Is a Meta-Analysis of Clinical Trial Outcomes for Ketogenic Diets Justifiable? A Critical Assessment Based on Systematic Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Screening and Data Extraction
3. Results
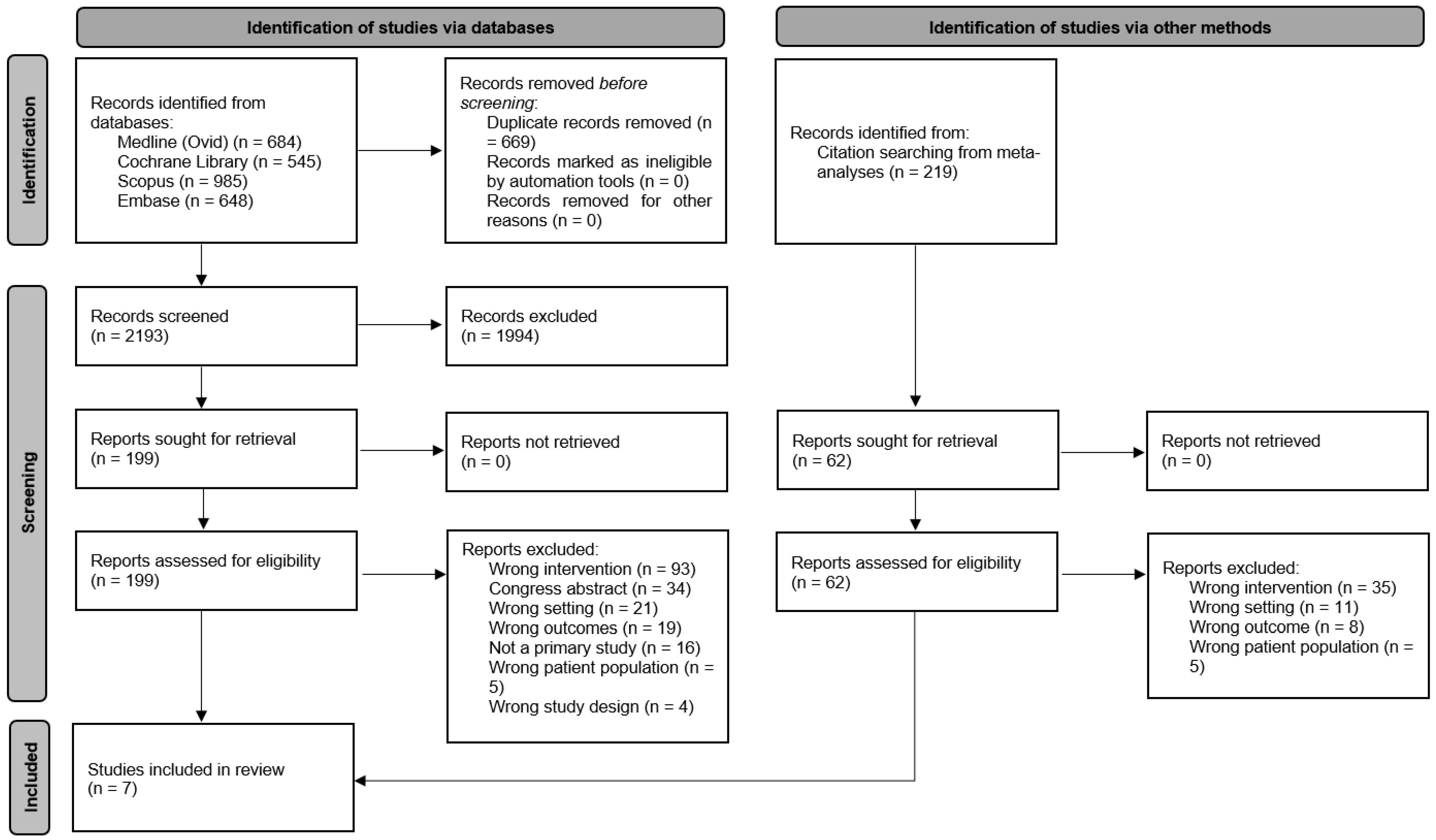
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Results of Individual Sources of Evidence
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Vining, E.P.; Freeman, J.M.; Ballaban-Gil, K.; Camfield, C.S.; Camfield, P.R.; Holmes, G.L.; Shinnar, S.; Shuman, R.; Trevathan, E.; Wheless, J.W. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol. 1998, 55, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Szendi, K.; Murányi, E.; Hunter, N.; Németh, B. Methodological Challenges and Confounders in Research on the Effects of Ketogenic Diets: A Literature Review of Meta-Analyses. Foods 2024, 13, 248. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e681. [Google Scholar] [CrossRef]
- Harvey, C.; Schofield, G.M.; Zinn, C.; Thornley, S.J.; Crofts, C.; Merien, F.L.R. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: A randomised clinical trial. PeerJ 2019, 7, e6273. [Google Scholar] [CrossRef]
- Saslow, L.R.; Kim, S.; Daubenmier, J.J.; Moskowitz, J.T.; Phinney, S.D.; Goldman, V.; Murphy, E.J.; Cox, R.M.; Moran, P.; Hecht, F.M. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 2014, 9, e91027. [Google Scholar] [CrossRef]
- Harvard, T.H.; Chan School of Public Health. Diet Review: Ketogenic Diet for Weight Loss. Available online: https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/ketogenic-diet/ (accessed on 8 September 2024).
