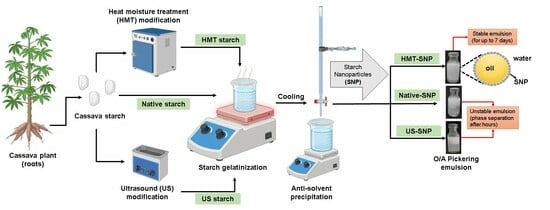
Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Pretreatments of Starch
2.3. Preparation of Starch Nanoparticles
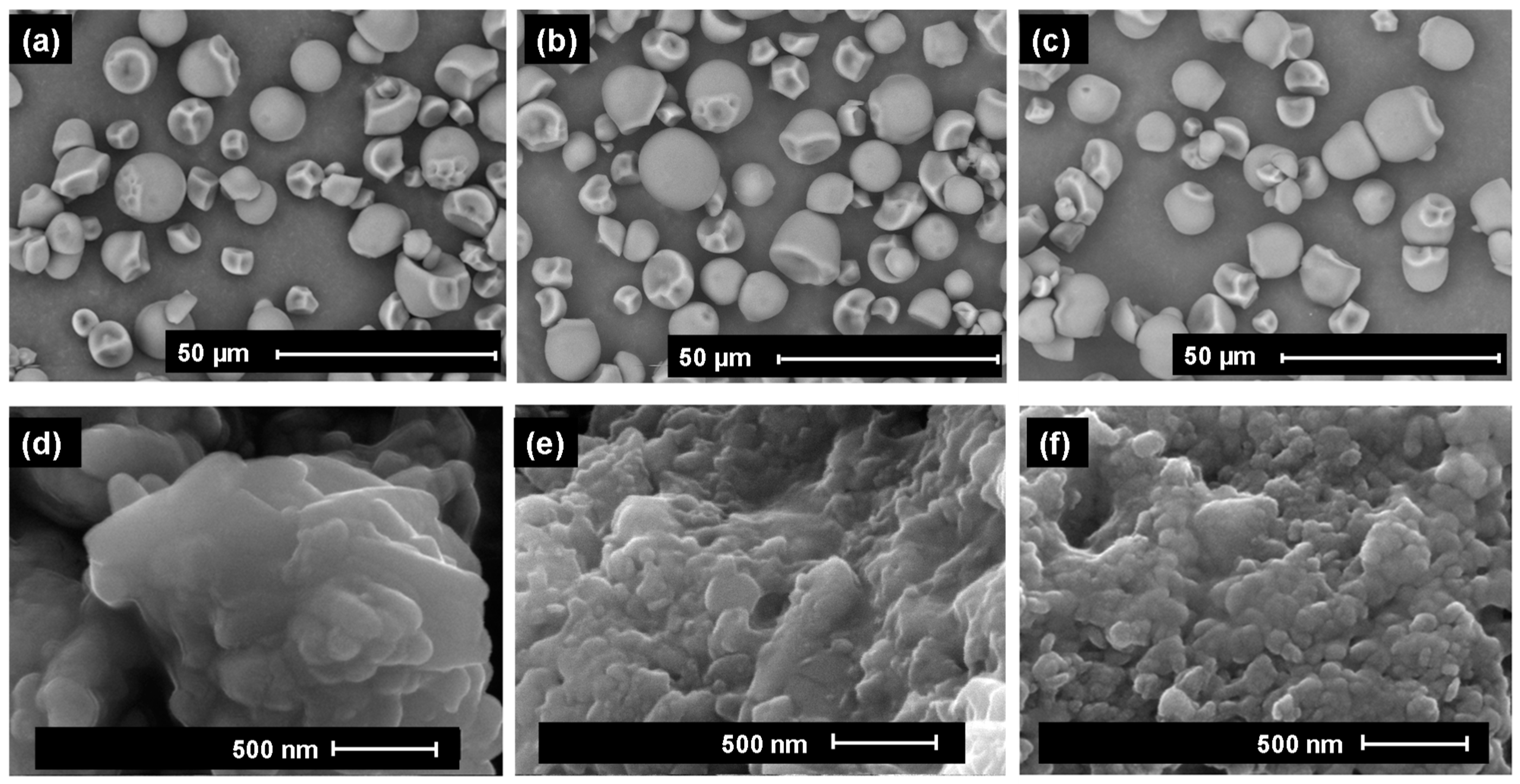
2.4. Scanning Electron Microscopy
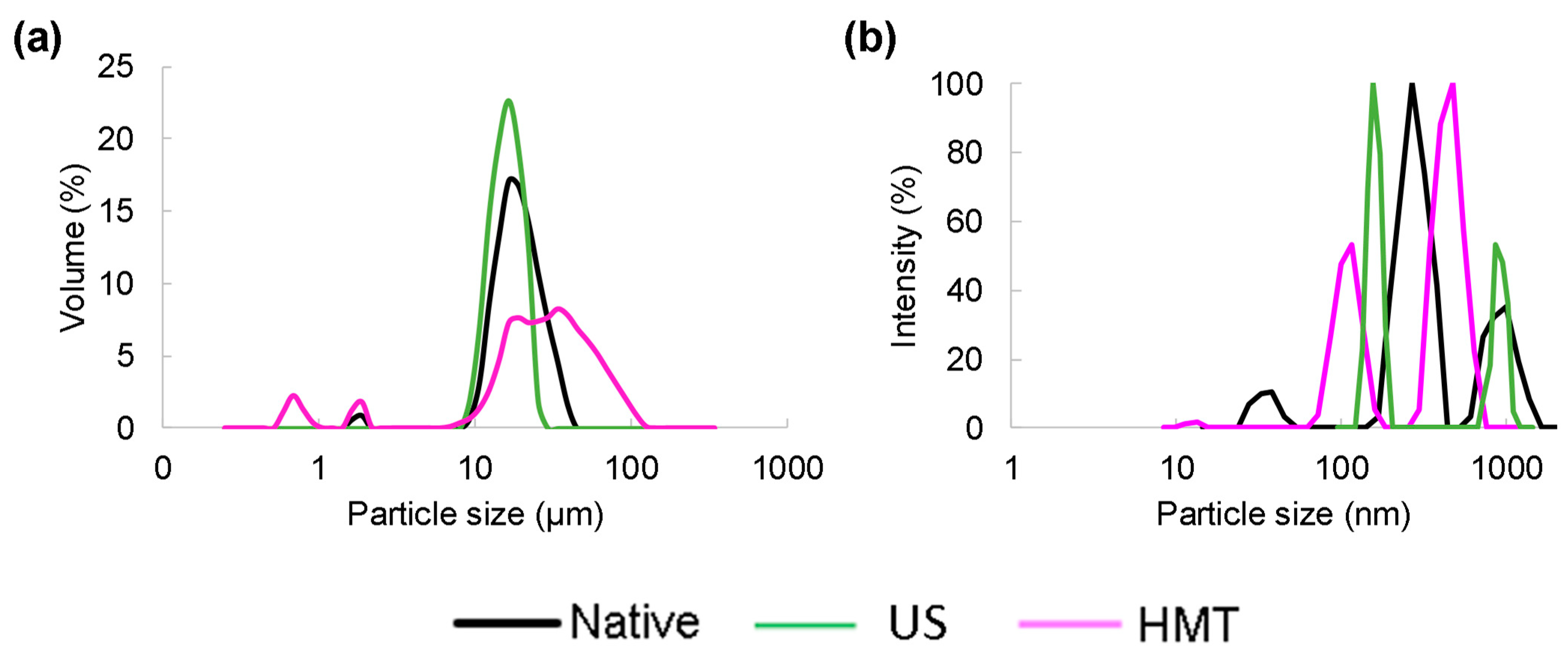
2.5. Particle Size Analysis and Zeta Potential Measurements
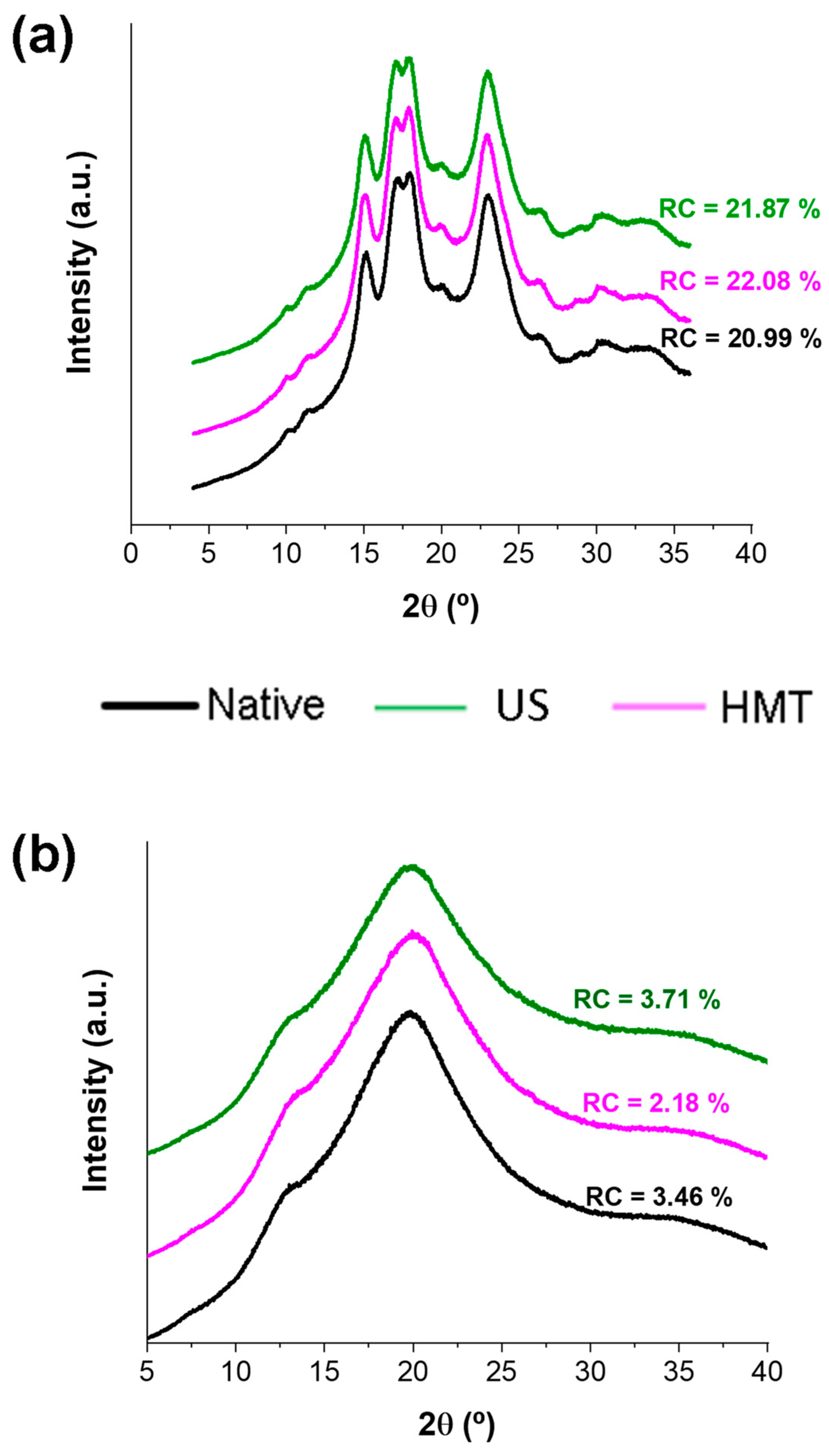
2.6. X-ray Diffraction (XRD)
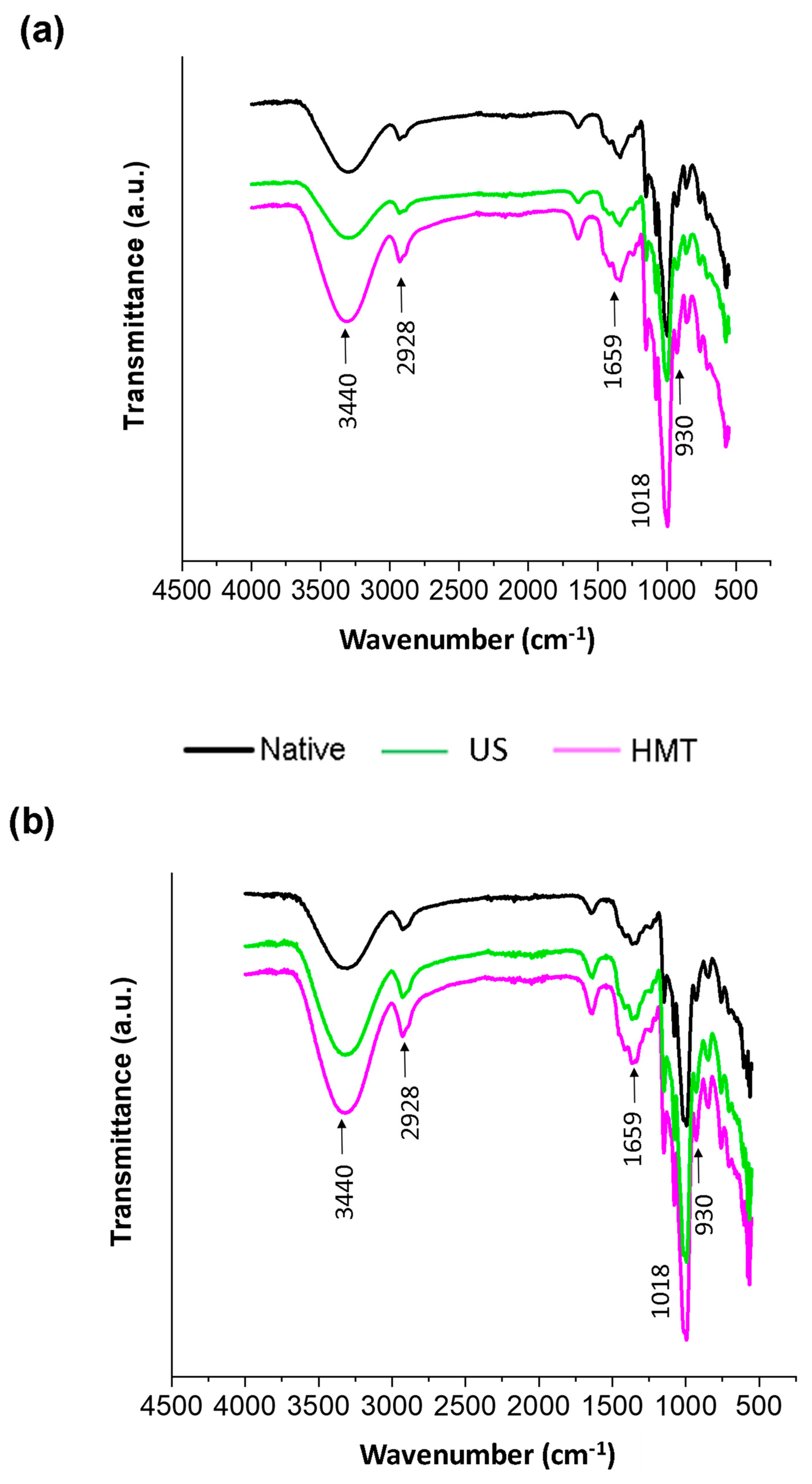
2.7. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
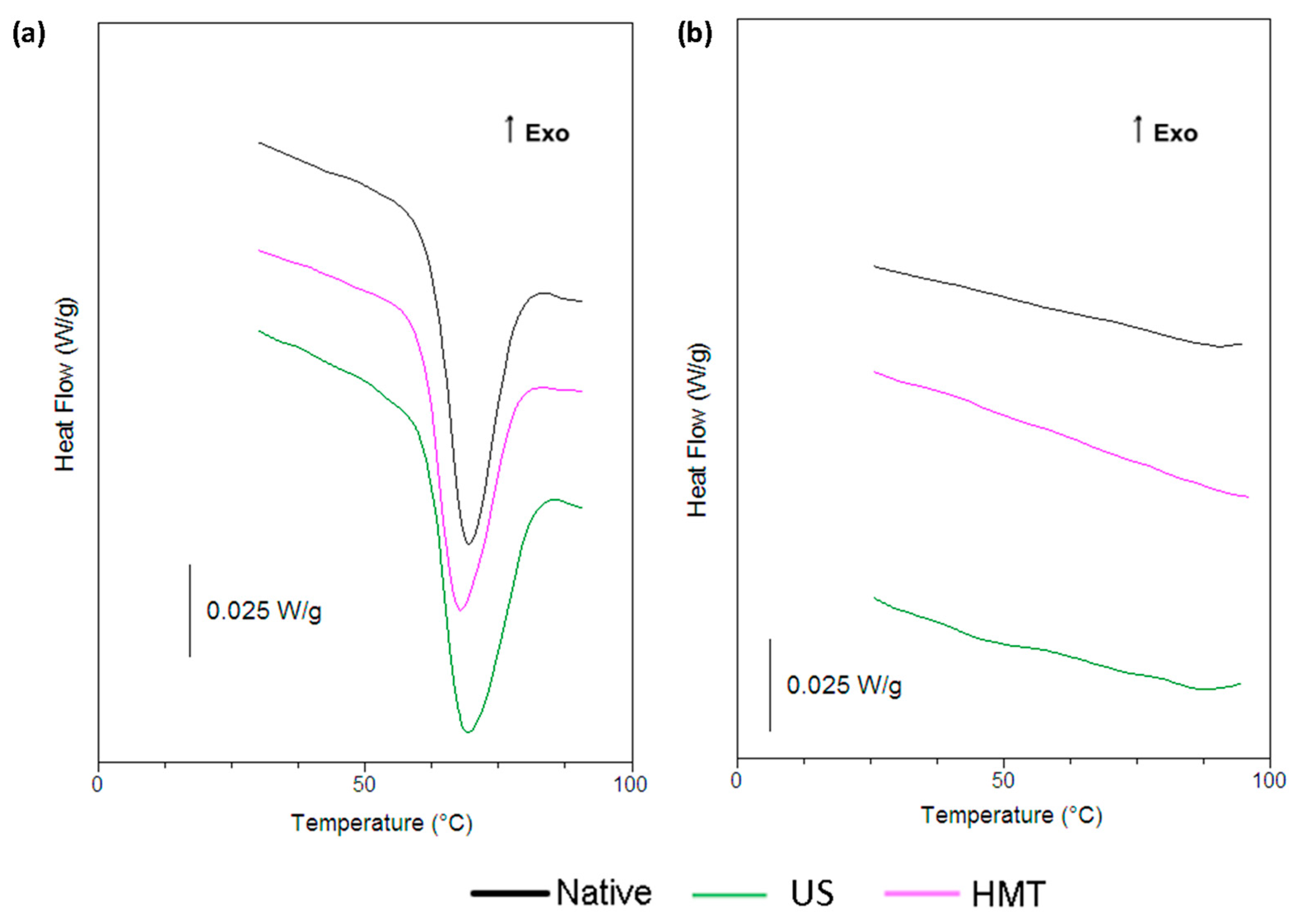
2.8. Differential Scanning Calorimeter (DSC)
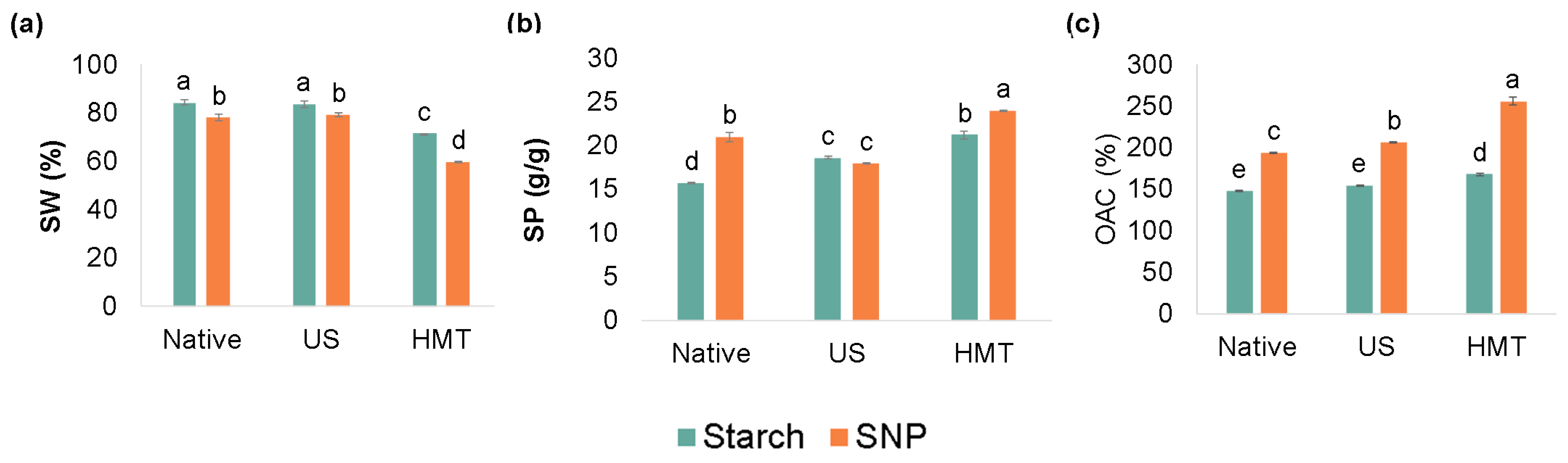
2.9. Solubility in Water (SW) and Swelling Power (SP)
2.10. Oil Absorption Capacity
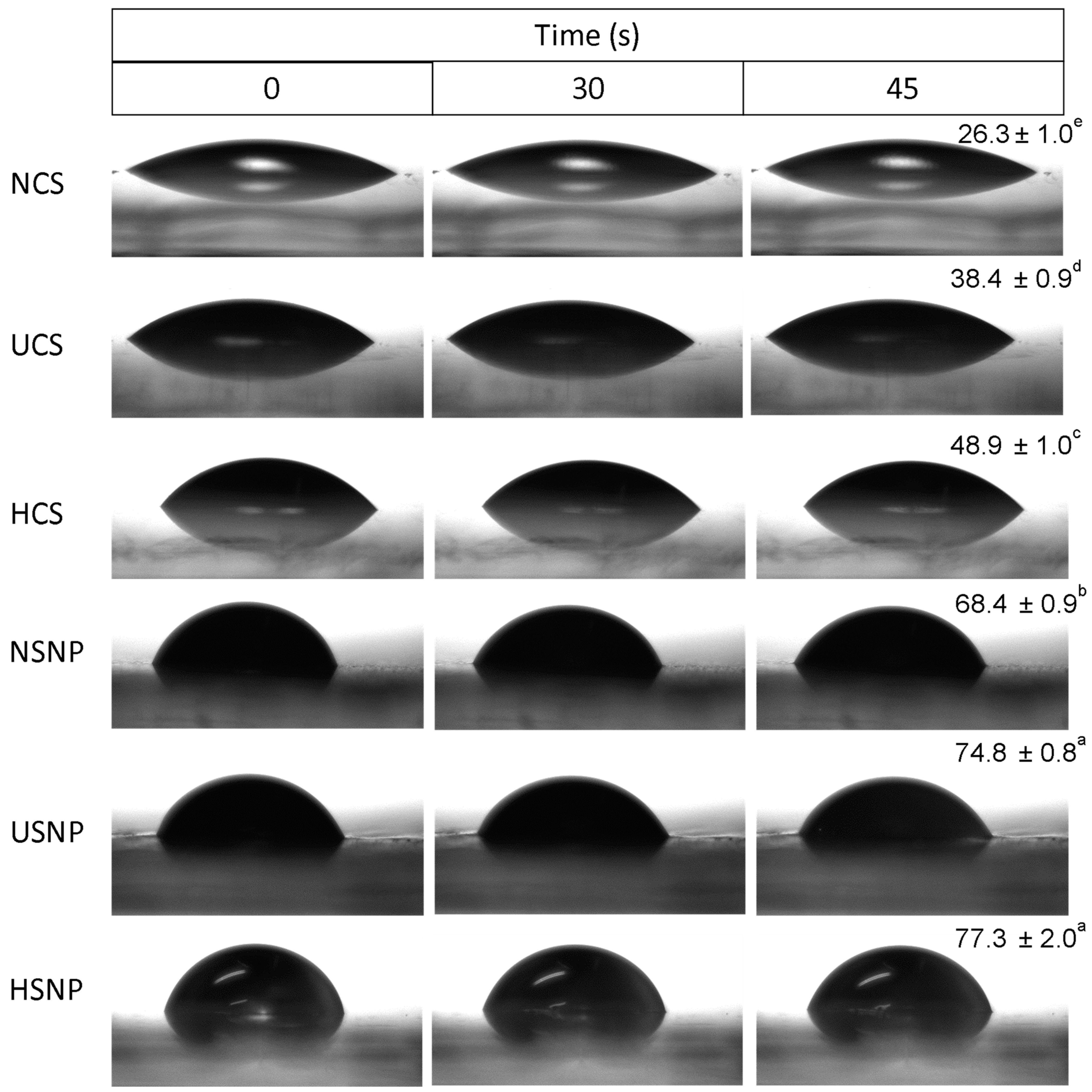
2.11. Contact-Angle Measurement
2.12. Production of Pickering Emulsions with SNP as Stabilizers
2.13. Statistical Analyses
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. Particle Size Analysis and Zeta Potential Measurement
3.3. X-ray Diffraction (XRD)
3.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.5. Differential Scanning Calorimetry (DSC)
3.6. Solubility in Water, Swelling Power and Oil Absorption Capacity
3.7. Water Contact Angle Measurement
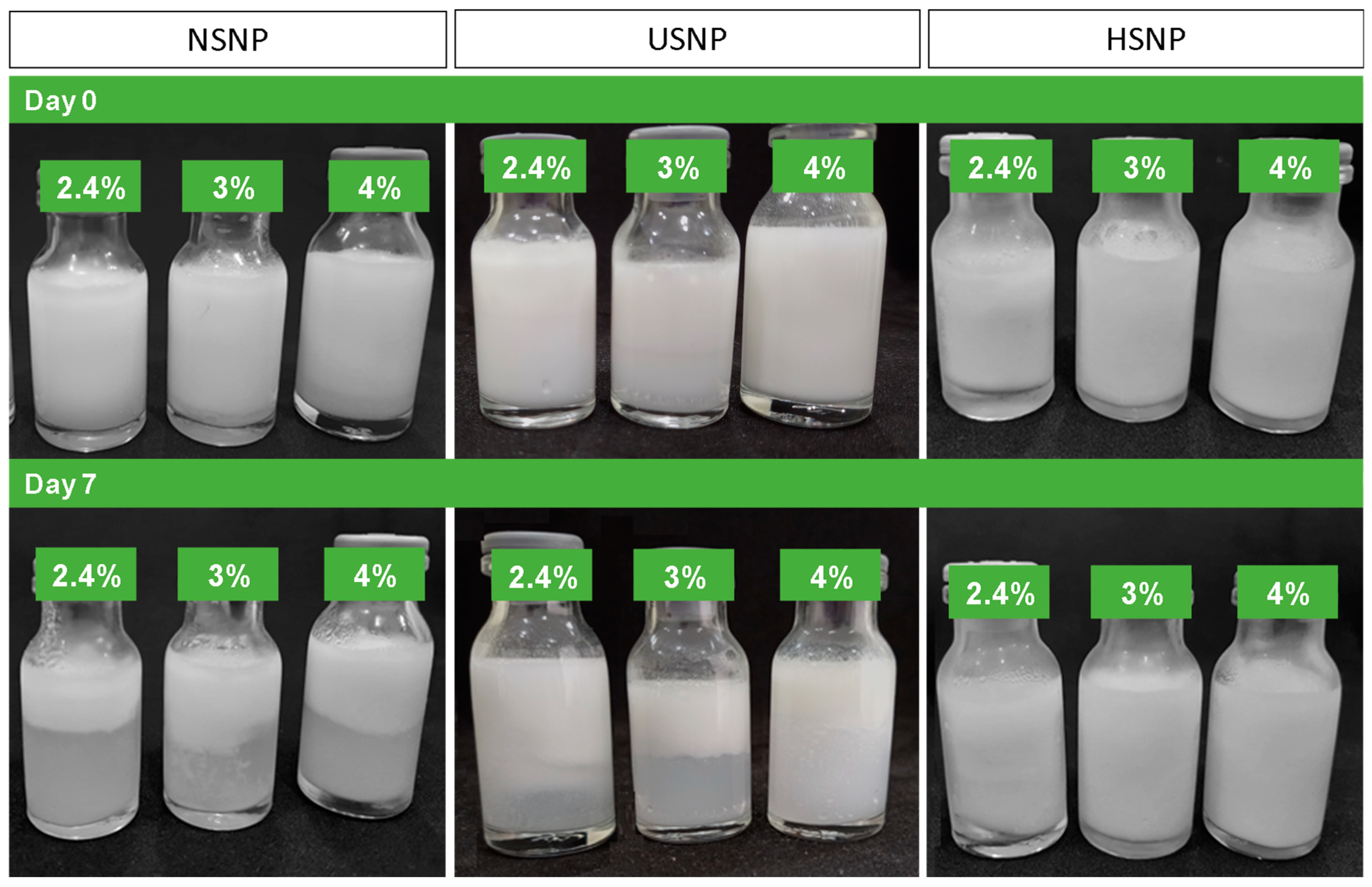
