Recent Advances in the CRISPR/Cas-Based Nucleic Acid Biosensor for Food Analysis: A Review
Abstract
1. Introduction
2. The CRISPR/Cas Systems
2.1. CRISPR-Cas9
2.2. CRISPR-Cas 12
2.3. CRISPR-Cas 13
2.4. CRISPR-Cas 14
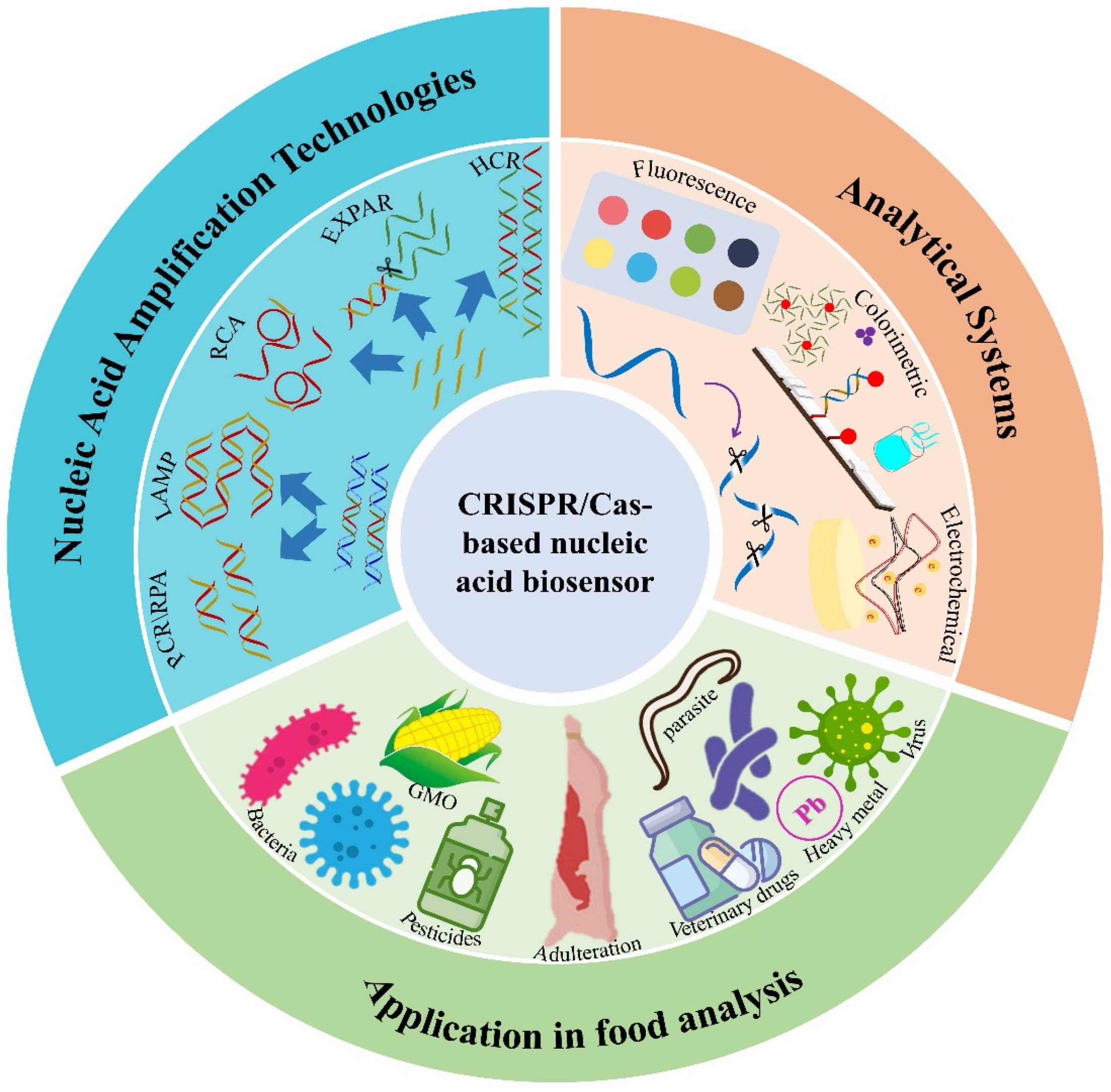
3. Combination with Nucleic Acid Amplification Technologies (NAATs)
3.1. Polymerase Chain Reaction
3.2. Loop-Mediated Isothermal Amplification
3.3. Recombinase Polymerase Amplification
3.4. Nucleic Acid Sequence-Based Amplification
3.5. Rolling Circle Amplification
3.6. Strand Displacement Amplification
3.7. Hybridization Chain Reaction
4. CRISPR/Cas Analytical Systems
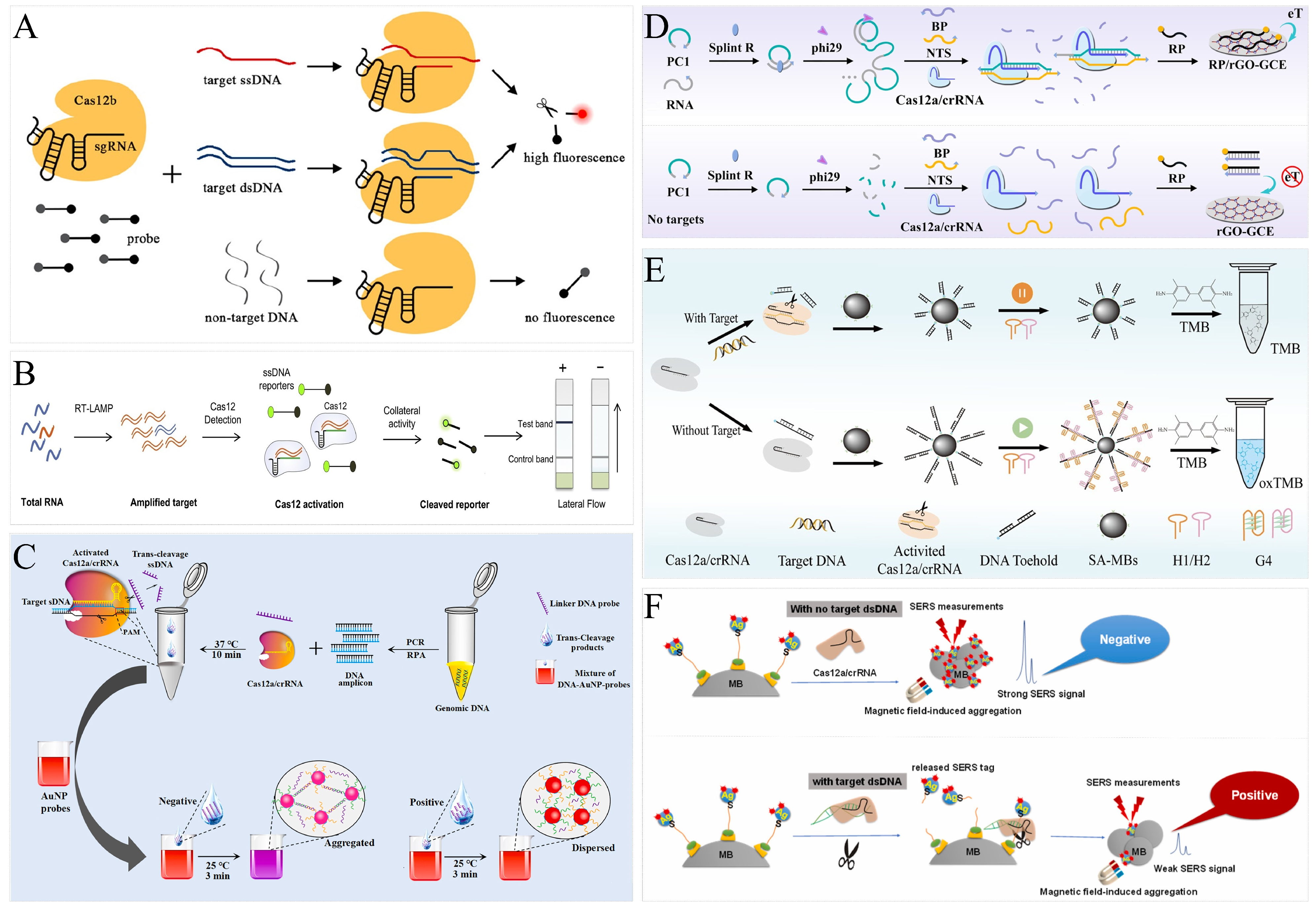
4.1. Fluorescence Analytical Systems
4.2. Colorimetric Analytical Systems
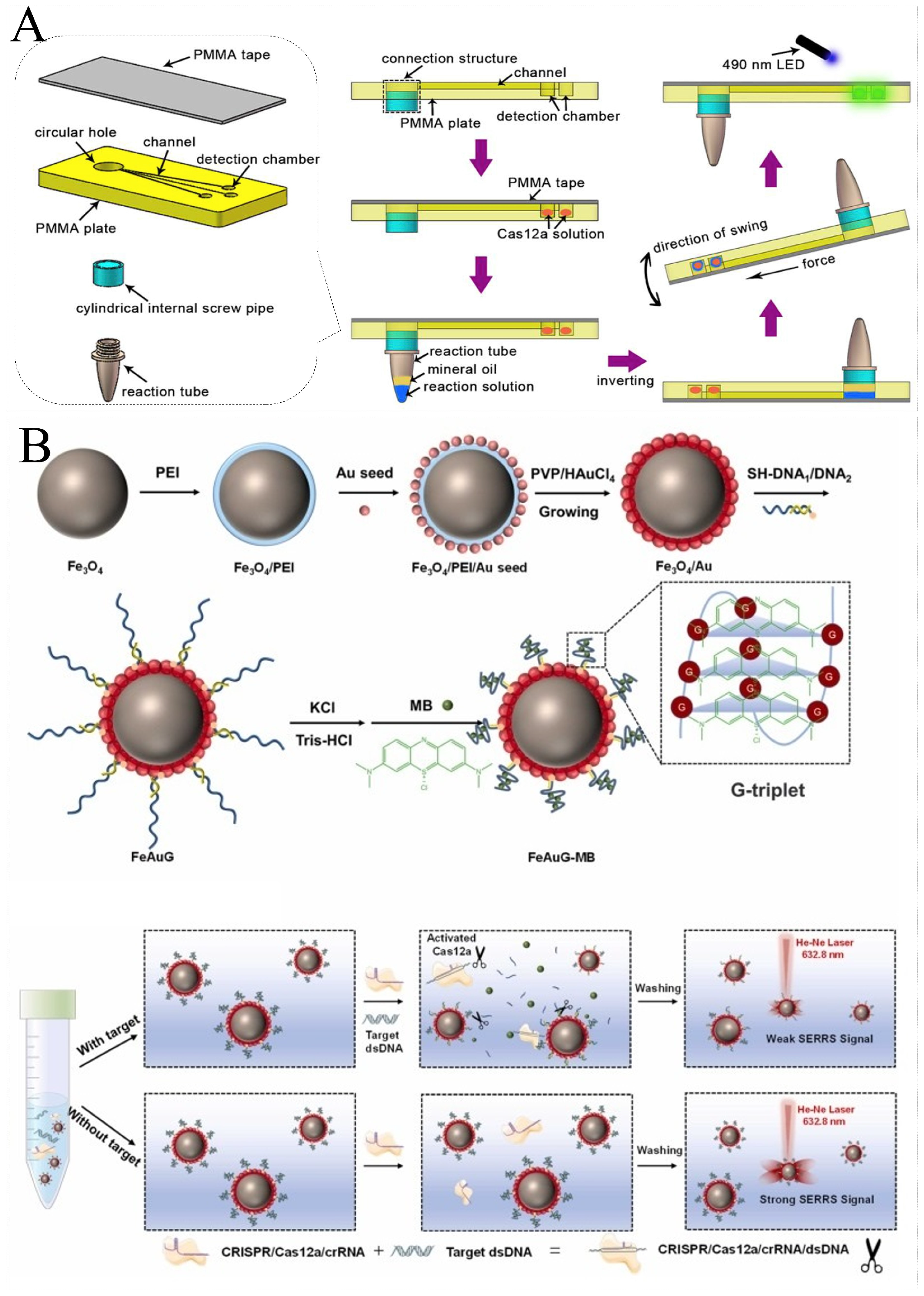
4.3. Electrochemical Analytical Systems
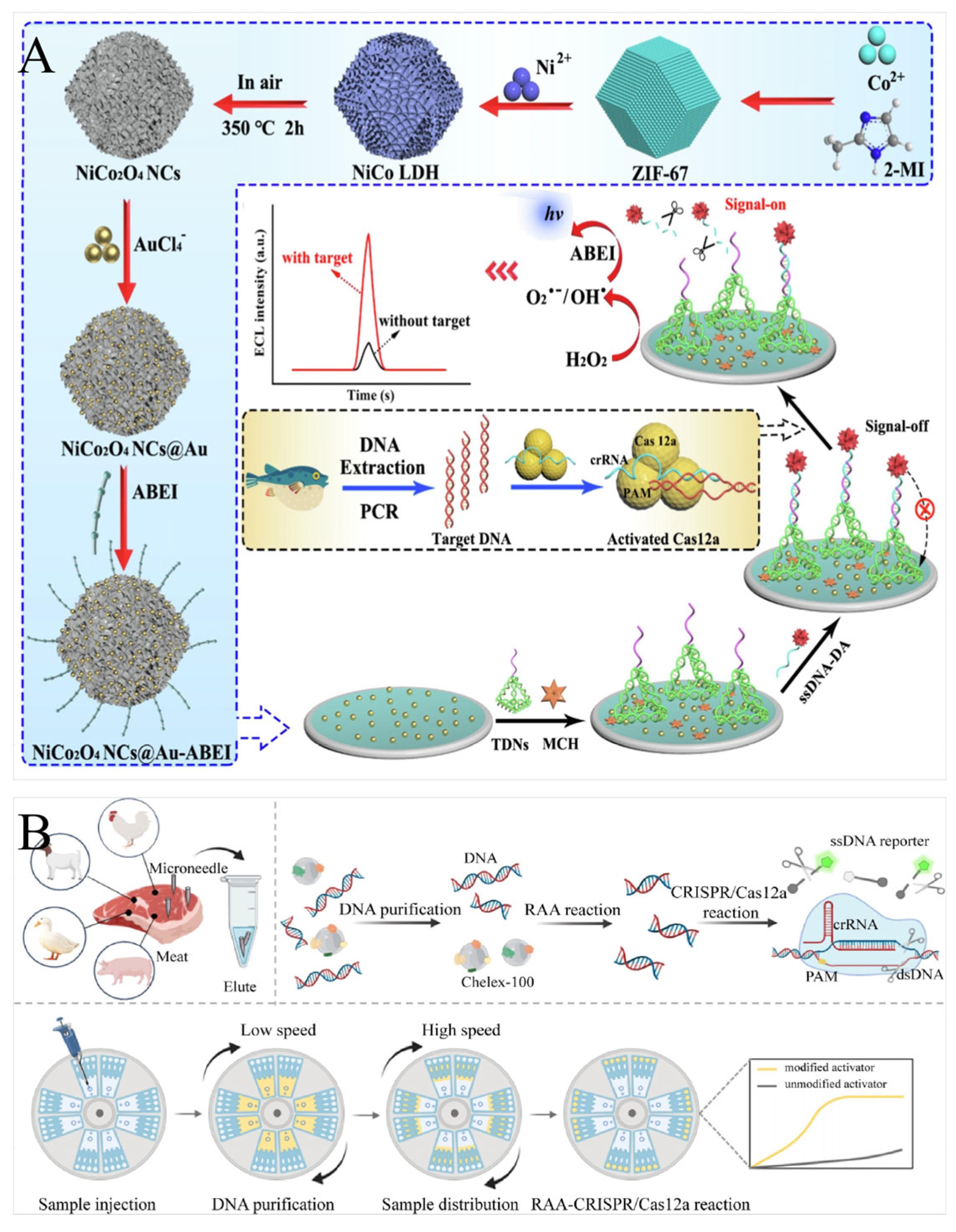
4.4. Other Analytical Systems
5. Applications in Rapid Food Analysis
5.1. Detection of Foodborne Pathogens
5.2. Detection of Biotech Foods
5.3. Detection of Food Adulteration
5.4. Detection of Biotoxins
5.5. Detection of Heavy Metal Ions
5.6. Detection of Pesticides and Veterinary Drugs
5.7. Detection of Others
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Yoon, Y. Etiological agents implicated in foodborne illness world wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Guo, L.; Xu, X.; Liu, L.; Xu, L.; Song, S.; Xu, C.; Kuang, H. Advances in immunoassays for organophosphorus and pyrethroid pesticides. TrAC Trends Anal. Chem. 2020, 131, 116022. [Google Scholar] [CrossRef]
- Ravariu, C. From Enzymatic Dopamine Biosensors to OECT Biosensors of Dopamine. Biosensors 2023, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lan, X.; Zhu, L.; Ru, P.; Liu, H.; Xu, W. Nucleic acid-aided molecular amplification techniques for food microorganism detection. TrAC Trends Anal. Chem. 2023, 165, 117116. [Google Scholar] [CrossRef]