- National Cancer Institute. Dictionary of Cancer Terms: Ketogenic Diet. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/ketogenic-diet (accessed on 9 September 2024).
- Albu, J.; Pi-Sunyer, F.X. Obesity and diabetes. In Handbook of Obesity; Bray, G.A., Bouchard, C., James, W.P.T., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 697–707. [Google Scholar]
- World Health Organization. Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 September 2024).
- Kossoff, E.H.; Zupec-Kania, B.A.; Rho, J.M. Ketogenic diets: An update for child neurologists. J. Child Neurol. 2009, 24, 979–988. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Buga, A.; Kackley, M.L.; Crabtree, C.D.; Bedell, T.N.; Robinson, B.T.; Stoner, J.T.; Decker, D.D.; Hyde, P.N.; LaFountain, R.A.; Brownlow, M.L.; et al. Fasting and diurnal blood ketonemia and glycemia responses to a six-week, energy-controlled ketogenic diet, supplemented with racemic R/S-BHB salts. Clin. Nutr. ESPEN 2023, 54, 277–287. [Google Scholar] [CrossRef]
- Crabtree, C.D.; Kackley, M.L.; Buga, A.; Fell, B.; LaFountain, R.A.; Hyde, P.N.; Sapper, T.N.; Kraemer, W.J.; Scandling, D.; Simonetti, O.P.; et al. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J.; Courville, A.B.; Boring, J.; Brychta, R.; Chen, K.Y.; Darcey, V.; Forde, C.G.; Gharib, A.M.; Gallagher, I.; et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 2021, 27, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kackley, M.L.; Buga, A.; Brownlow, M.L.; O’Connor, A.; Sapper, T.N.; Crabtree, C.D.; Robinson, B.T.; Stoner, J.T.; Decker, D.D.; Soma, L.; et al. Self-reported menses physiology is positively modulated by a well-formulated, energy-controlled ketogenic diet vs. low fat diet in women of reproductive age with overweight/obesity. PLoS ONE 2024, 19, e0293670. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kong, Z.; Shi, Q.; Hu, M.; Zhang, H.; Zhang, D.; Nie, J. Non-Energy-Restricted Low-Carbohydrate Diet Combined with Exercise Intervention Improved Cardiometabolic Health in Overweight Chinese Females. Nutrients 2019, 11, 3051. [Google Scholar] [CrossRef]
- White, A.M.; Johnston, C.S.; Swan, P.D.; Tjonn, S.L.; Sears, B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: A pilot study. J. Am. Diet Assoc. 2007, 107, 1792–1796. [Google Scholar] [CrossRef]
- Barbosa-Yañez, R.L.; Dambeck, U.; Li, L.; Machann, J.; Kabisch, S.; Pfeiffer, A.F.H. Acute Endothelial Benefits of Fat Restriction over Carbohydrate Restriction in Type 2 Diabetes Mellitus: Beyond Carbs and Fats. Nutrients 2018, 10, 1859. [Google Scholar] [CrossRef]
- Hanafy, H.M.; Mostafa, M.H.; Ramadan, N.M. Effect of aerobic exercise and ketogenic diet on type II diabetic obese pre menopausal women. Fizjoterapia Pol. 2022, 22, 160–164. [Google Scholar]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Govers, E.; Otten, A.; Schuiling, B.; Bouwman, W.; Lourens, A.; Visscher, T. Effectiveness of the 6 × 6 Dieet® in Obese DMT2 Patients Effectiveness of a Very Low Carbohydrate Ketogenic Diet Compared to a Low Carbohydrate and Energy-Restricted Diet in Overweight/Obese Type 2 Diabetes Patients. Int. J. Endocrinol. Metab. Disord. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Bengin, E.; Kırtepe, A.; Çınar, V.; Akbulut, T.; Russo, L.; Aydemir, İ.; Yücedal, P.; Aydın, S.; Migliaccio, G.M. Leptin, Ghrelin, Irisin, Asprosin and Subfatin Changes in Obese Women: Effect of Exercise and Different Nutrition Types. Medicina 2024, 60, 1118. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S., Jr.