3.8. SNP as Pickering Emulsion Stabilizers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bulatović, V.O.; Mandić, V.; Kučić Grgić, D.; Ivančić, A. Biodegradable Polymer Blends Based on Thermoplastic Starch. J. Polym. Environ. 2021, 29, 492–508. [Google Scholar] [CrossRef]
- Khakpour, F.; Pirsa, S.; Amiri, S. Modified Starch/CrO/Lycopene/Gum Arabic Nanocomposite Film: Preparation, Investigation of Physicochemical Properties and Ability to Use as Nitrite Kit. J. Polym. Environ. 2023, 31, 3875–3893. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Faisal Manzoor, M. Ultrasound Based Modification and Structural-Functional Analysis of Corn and Cassava Starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef] [PubMed]
- Otache, M.A.; Duru, R.U.; Achugasim, O.; Abayeh, O.J. Advances in the Modification of Starch via Esterification for Enhanced Properties. J. Polym. Environ. 2021, 29, 1365–1379. [Google Scholar] [CrossRef]
- Frost, K.; Kaminski, D.; Kirwan, G.; Lascaris, E.; Shanks, R. Crystallinity and Structure of Starch Using Wide Angle X-ray Scattering. Carbohydr. Polym. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical Characterization of Modified Lotus Seed Starch Obtained through Acid and Heat Moisture Treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef]