- Kumar, Y. Isothermal amplification-based methods for assessment of microbiological safety and authenticity of meat and meat products. Food Control 2021, 121, 107679. [Google Scholar] [CrossRef]
- Rakkhun, W.; Jantra, J.; Cheubong, C.; Teepoo, S. Colorimetric test strip cassette readout with a smartphone for on-site and rapid screening test of carbamate pesticides in vegetables. Microchem. J. 2022, 181, 107837. [Google Scholar] [CrossRef]
- Fan, K.; Wang, X.; Yang, H.; Gao, L.; Han, G.; Zhou, L.; Fang, S. Semiquantitative naked-eye detection of Cu (ii) with a standard colorimetric card via a hydrogel-coated paper sensor. Anal. Methods 2020, 12, 1561–1566. [Google Scholar] [CrossRef]
- Nirbhaya, V.; Chauhan, D.; Jain, R.; Chandra, R.; Kumar, S. Nanostructured graphitic carbon nitride based ultrasensing electrochemical biosensor for food toxin detection. Bioelectrochem. Bioenerg. 2021, 139, 107738. [Google Scholar] [CrossRef]
- Chakraborty, J.; Chaudhary, A.A.; Khan, S.-U.-D.; Rudayni, H.A.; Rahaman, S.M.; Sarkar, H. CRISPR/Cas-based biosensor as a new age detection method for pathogenic bacteria. ACS Omega 2022, 7, 39562–39573. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Zhang, M.; Wang, X.; Chen, Y.; Qian, C.; Wu, J.; Xu, J. Versatile detection with CRISPR/Cas system from applications to challenges. TrAC Trends Anal. Chem. 2021, 135, 116150. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Bonini, A.; Poma, N.; Vivaldi, F.; Kirchhain, A.; Salvo, P.; Bottai, D.; Tavanti, A.; Di Francesco, F. Advances in biosensing: The CRISPR/Cas system as a new powerful tool for the detection of nucleic acids. J. Pharmaceut. Biomed. 2021, 192, 113645. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Hess, G.T.; Frésard, L.; Han, K.; Lee, C.H.; Li, A.; Cimprich, K.A.; Montgomery, S.B.; Bassik, M.C. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 2016, 13, 1036–1042. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jinek, M. Mechanistic Insights into the cis-and trans-Acting DNase Activities of Cas12a. Mol. Cell 2019, 73, 589–600.e584. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Ji, Q. Miniature CRISPR-Cas12 Systems: Mechanisms, Engineering, and Genome Editing Applications. ACS Chem. Biol. 2024, 19, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Chen, R.; Wang, X.; Zhou, Z.; Peng, Y.; Li, S.; Han, D.; Li, S.; Wang, Y.; Han, T. CRISPR/Cas12a-based technology: A powerful tool for biosensing in food safety. Trends Food Sci. Technol. 2022, 122, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Strecker, J.; Jones, S.; Koopal, B.; Schmid-Burgk, J.; Zetsche, B.; Gao, L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. Engineering of CRISPR-Cas12b for human genome editing. Nat. Commun. 2019, 10, 212. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, Y.H.; Jang, Y.; Yu, J.; Goo, J.; Lee, G.; Jeong, Y.K.; Lee, S.H.; Kim, I.-S.; Kim, J.-S. Direct observation of DNA target searching and cleavage by CRISPR-Cas12a. Nat. Commun. 2018, 9, 2777. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Peng, S.; Xie, S.; Lei, C.; Huang, Y.; Nie, Z. PAM-less conditional DNA substrates leverage trans-cleavage of CRISPR-Cas12a for versatile live-cell biosensing. Chem. Sci. 2022, 13, 2011–2020. [Google Scholar] [CrossRef]
- Sun, A.; Li, C.-P.; Chen, Z.; Zhang, S.; Li, D.-Y.; Yang, Y.; Li, L.-Q.; Zhao, Y.; Wang, K.; Li, Z. The compact Casπ (Cas12l)‘ bracelet’ provides a unique structural platform for DNA manipulation. Cell Res. 2023, 33, 229–244. [Google Scholar] [CrossRef]
- Deng, X.; Osikpa, E.; Yang, J.; Oladeji, S.J.; Smith, J.; Gao, X.; Gao, Y. Structural basis for the activation of a compact CRISPR-Cas13 nuclease. Nat. Commun. 2023, 14, 5845. [Google Scholar] [CrossRef]