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Perissiou, M.; Borkoles, E.; Kobayashi, K.; Polman, R. The Effect of an 8 Week Prescribed Exercise and Low-Carbohydrate Diet on Cardiorespiratory Fitness, Body Composition and Cardiometabolic Risk Factors in Obese Individuals: A Randomised Controlled Trial. Nutrients 2020, 12, 482. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial1234. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Veum, V.L.; Laupsa-Borge, J.; Eng, Ø.; Rostrup, E.; Larsen, T.H.; Nordrehaug, J.E.; Nygård, O.K.; Sagen, J.V.; Gudbrandsen, O.A.; Dankel, S.N.; et al. Visceral adiposity and metabolic syndrome after very high-fat and low-fat isocaloric diets: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 85–99. [Google Scholar] [CrossRef]
- Luong, T.V.; Pedersen, M.G.B.; Abild, C.B.; Lauritsen, K.M.; Kjærulff, M.L.G.; Møller, N.; Gormsen, L.C.; Søndergaard, E. A 3-Week Ketogenic Diet Increases Skeletal Muscle Insulin Sensitivity in Individuals With Obesity: A Randomized Controlled Crossover Trial. Diabetes 2024, 73, 1631–1640. [Google Scholar] [CrossRef]
- Gibas, M.K.; Gibas, K.J. Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S385–S390. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Tjonn, S.L.; Swan, P.D.; White, A.; Hutchins, H.; Sears, B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am. J. Clin. Nutr. 2006, 83, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Gómez, A.L.; Scheett, T.P.; Volek, J.S. A Ketogenic Diet Favorably Affects Serum Biomarkers for Cardiovascular Disease in Normal-Weight Men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Klonek, G.; Maszczyk, A.; Zajac, A. The Effects of a Low Calorie Ketogenic Diet on Glycaemic Control Variables in Hyperinsulinemic Overweight/Obese Females. Nutrients 2020, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Pinsawas, B.; Surawit, A.; Mongkolsucharitkul, P.; Pongkunakorn, T.; Suta, S.; Manosan, T.; Ophakas, S.; Pumeiam, S.; Sranacharoenpong, K.; Mayurasakorn, K. Asian Low-Carbohydrate Diet with Increased Whole Egg Consumption Improves Metabolic Outcomes in Metabolic Syndrome: A 52-Week Intervention Study. J. Nutr. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Zomparelli, S.; De Santis, G.L.; Seraceno, S.; Zuena, C.; Frank, G.; Cianci, R.; Centofanti, D.; De Lorenzo, A. Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study. Nutrients 2023, 15, 3654. [Google Scholar] [CrossRef]
- Luong, T.V.; Pedersen, M.G.B.; Abild, C.B.; Cunnane, S.C.; Croteau, E.; Lauritsen, K.M.; Kjaerulff, M.L.G.; Tolbod, L.P.; Møller, N.; Søndergaard, E.; et al. A ketogenic diet lowers myocardial fatty acid oxidation but does not affect oxygen consumption: A study in overweight humans. Obesity 2024, 32, 506–516. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hall, K.D.; Guo, J.; Ravussin, E.; Mayer, L.S.; Reitman, M.L.; Smith, S.R.; Walsh, B.T.; Leibel, R.L. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Sahoo, P.; McGrail, E.; Francois, A.; Stratton, M.S. Modestly Increased Incidence of Ketosis in Caloric Restriction Does not Significantly Alter the Effects of Caloric Restriction. J. Nutr. Health Aging 2022, 26, 657–662. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series 916; WHO/FAO Expert Consultation: Geneva, Switzerland, 2003. [Google Scholar]