- dos Santos Lima, K.T.; Garcez, J.; Alves, M.J.S.; Monteiro, A.R.; Valencia, G.A. Physicochemical Properties of Modified Starches Obtained by Anti-Solvent Precipitation Containing Anthocyanins from Jambolan (Syzygium cumini) Fruit. Starch/Staerke 2021, 73, 2000221. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khodadadzadeh, L. Ultrasonic-Assisted Fabrication of Starch/MWCNT-Glucose Nanocomposites for Drug Delivery. Ultrason. Sonochem. 2018, 40, 402–409. [Google Scholar] [CrossRef]
- Amini, A.M.; Razavi, S.M.A.; Mortazavi, S.A. Morphological, Physicochemical, and Viscoelastic Properties of Sonicated Corn Starch. Carbohydr. Polym. 2015, 122, 282–292. [Google Scholar] [CrossRef]
- Ji, N.; Li, X.; Qiu, C.; Li, G.; Sun, Q.; Xiong, L. Effects of Heat Moisture Treatment on the Physicochemical Properties of Starch Nanoparticles. Carbohydr. Polym. 2015, 117, 605–609. [Google Scholar] [CrossRef]
- Li, S.; Ward, R.; Gao, Q. Effect of Heat-Moisture Treatment on the Formation and Physicochemical Properties of Resistant Starch from Mung Bean (Phaseolus radiatus) Starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Pratiwi, M.; Faridah, D.N.; Lioe, H.N. Structural Changes to Starch after Acid Hydrolysis, Debranching, Autoclaving-Cooling Cycles, and Heat Moisture Treatment (HMT): A Review. Starch—Stärke 2018, 70, 1700028. [Google Scholar] [CrossRef]
