | ||||
| 2000 | 7.64 | 98 ± 0.1 | 11.6 |
| Experimental Method | Marking Material | Target Analyte | LOD (μg/kg) | Sample Preparation | Total Analysis Time (min) | Reference |
|---|---|---|---|---|---|---|
| ELISA | - | AFB1 | 4.36 | feed | 15 | [19] |
| Ic-ELISA | - | AFB1 | 0.008–0.020 | animal-derived medicine | 135 | [20] |
| MBs-ic-ELISA | - | AFB1 | 0.0013 | buffer solution | 85 | [21] |
| Ic-ELISA | - | AFB1 | 0.0386 | feed and maize | 85 | This study |
| GICA | gold nanoparticles | AFB1 | 10 | animal feeds or food | 10 | [22] |
| ICS | gold nanoparticles | AFB1, ZEN, and T2 | 0.5, 5.0, and 5.0 | medicinal and edible food | 10 | [23] |
| LFIA | QDs | DON, ZEN, and T2/HT2 | 1000, 80, and 80 | barley | 15 | [24] |
| LFIA | NU66@QD | AFB1, FB1, DON, T-2, and ZEN | 0.04, 0.28, 0.25, 0.09, and 0.08 | cereals and feed | 8 | [25] |
| LFIA | fluorescent microsphere | AFB1 | 3.4 | distillers’ grains | 15 | [26] |
| TRFICA | TRFMs nanospheres | AFB1 + B2 + G1 + G2 | 0.16 | Feed | 12 | [27] |
| TRFICA | TRFMs nanospheres | AFB1, ZEN | 0.05 and 0.07 | maize | 8 | [13] |
| TRFICA | TRFMs | AFB1 and ZEN | 0.60 and 0.40 | Chinese herbal medicines | 15 | [28] |
| TRFICA | TRFMs nanoparticle | AFTs, carbaryl, and carbofuran | 0.03, 0.02, and 60.2 | maize | 10 | [29] |
| TRFICA | TRFMs | AFB1, ZEN, DON, T-2, FB1 | 2.5, 0.5, 0.5, 2.5, and 0.5 | maize, wheat, bran | 8 | [30] |
| TRFICA | TRFMs | AFB1 | 0.0627 and 0.121 | maize and feed | 6 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Yang, Y.; Chen, T.; Yang, B.; Ding, M.; Wen, H.; Xiao, J.; Cheng, G.; Tao, Y.; Hao, H.; et al. Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies. Foods 2024, 13, 319. https://doi.org/10.3390/foods13020319
Han S, Yang Y, Chen T, Yang B, Ding M, Wen H, Xiao J, Cheng G, Tao Y, Hao H, et al. Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies. Foods. 2024; 13(2):319. https://doi.org/10.3390/foods13020319
Chicago/Turabian StyleHan, Shiyun, Yalin Yang, Ting Chen, Bijia Yang, Mingyue Ding, Hao Wen, Jiaxu Xiao, Guyue Cheng, Yanfei Tao, Haihong Hao, and et al. 2024. "Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies" Foods 13, no. 2: 319. https://doi.org/10.3390/foods13020319
APA StyleHan, S., Yang, Y., Chen, T., Yang, B., Ding, M., Wen, H., Xiao, J., Cheng, G., Tao, Y., Hao, H., & Peng, D. (2024). Quantitative Determination of Aflatoxin B1 in Maize and Feed by ELISA and Time-Resolved Fluorescent Immunoassay Based on Monoclonal Antibodies. Foods, 13(2), 319. https://doi.org/10.3390/foods13020319