- Anderson, K.M.; Poosala, P.; Lindley, S.R.; Anderson, D.M. Targeted cleavage and polyadenylation of RNA by CRISPR-Cas13. arXiv 2019. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, R.; Sohail, M.; Kong, X.; Zhang, X.; Fu, N.; Li, B. CRISPR/Cas14 provides a promising platform in facile and versatile aptasensing with improved sensitivity. Talanta 2023, 254, 124120. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. A review on the mechanism and applications of CRISPR/Cas9/Cas12/Cas13/Cas14 proteins utilized for genome engineering. Mol. Biotechnol. 2023, 65, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Cheng, Q.-X.; Wang, J.-M.; Li, X.-Y.; Zhang, Z.-L.; Gao, S.; Cao, R.-B.; Zhao, G.-P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, R.; Qian, C.; Pang, Y.; Wu, J.; Li, F. Ultrafast visual nucleic acid detection with CRISPR/Cas12a and rapid PCR in single capillary. Sens. Actuators B Chem. 2021, 326, 128618. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Zhang, L.; Yang, Y.; Yang, S.; Yang, L.; Chen, H.; Liu, C.; Li, J.; Xie, G. Integration of multiplex PCR and CRISPR-Cas allows highly specific detection of multidrug-resistant Acinetobacter Baumannii. Sens. Actuators B Chem. 2021, 334, 129600. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Wu, N.; Wu, J.; Wang, G.; Zhao, G.; Wang, J. HOLMESv2: A CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth. Biol. 2019, 8, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Sam, I.K.; Chen, Y.-Y.; Ma, J.; Li, S.-Y.; Ying, R.-Y.; Li, L.-X.; Ji, P.; Wang, S.-j.; Xu, J.; Bao, Y.-j. TB-QUICK: CRISPR-Cas12b-assisted rapid and sensitive detection of Mycobacterium tuberculosis. J. Infect. 2021, 83, 54–60. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wang, H.; Wang, F.; Li, Z. CRISPR/Cas12a-assisted ligation-initiated loop-mediated isothermal amplification (CAL-LAMP) for highly specific detection of microRNAs. Anal. Chem. 2021, 93, 7942–7948. [Google Scholar] [CrossRef]
- Qian, C.; Wang, R.; Wu, H.; Zhang, F.; Wu, J.; Wang, L. Uracil-mediated new photospacer-adjacent motif of Cas12a to realize visualized DNA detection at the single-copy level free from contamination. Anal. Chem. 2019, 91, 11362–11366. [Google Scholar] [CrossRef]