- Zhu, F. Starch Based Pickering Emulsions: Fabrication, Properties, and Applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-Treated Starch: SEM and TEM Imaging, and Functional Behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Ge, X.; Shen, H.; Sun, X.; Liang, W.; Zhang, X.; Sun, Z.; Lu, Y.; Li, W. Insight into the Improving Effect on Multi-Scale Structure, Physicochemical and Rheology Properties of Granular Cold Water Soluble Rice Starch by Dielectric Barrier Discharge Cold Plasma Processing. Food Hydrocoll. 2022, 130, 107732. [Google Scholar] [CrossRef]
- Piecyk, M.; Domian, K. Effects of Heat–Moisture Treatment Conditions on the Physicochemical Properties and Digestibility of Field Bean Starch (Vicia faba Var. Minor). Int. J. Biol. Macromol. 2021, 182, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Xiong, L.; Li, M.; Liu, J.; Yang, J.; Chang, R.; Liang, C.; Sun, Q. Characterizations of Pickering Emulsions Stabilized by Starch Nanoparticles: Influence of Starch Variety and Particle Size. Food Chem. 2017, 234, 339–347. [Google Scholar] [CrossRef]
- Velásquez-Castillo, L.E.; Leite, M.A.; Tisnado, V.J.A.; Ditchfield, C.; do Amaral Sobral, P.J.; Moraes, I.C.F. Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties. Foods 2023, 12, 576. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA; University of Massachusetts: Amherst, MA, USA, 2004; ISBN 0849320232. [Google Scholar]
