Fucoidan–Vegetable Oil Emulsion Applied to Myosin of Silver Carp: Effect on Protein Conformation and Heat-Induced Gel Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the FU–Vegetable Oil–Myosin Emulsion
2.3. Preparation of the Myosin Gel
2.4. SDS-PAGE
2.5. Measurement of Determination of Turbidity
2.6. Measurement of Particle Size
2.7. Measurement of UV Absorption Spectroscopy
2.8. Measurement of Fluorescence Spectroscopy
2.9. Measurement of Total Sulfhydryl (SH) Content
2.10. Measurement of Surface Hydrophobicity
2.11. Measurement of Dynamic Rheology
2.12. Measurement of Gel Strength and Texture
2.13. Measurement of Whiteness
2.14. Measurement of Water-Holding Capacity (WHC)
2.15. Measurement of Chemical Forces
2.16. Measurement of Raman Spectroscopy
2.17. Measurement of Confocal Laser Scanning Microscopy (CLSM)
2.18. Measurement of Optical Microscopy
2.19. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Emulsion on Morphology and Conformation of Myosin
3.1.1. Protein Patterns
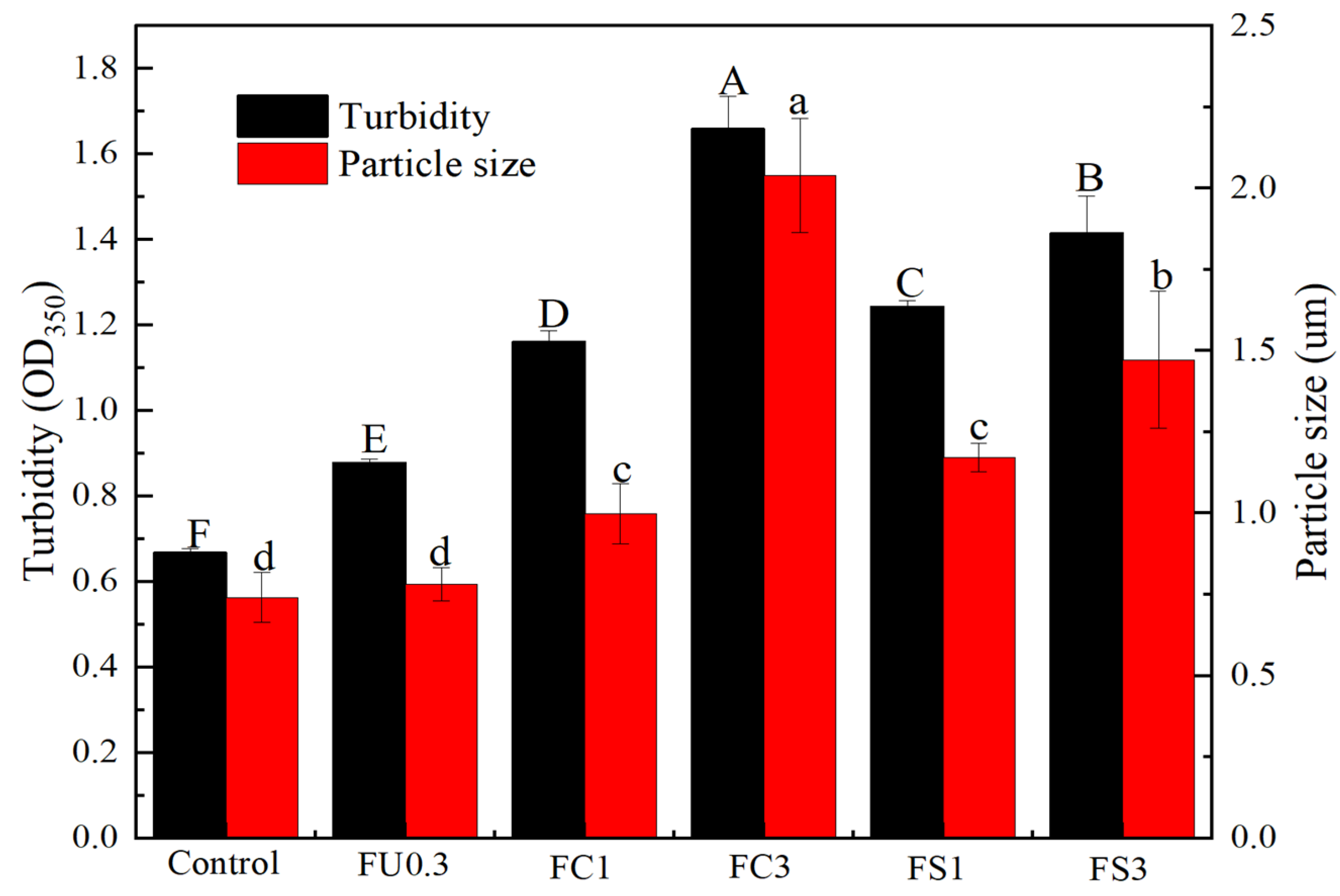
3.1.2. Turbidity
3.1.3. Particle Size
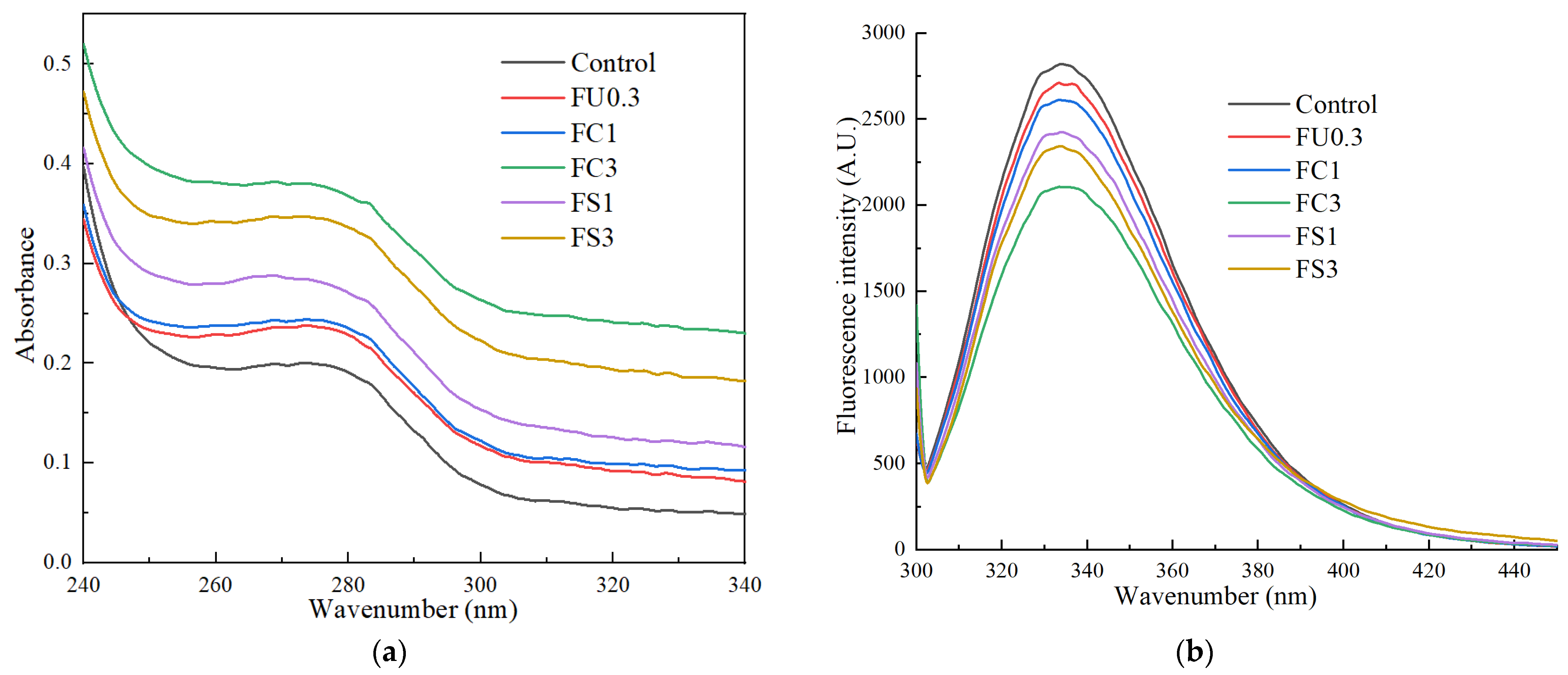
3.1.4. UV Absorption
3.1.5. Intrinsic Fluorescence Spectrum
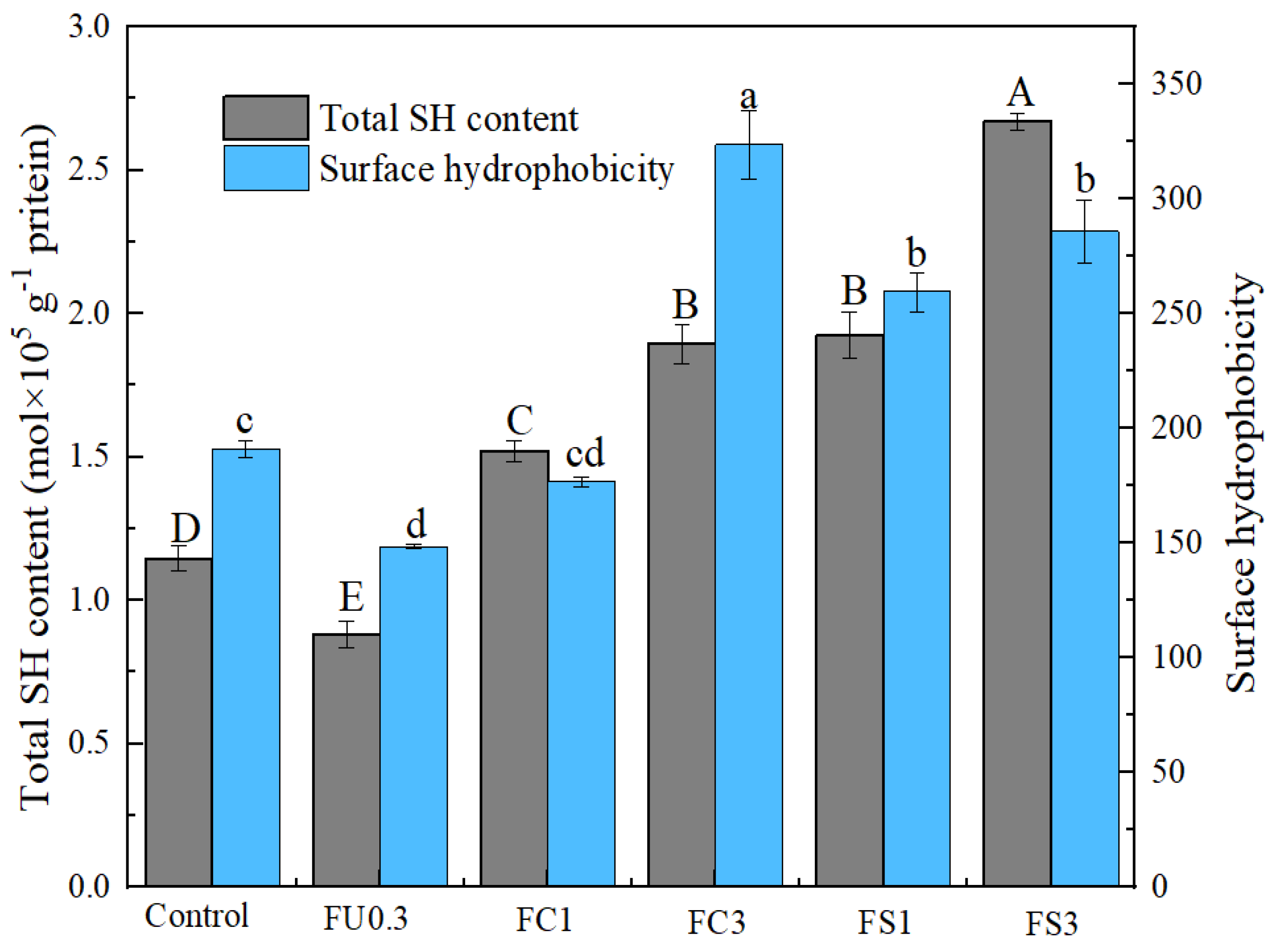
3.1.6. Total Sulfhydryl (SH)
3.1.7. Surface Hydrophobicity
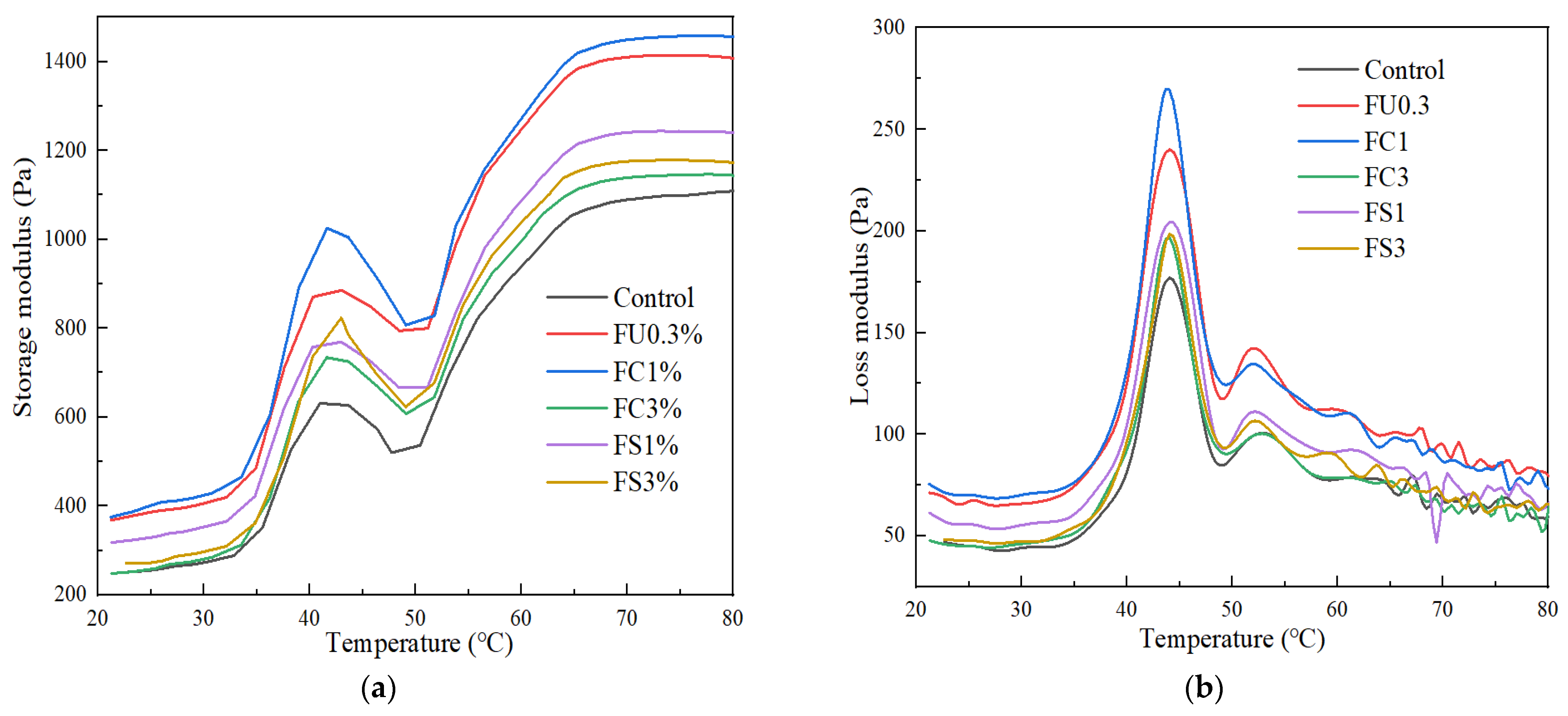
3.1.8. Dynamic Rheometry
3.2. Effect of the Emulsion on the Gel Properties of Myosin
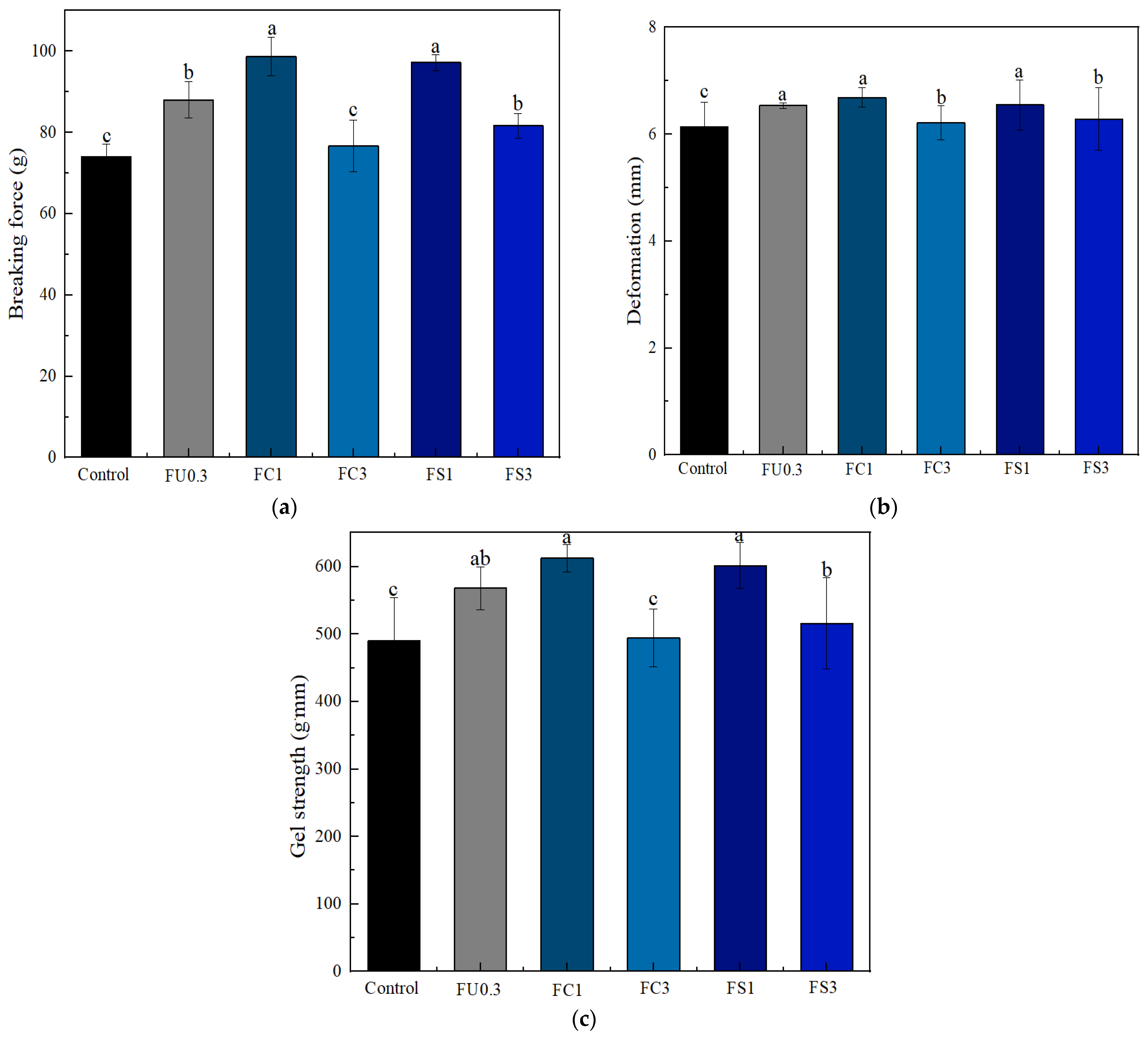
3.2.1. Gel Strength of the Myosin Gel
3.2.2. TPA of Myosin Gel
3.2.3. Whiteness and WHC of the Myosin Gel
3.2.4. Chemical Interactions of Myosin Gel
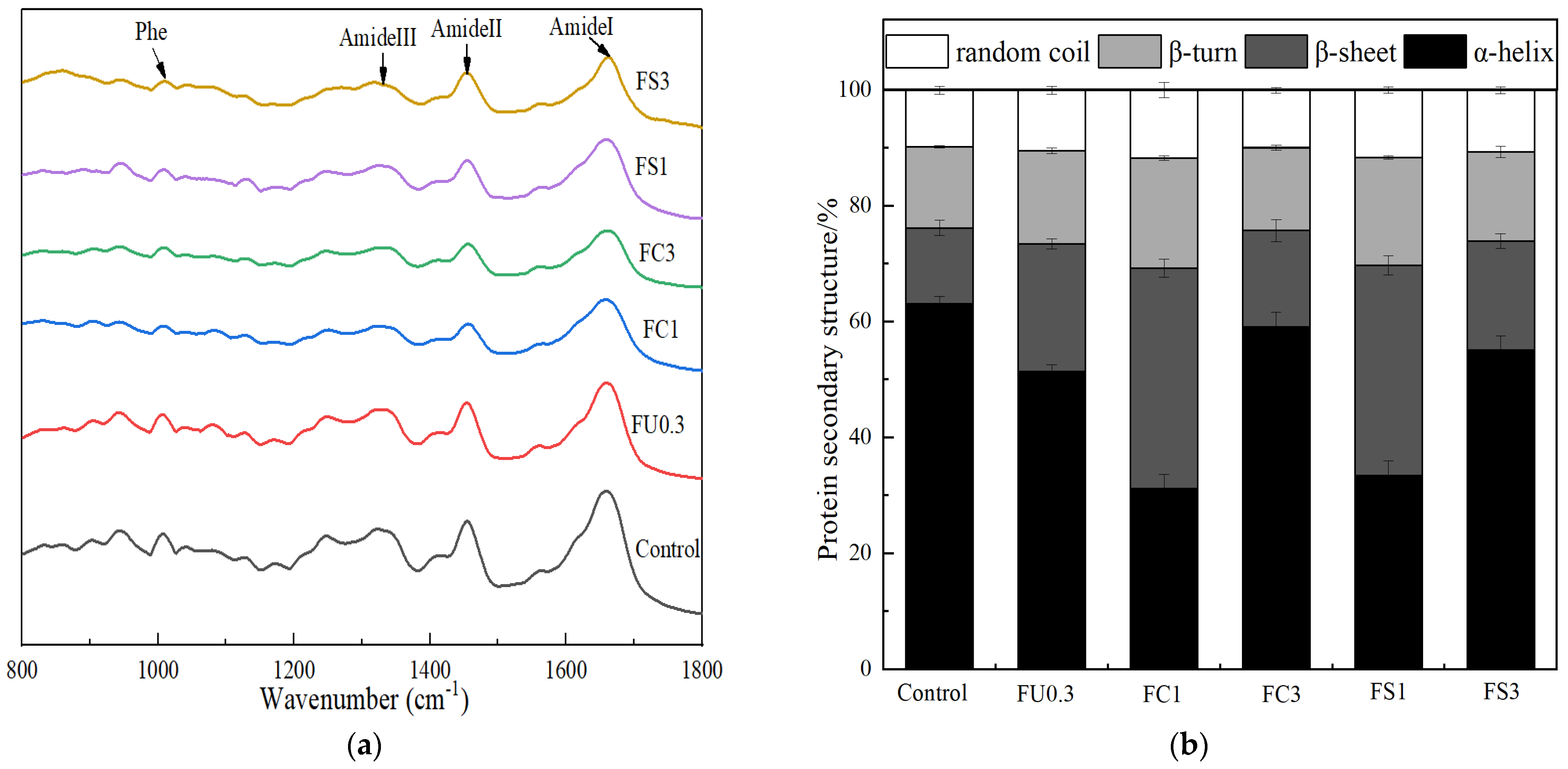
3.2.5. Raman Spectroscopy of the Myosin Gel
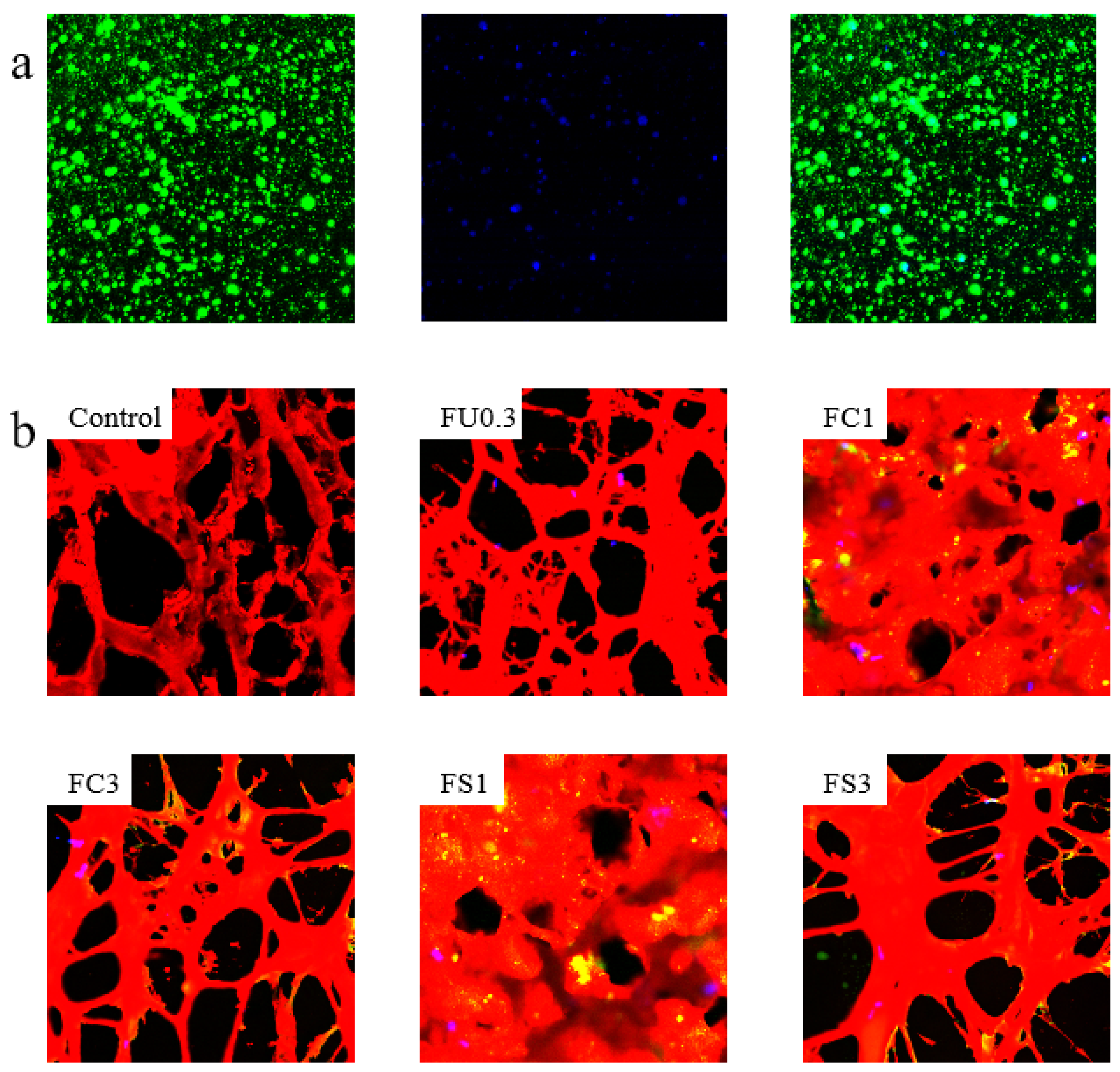
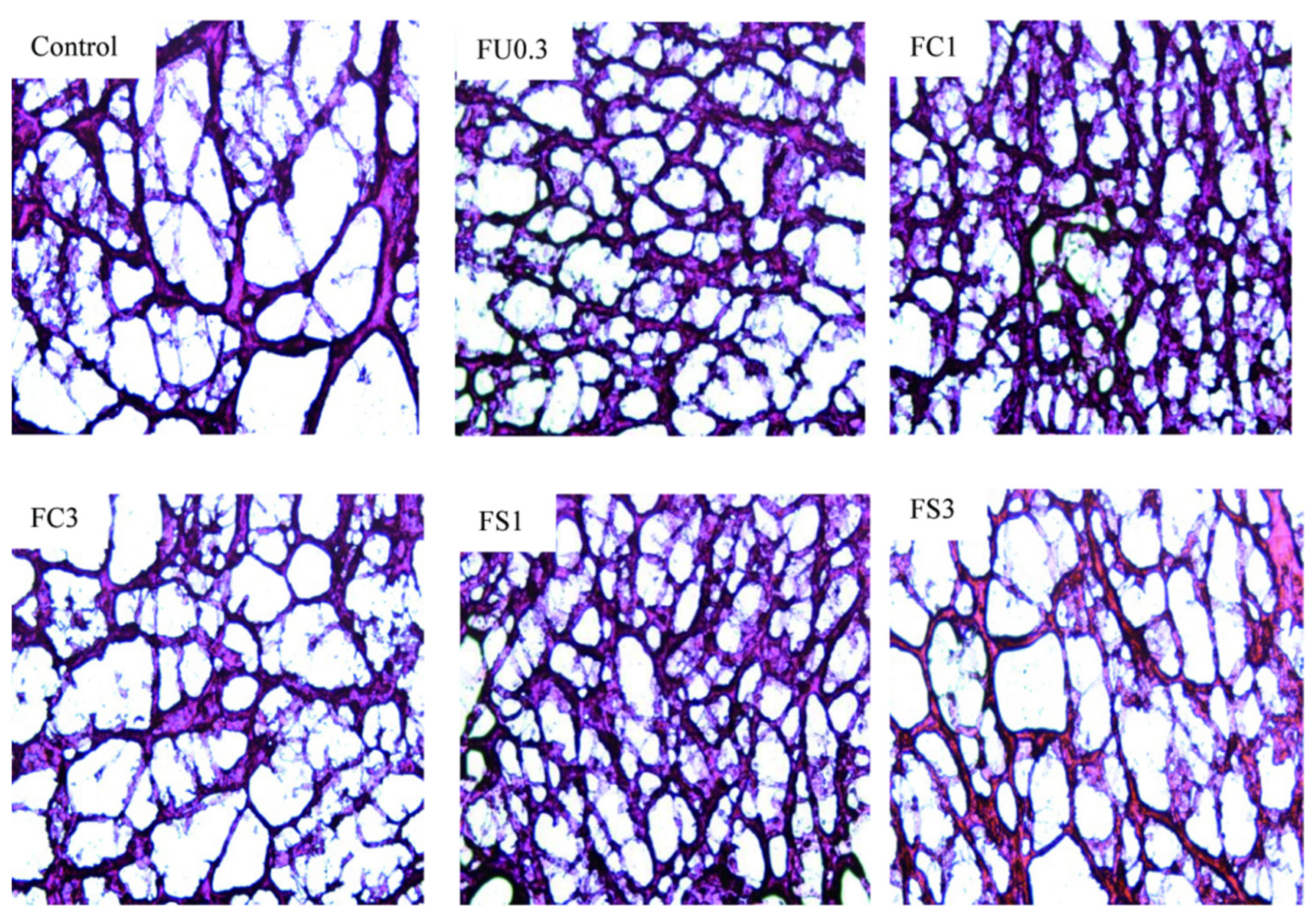
3.2.6. Microstructure of Myosin Gels
Examination by Confocal Laser Scanning Microscopy (CLSM)