- Bao, Y.; Jiang, Y.; Xiong, E.; Tian, T.; Zhang, Z.; Lv, J.; Li, Y.; Zhou, X. CUT-LAMP: Contamination-free loop-mediated isothermal amplification based on the CRISPR/Cas9 cleavage. ACS Sens. 2020, 5, 1082–1091. [Google Scholar] [CrossRef]
- Ali, Z.; Aman, R.; Mahas, A.; Rao, G.S.; Tehseen, M.; Marsic, T.; Salunke, R.; Subudhi, A.K.; Hala, S.M.; Hamdan, S.M. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020, 288, 198129. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ye, Q.; Chen, M.; Wang, C.; Xiang, X.; Li, Y.; Zhang, J.; Zhang, Y.; Wang, J.; Wu, S. A label-free AuNP bioprobe-assisted CRISPR/Cas12a colorimetric platform for high-throughput detection of Staphylococcus aureus ST398. Food Control 2023, 145, 109451. [Google Scholar] [CrossRef]
- Qing, M.; Chen, S.L.; Sun, Z.; Fan, Y.; Luo, H.Q.; Li, N.B. Universal and programmable rolling circle amplification-CRISPR/Cas12a-mediated immobilization-free electrochemical biosensor. Anal. Chem. 2021, 93, 7499–7507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, C.; Jin, M.; Zhou, R.; Wu, Q.; Huang, F.; Lou, Y.; Zheng, L. An Ultrasensitive Colorimetric Foodborne pathogenic detection method using a CRISPR/Cas12a mediated strand Displacement/Hybridization chain reaction. J. Agric. Food Chem. 2023, 71, 4193–4200. [Google Scholar] [CrossRef]
- Wang, H.; Su, A.; Bao, C.; Liang, C.; Xu, W.; Chang, J.; Xu, S. A CRISPR/Cas12a-SERS platform for amplification-free detection of African swine fever virus genes. Talanta 2024, 267, 125225. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef]