- Jiang, S.; Liu, C.; Han, Z.; Xiong, L.; Sun, Q. Evaluation of Rheological Behavior of Starch Nanocrystals by Acid Hydrolysis and Starch Nanoparticles by Self-Assembly: A Comparative Study. Food Hydrocoll. 2016, 52, 914–922. [Google Scholar] [CrossRef]
- Velásquez-Castillo, L.E.; Leite, M.A.; Ditchfield, C.; do Amaral Sobral, P.J.; Moraes, I.C.F. Quinoa Starch Nanocrystals Production by Acid Hydrolysis: Kinetics and Properties. Int. J. Biol. Macromol. 2020, 143, 93–101. [Google Scholar] [CrossRef]
- Uzomah, A.; Ibe, C. The Functional Properties, Pasting and Baking Behaviour of Chemically Modified Sour Cassava Starches. Afr. J. Food Sci. 2011, 5, 686–694. [Google Scholar]
- Dewi, A.M.P.; Santoso, U.; Pranoto, Y.; Marseno, D.W. Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polymers 2022, 14, 1086. [Google Scholar] [CrossRef]
- Ramos, G.V.C.; Suzigan, A.R.; Pinho, S.C.; Moraes, I.C.F. Impact of Emulsification Time and Concentration of Modified Starch Nanoparticles on Pickering Stability. Chem. Eng. Trans. 2023, 102, 247–252. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of Heat-Moisture Treatment on the Structure and Physicochemical Properties of Tuber and Root Starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent Advances in Heat-Moisture Modified Cereal Starch: Structure, Functionality and Its Applications in Starchy Food Systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, C.; Junejo, S.A.; Zhang, B.; Fu, X.; Tan, C.P.; Huang, Q. Effect of V-Type Crystallinity and Starch Particle Structure on the Oil Loading Capacity and Anti-Oxidation. Carbohydr. Polym. 2022, 297, 120015. [Google Scholar] [CrossRef]