Examination by Light Microscopic Observation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.X.; Jiang, S.; Zhao, D.D.; Zhang, J.Y.; Gu, S.Q.; Pan, Z.Y.; Ding, Y.T. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Yang, Y.L.; Meng, L.L.; Wang, Y.X.; Yan, B.W. Effects of exogenous lipids on gelling properties of silver carp surimi gel subjected to microwave heating. Food Sci. Nutr. 2022, 10, 4296–4307. [Google Scholar] [CrossRef]
- Jiao, X.D.; Cao, H.W.; Fan, D.M.; Huang, J.L.; Zhao, J.X.; Yan, B.W.; Zhou, W.G.; Zhang, W.H.; Ye, W.J.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Zhang, L.; Li, S.; Cheng, Q.; Zhu, B.; Dong, X. Effects of pork fat and linseed oil as additives on gel quality of fish cake. J. Texture Stud. 2023, 54, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, X.F.; Chang, T.; Wang, C.J.; Yang, H.; Cui, M. Effects of vegetable oils on gel properties of surimi gels. LWT Food Sci. Technol. 2014, 57, 586–593. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Jolliet, O.; Meijaard, E.; Slavin, J.; Rasetti, M.; Aleta, A.; Moreno, Y.; Agostoni, C. Sustainable nutrition and the case of vegetable oils to match present and future dietary needs. Front. Public Health 2023, 11, 1106083. [Google Scholar] [CrossRef]
- Shin, D.-M.; Yune, J.H.; Kim, T.-K.; Kim, Y.J.; Kwon, H.C.; Kim, D.H.; Jeong, C.H.; Choi, Y.-S.; Han, S.G. Physicochemical properties and oxidative stability of duck fat-added margarine for reducing the use of fully hydrogenated soybean oil. Food Chem. 2021, 363, 130260. [Google Scholar] [CrossRef]
- Gao, L.; Jin, L.; Liu, Q.; Zhao, K.; Lin, L.; Zheng, J.; Li, C.; Chen, B.; Shen, Y. Recent advances in the extraction, composition analysis and bioactivity of Camellia (Camellia oleifera Abel.) oil. Trends Food Sci. Technol. 2024, 143, 104211. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S.; Smith, A. Effects of pre-emulsifying fat/oil on meat batter stability, texture and microstructure. Int. J. Food Sci. Technol. 2011, 46, 1216–1224. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Chuai, P.; Jiang, Z.; Zhu, Y.; Zhang, B.; Ni, H.; Li, Q. Effects of crude fucoidan on physicochemical properties, antioxidation and bacteriostasis of surimi products. Food Control 2021, 122, 107806. [Google Scholar] [CrossRef]