- Arizti-Sanz, J.; Freije, C.A.; Stanton, A.C.; Boehm, C.K.; Petros, B.A.; Siddiqui, S.; Shaw, B.M.; Adams, G.; Kosoko-Thoroddsen, T.-S.F.; Kemball, M.E. Integrated sample inactivation, amplification, and Cas13-based detection of SARS-CoV-2. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, B.; Wang, R.; Wang, D.; Wu, J.; Li, J.; Wang, J.; Liu, H.; Wang, Y. Cas12aVDet: A CRISPR/Cas12a-based platform for rapid and visual nucleic acid detection. Anal. Chem. 2019, 91, 12156–12161. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Shen, H.; Lu, Q.; Li, B.; Liu, X.; Gao, S. A one-pot RPA-CRISPR detection method for point-of-care testing of Enterocytozoon hepatopenaei infection in shrimp. Sens. Actuators B Chem. 2023, 374, 132853. [Google Scholar] [CrossRef]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F. Cas14: Big Advances from Small CRISPR Proteins. Biochemistry 2019, 58, 1024–1025. [Google Scholar] [CrossRef]
- Teng, F.; Guo, L.; Cui, T.; Wang, X.-G.; Xu, K.; Gao, Q.; Zhou, Q.; Li, W. CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019, 20, 1–7. [Google Scholar] [CrossRef]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous dual-gene diagnosis of SARS-CoV-2 based on CRISPR/Cas9-mediated lateral flow assay. Angew. Chem. Int. Ed. 2021, 60, 5307–5315. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef]
- Ke, X.; Liang, A.; Chen, C.; Hu, T. A one-pot CRISPR-RCA strategy for ultrasensitive and specific detection of circRNA. Anal. Methods 2024, 16, 3256–3262. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, S.; Zhang, T.; Xia, X.; Zhang, K.; Deng, S.; He, G.; Gao, H.; He, Q. CRISPR/Cas14a-based isothermal amplification for profiling plant microRNAs. Anal. Chem. 2021, 93, 12602–12608. [Google Scholar] [CrossRef]
- Xu, L.; Dai, Q.; Shi, Z.; Liu, X.; Gao, L.; Wang, Z.; Zhu, X.; Li, Z. Accurate MRSA identification through dual-functional aptamer and CRISPR-Cas12a assisted rolling circle amplification. J. Microbiol. Methods 2020, 173, 105917. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Chen, X.; Qiu, X.; Qing, G.; Zhang, H.; Zhang, L.; Hu, X.; He, Z.; Zhong, D. Rolling circular amplification (RCA)-assisted CRISPR/Cas9 cleavage (RACE) for highly specific detection of multiple extracellular vesicle MicroRNAs. Anal. Chem. 2019, 92, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, Y.; Huang, Y.; Hu, K.; Zhao, S.; Tian, J. A sensitive and facile microRNA detection based on CRISPR-Cas12a coupled with strand displacement amplification. Spectrochim. Acta A 2022, 279, 121476. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, S.; Chu, C.; Yang, M.; Huo, D.; Hou, C. Target-induced transcription amplification to trigger the trans-cleavage activity of CRISPR/Cas13a (TITAC-Cas) for detection of alkaline phosphatase. Biosens. Bioelectron. 2021, 185, 113281. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, X.; Wang, H.; Xing, D. Clustered regularly interspaced short palindromic repeats/Cas9 triggered isothermal amplification for site-specific nucleic acid detection. Anal. Chem. 2018, 90, 2193–2200. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, H.; Wang, T.; Chen, P.; Yin, B.; Ye, B. A programmable and sensitive CRISPR/Cas12a-based MicroRNA detection platform combined with hybridization chain reaction. Biosens. Bioelectron. 2022, 211, 114382. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Li, X.; Lin, C.; Wang, X.; Liu, J.; Ling, L.; Wang, J. CRISPR/Cas12a-based biosensor for colorimetric detection of serum prostate-specific antigen by taking nonenzymatic and isothermal amplification. Sens. Actuators B Chem. 2022, 354, 131228. [Google Scholar] [CrossRef]
- Ke, X.; Ou, Y.; Lin, Y.; Hu, T. Enhanced chemiluminescence imaging sensor for ultrasensitive detection of nucleic acids based on HCR-CRISPR/Cas12a. Biosens. Bioelectron. 2022, 212, 114428. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X. A CRISPR-based and post-amplification coupled SARS-CoV-2 detection with a portable evanescent wave biosensor. Biosens. Bioelectron. 2021, 190, 113418. [Google Scholar] [CrossRef]