- Chandla, N.K.; Saxena, D.C.; Singh, S. Processing and Evaluation of Heat Moisture Treated (HMT) Amaranth Starch Noodles; An Inclusive Comparison with Corn Starch Noodles. J. Cereal Sci. 2017, 75, 306–313. [Google Scholar] [CrossRef]
- Nikolic, G.S.; Cakic, M.D. Physical Investigation of the Colloidal Iron-Inulin Complex. Colloid J. 2007, 69, 464–473. [Google Scholar] [CrossRef]
- Sammon, C.; Bajwa, G.; Timmins, P.; Melia, C.D. The Application of Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy to Monitor the Concentration and State of Water in Solutions of a Thermally Responsive Cellulose Ether during Gelation. Polymer 2006, 47, 577–584. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R.P. Cationic Starch: An Effective Flocculating Agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.; Ding, H. Some Physicochemical Properties of Sage (Salvia macrosiphon) Seedgum. Food Hydrocoll. 2014, 35, 453–462. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and Characterization of Highly Lipophilic Modified Potato Starch by Ultrasound and Freeze-Thaw Treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, P.N.; Akonor, P.T.; Tortoe, C.; Johnsona, P.N.T.; Manu-Aduening, J. Effect of the Use of Starches of Three New Ghanaian Cassava Varieties as a Thickener on the Physicochemical, Rheological and Sensory Properties of Yoghurt. Sci. Afr. 2020, 9, e00521. [Google Scholar] [CrossRef]
- Dudu, O.E.; Li, L.; Oyedeji, A.B.; Oyeyinka, S.A.; Ma, Y. Structural and Functional Characteristics of Optimised Dry-Heat-Moisture Treated Cassava Flour and Starch. Int. J. Biol. Macromol. 2019, 133, 1219–1227. [Google Scholar] [CrossRef]
- Agnes, A.C.; Felix, E.C.; Ugochukwu, N.T. Morphology, Rheology and Functional Properties of Starch from Cassava, Sweet Potato and Cocoyam. Asian J. Biol. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Wang, L.J.; Hu, Y.Q.; Yin, S.W.; Yang, X.Q.; Lai, F.R.; Wang, S.Q. Fabrication and Characterization of Antioxidant Pickering Emulsions Stabilized by Zein/Chitosan Complex Particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef]
- Zanini, M.; Marschelke, C.; Anachkov, S.E.; Marini, E.; Synytska, A.; Isa, L. Universal emulsion stabilization from the arrested adsorption of rough particles at liquid-liquid interfaces. Nat. Commun. 2017, 8, 15701. [Google Scholar] [CrossRef]
| Sample | PDI | Zeta Potential (mV) |
|---|---|---|
| NCS | - | −50.5 ± 1.3 c |
| UCS | - | −44.0 ± 1.0 b |
| HCS | - | −45.3 ± 2.9 b |
| NSNP | 0.80 ± 0.11 a | −1.0 ± 0.2 a |