- Yao, Y.; Zaw, A.M.; Anderson, D.E.J.; Hinds, M.T.; Yim, E.K.F. Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility. Biomaterials 2020, 249, 120010. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, X.; Qiao, M.; Yi, S.; Li, X.; Li, J. Effects of chickpea and peanut protein isolates on the gelling properties of hairtail (Trichiurus haumela) myosin. LWT Food Sci. Technol. 2022, 163, 113562. [Google Scholar] [CrossRef]
- Yi, S.; Wu, Q.; Tong, S.; Wang, W.; Li, X.; Mi, H.; Xu, Y.; Li, J. Thermal aggregation behavior of egg white protein and blue round scad (Decapterus maruadsi) myofibrillar protein. J. Food Sci. 2022, 87, 3900–3912. [Google Scholar] [CrossRef]
- Mi, H.; Liang, S.; Li, Z.; Yi, S.; Chen, J.; Li, X.; Li, J. Effect of corn starch on the structure, physicochemical and gel properties of hairtail (Trichiurus haumela) myosin. Int. J. Food Sci. Technol. 2020, 56, 2843–2852. [Google Scholar] [CrossRef]
- Xu, H.; Yang, L.; Jin, J.; Zhang, J.; Xie, P.; Chen, Y.; Shi, L.; Wei, W.; Jin, Q.; Wang, X. Elucidation on the destabilization mechanism of whipping creams during static storage. Food Hydrocoll. 2022, 129, 107613. [Google Scholar] [CrossRef]
- Yi, S.; Ye, B.; Li, J.; Wang, W.; Li, X. Physicochemical properties, protein conformation, and aggregate morphology of heated myosin from Hypophthalmichthys molitrix and Nemipterus virgatus mixtures. Food Front. 2020, 1, 473–483. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Q.; Chen, L.; Chen, N.; Gao, H. Interaction and action mechanism of quercetin and myofibrillar protein and its effects on the quality of cured meat. J. Food Process. Preserv. 2021, 45, e16020. [Google Scholar] [CrossRef]
- Wei, L.; Cao, L.; Xiong, S.; You, J.; Hu, Y.; Liu, R. Effects of pH on self-assembly of silver carp myosin at low temperature. Food Biosci. 2019, 30, 100420. [Google Scholar] [CrossRef]