| Reference, Type of Clinical Trial | Disease/Test Parameters | Duration (Day/Week) | Planned (and Real) Macronutrient Ratio (%/g) of Experimental Group | Planned (and Real) Macronutrient Ratio (%) of Control Group | Controlling and Monitoring of Food Intake | Average of Real Calorie Intake of Experimental Group | Average of Real Calorie Intake of Control Group | Fatty Acid Type | Results of Ketone Body Measurements | Liver and Kidney Function Measurement | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buga et al. [14], RCT | Overweight and obesity | 6 weeks | Ketogenic diet (KD): Carbohydrate <50 g, protein 1.5 g/kg of ideal bodyweight; the remainder of individual energy needs were derived from lipids (No data) | Low-fat diet (LFD): USDA’s Dietary Guidelines for Healthy Americans 2015–2020 (No data) | All the food was prepared that was provided to participants. Ingredients were precisely weighed on electronically calibrated scales. | 75% of estimated energy requirements for weight-maintenance | 75% of estimated energy requirements for weight-maintenance | Emphasis on monounsaturated and saturated fat sources. KD group: + two servings of 10 g medium-chain fatty acid (MCT) oil | Nutritional ketosis was attained after day 5 (1.0 ± 0.04 mM BHB) in the KD group (daily fasting capillary ketone measurement). LFD had no impact on BHB. | No data. | Similar weight loss in both groups. |
| Crabtree et al. [15], RCT | Overweight | 6 weeks | Ketogenic diet (KD): No data (Carbohydrate 38 ± 7 g, fat 131 ± 8 g, protein 100 ± 3 g) | Low-fat diet (LFD): No data (Carbohydrate 259 ± 8 g, fat 51 ± 9 g, protein 100 ± 3 g) | Each meal was prepared and provided to the participants. Each ingredient was precisely weighed (±0.1 g) with custom macro-and micronutrient composition personalized to each participant. | 1752 ± 98 kcal (75% of energy expenditure) | 1900 ± 102 kcal (75% of energy expenditure) | Rich in saturated fat | The KD group reached the 0.5 mM threshold indicating ketosis by day 3 and remained in nutritional ketosis throughout the intervention (1.12 ± 0.12 mmol/L BHB). | There were no differences in other major liver function enzymes (AST, ALT, AST/ALT, and bilirubin) from baseline to post-intervention. | Weight loss was similar between groups. Hypocaloric low-fat diet and KD can both be used in the short term to significantly reduce liver fat in individuals with NAFLD. |
| Hall et al. [16], RCT | Overweight | 2 weeks | Animal-based, ketogenic, low-carbohydrate diet (LC): No data (Carbohydrate 10.0%, fat 75.8%, protein 14.2%) | Plant-based, low-fat diet (LF): No data (Carbohydrate 75.2%, fat 10.3%, protein 14.5%) | Inpatient study. Participants were presented with three daily meals and a continuous supply of snacks and bottled water. | Ad libitum | Ad libitum (689 ± 73 kcal d−1 less energy intake) | Animal-based | Daily capillary BHB: average concentration of 1.8 ± 0.1 mM. Daily urinary excretion of ketones was 1.44 ± 0.06 g d−1. | Daily excretion of urea was lower during the LF diet, as was excretion of ammonia and creatinine. | LF diet led to 689 ± 73 kcal d−1 less energy intake than the LC diet. Body weight decreased during both diets. Total cholesterol, LDL, and HDL were significantly higher in the LC group compared to the LF group. |
| Johnstone et al. [17], RCT | Obesity | 4 weeks | Low-carbohydrate ketogenic diet (LC): Carbohydrate 4%, fat 66%, protein 30% (Carbohydrate 5%, fat 66%, protein 30%) | Medium-carbohydrate nonketogenic diet (MC): Carbohydrate 35%, fat 35%, protein 30% (Carbohydrate 36%, fat 34%, protein 30%) | Food was provided daily. Food was weighed before and after consumption to measure intake. Subjects completed food diaries. | 1732 kcal | 1900 kcal | Mostly saturated fats | Mean 3-hydroxybutyrate concentrations: 1.52 mmol/L in plasma and 2.99 mmol/L in urine. All subjects became ketotic after 1–3 d of the LC diet and remained so for the duration of the dietary period. | No significant alteration in plasma urea. | Energy intake and hunger was significantly lower and weight loss was significantly greater with the LC diet. |