| USNP | 0.65 ± 0.12 a,b | −1.7 ± 0.5 a |
| HSNP | 0.42 ± 0.01 b | −3.4 ±0.6 a |
| Sample | T0 (°C) | Tp (°C) | Tc (°C) | ∆H (J/g) |
|---|---|---|---|---|
| NCS | 62.8 ± 0.2 a | 69.3 ± 0.1 a | 83.5 ± 0.9 a | 14.4 ± 0.8 a |
| UCS | 62.2 ± 0.3 a | 69.3 ± 0.1 a | 84.8 ± 1.0 a | 13.9 ± 0.3 a |
| HCS | 61.0 ± 0.1 b | 67.8 ± 0.2 b | 83.1 ± 0.9 a | 13.6 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, G.V.C.; Rabelo, M.E.A.; Pinho, S.C.d.; Valencia, G.A.; Sobral, P.J.d.A.; Moraes, I.C.F. Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers. Foods 2024, 13, 327. https://doi.org/10.3390/foods13020327
Ramos GVC, Rabelo MEA, Pinho SCd, Valencia GA, Sobral PJdA, Moraes ICF. Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers. Foods. 2024; 13(2):327. https://doi.org/10.3390/foods13020327
Chicago/Turabian StyleRamos, Giselle Vallim Correa, Marya Eduarda Azelico Rabelo, Samantha Cristina de Pinho, Germán Ayala Valencia, Paulo José do Amaral Sobral, and Izabel Cristina Freitas Moraes. 2024. "Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers" Foods 13, no. 2: 327. https://doi.org/10.3390/foods13020327
APA StyleRamos, G. V. C., Rabelo, M. E. A., Pinho, S. C. d., Valencia, G. A., Sobral, P. J. d. A., & Moraes, I. C. F. (2024). Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers. Foods, 13(2), 327. https://doi.org/10.3390/foods13020327