- Cheng, W.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Cao, Y.; Li, B.; Fan, X.; Wang, J.; Zhu, Z.; Huang, J.; Xiong, Y.L. Synergistic recovery and enhancement of gelling properties of oxidatively damaged myofibrillar protein by -lysine and transglutaminase. Food Chem. 2021, 358, 129860. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of high intensity ultrasound on gelation properties of silver carp surimi with different salt contents. Ultrason. Sonochemistry 2021, 70, 105326. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Yu, Y.; Wei, Y.; Tan, H.; Tang, M.; Ma, L.; Zhang, Y. Adjusting the interfacial property and emulsifying property of cellulose nanofibrils by ultrasonic treatment combined with gelatin addition. Food Hydrocoll. 2022, 133, 107905. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Xu, X.; Zhuang, X.; Chen, Y.; Xue, Y.; Xue, C.; Jiang, N. Effects of konjac glucomannan on physical properties and microstructure of fish myofibrillar protein gel: Phase behaviours involved. Food Hydrocoll. 2023, 134, 108034. [Google Scholar] [CrossRef]
- Huang, J.; Ye, B.; Wang, W.; Li, J.; Yi, S.; Li, X.; Xu, Y.; Mi, H. Incorporation effect of inulin and microbial transglutaminase on the gel properties of silver carp (Hypophthalmichthys molitrix) surimi. J. Food Meas. Charact. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Combination effect of high pressure treatment and ethanolic extract from coconut husk on gel properties of sardine surimi. Lebensm. Wiss. Technol. 2018, 91, 361–367. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Pan, N.; Wang, S.; Xia, X.; Zhang, D. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Zhou, X.; Gao, K.; Liu, X. Interactions of heat-induced myosin with hsian-tsao polysaccharide to affect the fishy odor adsorption capacity. Food Hydrocoll. 2023, 136, 108282. [Google Scholar] [CrossRef]
- Teng, H.; He, Y.; Fu, L.; Xiong, H.; Lu, M.; Zhang, C.; Ai, C.; Cao, H.; Zhong, S.; Chen, L. Effects of blackberry (Rubus spp.) polysaccharide on the structure and thermal behavior of the myofibrillar protein of chicken breast meat. Food Chem. X 2023, 20, 100914. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Wu, D.; Gong, H.; Guo, L.; Ma, J.; Sun, W. Study on the interaction and gel properties of pork myofibrillar protein with konjac polysaccharides. J. Sci. Food Agric. 2024, 104, 2284–2293. [Google Scholar] [CrossRef]