| Kackley et al. [18], RCT | Overweight/Obesity | 6 weeks | Ketogenic diet (KD): Carbohydrate 40 g/day (Carbohydrate 9%, fat 67%, protein 23%) | Low-fat diet (LFD): Fat 25% (Carbohydrate 55%, fat 24%, protein 21%) | All the food was prepared in a metabolic kitchen and 100% of the food containers were empty for both the LFD and KD when returned to the testing facility. Daily blood ketones were measured. | 1752 ± 350 | 1900 ± 296 | Emphasis on monounsaturated and saturated fat sources | KD elevated R-βHB into the range of nutritional ketosis (>0.5 mM R-βHB) throughout the experiment. | No data. | Both diets elicited clinically significant weight loss and improved insulin sensitivity and serum lipids. Fasting plasma glucose and inflammatory markers were not different between diets. |
| Sun et al. [19], RCT | Overweight | 4 weeks | Low-carbohydrate diet (LC): Carbohydrate 10%, fat 65%, protein 25% (Carbohydrate 9.3 ± 5.5%, fat 68.1 ± 4.6%, protein 22.8 ± 3.2%) | Control diet (CON): “Normal diet” (Carbohydrate 43.1 ± 7.9%, fat 40.2 ± 5.7%, protein 15.9 ± 3.6%) | Food records for 2 weekdays and 1 weekend day. Self-testing reagent strips to measure urinary ketones daily in the early morning or after dinner. Weekly follow-up. | 1776 ± 284 kcal | 1990 ± 345 kcal | Types of fat from saturated or unsaturated sources were not restricted | Urinary ketosis was detected on 97.6 ± 4.5% of the days in the LC group. | No data. | Significant reductions in body weight, BMI, and waist-to-hip ratio in the LC group. Fasting glucose and blood lipid levels remained unchanged in groups. |
| White et al. [20], RCT | Overweight | 14 days | Ketogenic diet: Carbohydrate 5%, fat 65%, protein 30% (No data) | Nonketogenic diet: Carbohydrate 40%, fat 30%, protein 30% (No data) | Participants were served a hot lunch daily Monday through Friday; all other meals and snacks were packaged and consumed at home. Energy intake was strictly controlled. | 70% of that needed for weight maintenance | 70% of that needed for weight maintenance | No data | Beta-hydroxybutyrate concentration at week 2: 0.72 ± 0.18 mmol/L. | Elevations in urinary urea. | No difference in weight loss or reductions in fat mass between groups. Ketogenic diet enhances fatigability and can reduce the desire to exercise. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter, N.; Czina, L.; Murányi, E.; Németh, B.; Varjas, T.; Szendi, K. Is a Meta-Analysis of Clinical Trial Outcomes for Ketogenic Diets Justifiable? A Critical Assessment Based on Systematic Research. Foods 2024, 13, 3219. https://doi.org/10.3390/foods13203219
Hunter N, Czina L, Murányi E, Németh B, Varjas T, Szendi K. Is a Meta-Analysis of Clinical Trial Outcomes for Ketogenic Diets Justifiable? A Critical Assessment Based on Systematic Research. Foods. 2024; 13(20):3219. https://doi.org/10.3390/foods13203219
Chicago/Turabian StyleHunter, Nicole, László Czina, Edit Murányi, Balázs Németh, Tímea Varjas, and Katalin Szendi. 2024. "Is a Meta-Analysis of Clinical Trial Outcomes for Ketogenic Diets Justifiable? A Critical Assessment Based on Systematic Research" Foods 13, no. 20: 3219. https://doi.org/10.3390/foods13203219