- Feng, X.; Sun, Y.; Tan, H.; Ma, L.; Dai, H.; Zhang, Y. Effect of oil phases on the stability of myofibrillar protein microgel particles stabilized Pickering emulsions: The leading role of viscosity. Food Chem. 2023, 413, 135653. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Zaaboul, F.; Sun, Y.; Tan, C.-P.; Liu, Y. Synergistic effect of hydrophilic polyglycerol fatty acid esters and protein on the stability of interfacial membrane in low-fat aerated emulsions with different homogenization conditions. Food Chem. 2024, 435, 137584. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, J.D.; Ricaurte, L.; Estrada, K.B.; Quintanilla-Carvajal, M.X. Effect of homogenization methods on the physical stability of nutrition grade nanoliposomes used for encapsulating high oleic palm oil. Lebensm. Wiss. Technol. 2020, 118, 108801. [Google Scholar] [CrossRef]
- Casals, E.; Galán, A.M.a.; Escolar, G.; Gallardo, M.; Estelrich, J. Physical stability of liposomes bearing hemostatic activity. Chem. Phys. Lipids 2003, 125, 139–146. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, R.; Tu, M.; Zhao, J. A novel biomimetic chitosan-based nanocarrier with suppression of the protein-nanocarrier interactions. Mater. Lett. 2012, 77, 38–40. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Yin, Y.; Zhang, W.; Yang, Y. Effects of ultrasound-assisted emulsification on the emulsifying and rheological properties of myofibrillar protein stabilized pork fat emulsions. Foods 2021, 10, 1201. [Google Scholar] [CrossRef]
- Martinez, K.; Carrerasanchez, C.; Pizonesruizhenestrosa, V.; Rodriguezpatino, J.; Pilosof, A. Soy protein–polysaccharides interactions at the air–water interface. Food Hydrocoll. 2007, 21, 804–812. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Kotwal, S. Influence of high pressure on conformational changes of soybean glycinin. Innov. Food Sci. Emerg. Technol. 2003, 4, 269–275. [Google Scholar] [CrossRef]
- Jia, N.; Wang, L.; Shao, J.; Liu, D.; Kong, B. Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 2017, 127, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, X.; Li, L. Physicochemical properties and microstructure of soybean protein isolate-vegetable oil complex gels induced by lactic acid bacteria: Effects of vegetable oil types and concentrations. Food Hydrocoll. 2023, 145, 109109. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of interactions between gelatin aggregates and hsian-tsao gum in aqueous solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Wang, J.; Wei, S.; Li, S. Effects of low fat addition on chicken myofibrillar protein gelation properties. Food Hydrocoll. 2019, 90, 126–131. [Google Scholar] [CrossRef]
- Romani, V.P.; Machado, A.V.; Olsen, B.D.; Martins, V.G. Effects of pH modification in proteins from fish (Whitemouth croaker) and their application in food packaging films. Food Hydrocoll. 2018, 74, 307–314. [Google Scholar] [CrossRef]
- Marchetti, L.; Muzzio, B.; Cerrutti, P.; Andrés, S.C.; Califano, A.N. Bacterial nanocellulose as novel additive in low-lipid low-sodium meat sausages. Effect on quality and stability. Food Struct. 2017, 14, 52–59. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Zhang, X.-h.; Li, K.; Li, Y.-p.; Lu, F.; Ma, H.-j.; Song, Z.-j.; Zhao, S.-m.; Zhu, M.-m. Effects of sodium bicarbonate on the gel properties, water distribution and mobility of low-salt pork batters. Lebensm. Wiss. Technol. 2021, 139, 110567. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Effects of emulsified lard and TGase on gel properties of threadfin bream (Nemipterus virgatus) surimi. Lebensm. Wiss. Technol. 2021, 146, 111513. [Google Scholar] [CrossRef]
- Wu, D.; Xiong, J.; Li, P.; Zhang, Y.; Li, F.; Yin, T.; Huang, Q. Dual enhancement effects of different yeast extract on gel properties and saltiness perception of low-salt surimi gel from silver carp. Food Hydrocoll. 2024, 152, 109925. [Google Scholar] [CrossRef]
- Zhao, Y.; Piao, X.; Zheng, B.; Gao, P.; Miao, W.; Wen, Z.; Zhang, X.; Mei, G.; Zhou, R.; Deng, S. Enhancement of surimi gel properties through the synergetic effect of fucoidan and oligochitosan. Food Hydrocoll. 2023, 140, 108626. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, W.; Liang, Q.; Jiang, X.; Zhang, Z.; Shi, W. High inner phase emulsion of fish oil stabilized with rutin-grass carp (Ctenopharyngodon idella) myofibrillar protein: Application as a fat substitute in surimi gel. Food Hydrocoll. 2023, 145, 109115. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Xu, Y.; Mi, H.; Yi, S.; Gao, R.; Li, X.; Li, J. Effects of chickpea protein-stabilized Pickering emulsion on the structure and gelling properties of hairtail fish myosin gel. Food Chem. 2023, 417, 135821. [Google Scholar] [CrossRef]
- Panpipat, W.; Cheong, L.Z.; Chaijan, M. Impact of lecithin incorporation on gel properties of bigeye snapper (Priacanthus tayenus) surimi. Int. J. Food Sci. Technol. 2020, 56, 2481–2491. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Ding, N.; Liang, S.; Mubango, E.; Zheng, Y.; Yu, Q.; Dai, R.; Tan, Y.; Luo, Y.; et al. Cryoprotective effect of fistular onion stalk polysaccharide on frozen surimi derived from bighead carp: Physicochemical properties and gel quality during storage. Food Hydrocoll. 2024, 148, 109404. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Bai, Y.; Liu, Y.; Xing, L.; Xu, X.-l.; Zhou, G.-h. Insight into the mechanism of myofibrillar protein gel improved by insoluble dietary fiber. Food Hydrocoll. 2018, 74, 219–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohydr. Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Tong, Y.; Zhong, Q.; Zhuang, Y.; Yang, H. Physicochemical properties of surimi gel from silver carp as affected by ultrasonically emulsified vegetable oils. Int. J. Food Prop. 2023, 26, 1214–1229. [Google Scholar] [CrossRef]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Wu, M.; Hu, J.; Gu, X.; Wang, Q.; Wei, R.; Wang, J.; Li, Z.; Liu, R.; Ge, Q.; Yu, H. Myofibrillar protein composite gels: Effect of esterified potato starch, lard and peanut oil on the gel properties. J. Sci. Food Agric. 2021, 102, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]


| Sample | Hardness (g) | Springiness (%) | Cohesiveness (g) | Chewiness (g) |
|---|---|---|---|---|
| Control | 343.88 ± 4.57 d | 0.91 ± 0.03 b | 0.62 ± 0.02 b | 193.11 ± 4.03 c |
| FU0.3 | 387.41 ± 3.26 b | 0.92 ± 0.01 b | 0.65 ± 0.01 ab | 230.42 ± 3.67 b |
| FC1 | 406.80 ± 5.90 a | 0.94 ± 0.01 a | 0.66 ± 0.01 a | 252.75 ± 8.45 a |
| FC3 | 364.30 ± 2.29 c | 0.91 ± 0.01 b | 0.62 ± 0.01 b | 213.53 ± 4.17 b |
| FS1 | 399.33 ± 9.66 ab | 0.93 ± 0.01 ab | 0.65 ± 0.02 ab | 249.72 ± 4.12 a |
| FS3 | 368.02 ± 3.22 c | 0.91 ± 0.02 b | 0.62 ± 0.01 b | 212.87 ± 5.91 b |
| Sample | L* | a* | b* | Whiteness | WHC (%) |
|---|---|---|---|---|---|
| Control | 84.05 ± 0.57 a | −1.86 ± 0.04 d | −4.14 ± 0.49 c | 83.40 ± 0.63 ab | 44.82 ± 3.87 b |
| FU0.3 | 83.21 ± 0.71 b | −1.55 ± 0.26 bc | −2.64 ± 0.80 ab | 82.91 ± 0.72 b | 52.90 ± 2.69 a |
| FC1 | 83.54 ± 0.11 ab | −1.32 ± 0.29 a | −2.94 ± 0.38 ab | 83.22 ± 0.05 ab | 53.46 ± 2.68 a |
| FC3 | 83.99 ± 0.68 a | −1.56 ± 0.12 bc | −2.24 ± 0.51 a | 83.75 ± 0.70 a | 47.78 ± 3.91 b |
| FS1 | 83.76 ± 0.11 ab | −1.49 ± 0.01 b | −3.1 ± 0.24 b | 83.40 ± 0.12 ab | 53.31 ± 1.93 a |
| FS3 | 84.02 ± 0.22 a | −1.69 ± 0.12 c | −2.68 ± 0.39 ab | 83.70 ± 0.70 a | 48.83 ± 3.52 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, L.; Yi, S. Fucoidan–Vegetable Oil Emulsion Applied to Myosin of Silver Carp: Effect on Protein Conformation and Heat-Induced Gel Properties. Foods 2024, 13, 3220. https://doi.org/10.3390/foods13203220
Wang W, Yan L, Yi S. Fucoidan–Vegetable Oil Emulsion Applied to Myosin of Silver Carp: Effect on Protein Conformation and Heat-Induced Gel Properties. Foods. 2024; 13(20):3220. https://doi.org/10.3390/foods13203220
Chicago/Turabian StyleWang, Wei, Lijuan Yan, and Shumin Yi. 2024. "Fucoidan–Vegetable Oil Emulsion Applied to Myosin of Silver Carp: Effect on Protein Conformation and Heat-Induced Gel Properties" Foods 13, no. 20: 3220. https://doi.org/10.3390/foods13203220