- Tao, D.; Liu, J.; Nie, X.; Xu, B.; Tran-Thi, T.-N.; Niu, L.; Liu, X.; Ruan, J.; Lan, X.; Peng, G. Application of CRISPR-Cas12a enhanced fluorescence assay coupled with nucleic acid amplification for the sensitive detection of African swine fever virus. ACS Synth. Biol. 2020, 9, 2339–2350. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q. A novel fluorescence biosensor based on CRISPR/Cas12a integrated MXenes for detecting Aflatoxin B1. Talanta 2023, 252, 123773. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Li, F.; Zheng, X.; Xing, X.; Zhang, C. Aptamer assisted CRISPR-Cas12a strategy for small molecule diagnostics. Biosens. Bioelectron. 2021, 183, 113196. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Huang, R.; Huang, M.; Yue, H.; Shan, Y.; Hu, J.; Xing, D. Cascade CRISPR/cas enables amplification-free microRNA sensing with fM-sensitivity and single-base-specificity. Chem. Commun. 2021, 57, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lim, J.; Shin, M.; Paek, S.-H.; Choi, J.-W. CRISPR-Cas12a-based nucleic acid amplification-free DNA biosensor via Au nanoparticle-assisted metal-enhanced fluorescence and colorimetric analysis. Nano Lett. 2020, 21, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, B.; Wang, Z.; Wang, Q.; Bi, J.; Sun, X. Multiple Isothermal Amplification Coupled with CRISPR–Cas14a for the Naked-eye and Colorimetric Detection of Aflatoxin B1. ACS Appl. Mater. Interfaces 2023, 15, 55423–55432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Q.; Li, Y.; Pang, X.; Cheng, N. A 3D-Printed Integrated Handheld Biosensor for the Detection of Vibrio parahaemolyticus. Foods 2024, 13, 1775. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Chen, Y.; Zhu, X.; Wu, Q.; Chen, W.; Zhao, Q.; Wang, J.; Qin, P. CRISPR/Cas9-mediated SERS/colorimetric dual-mode lateral flow platform combined with smartphone for rapid and sensitive detection of Staphylococcus aureus. Biosens. Bioelectron. 2024, 249, 116046. [Google Scholar] [CrossRef]
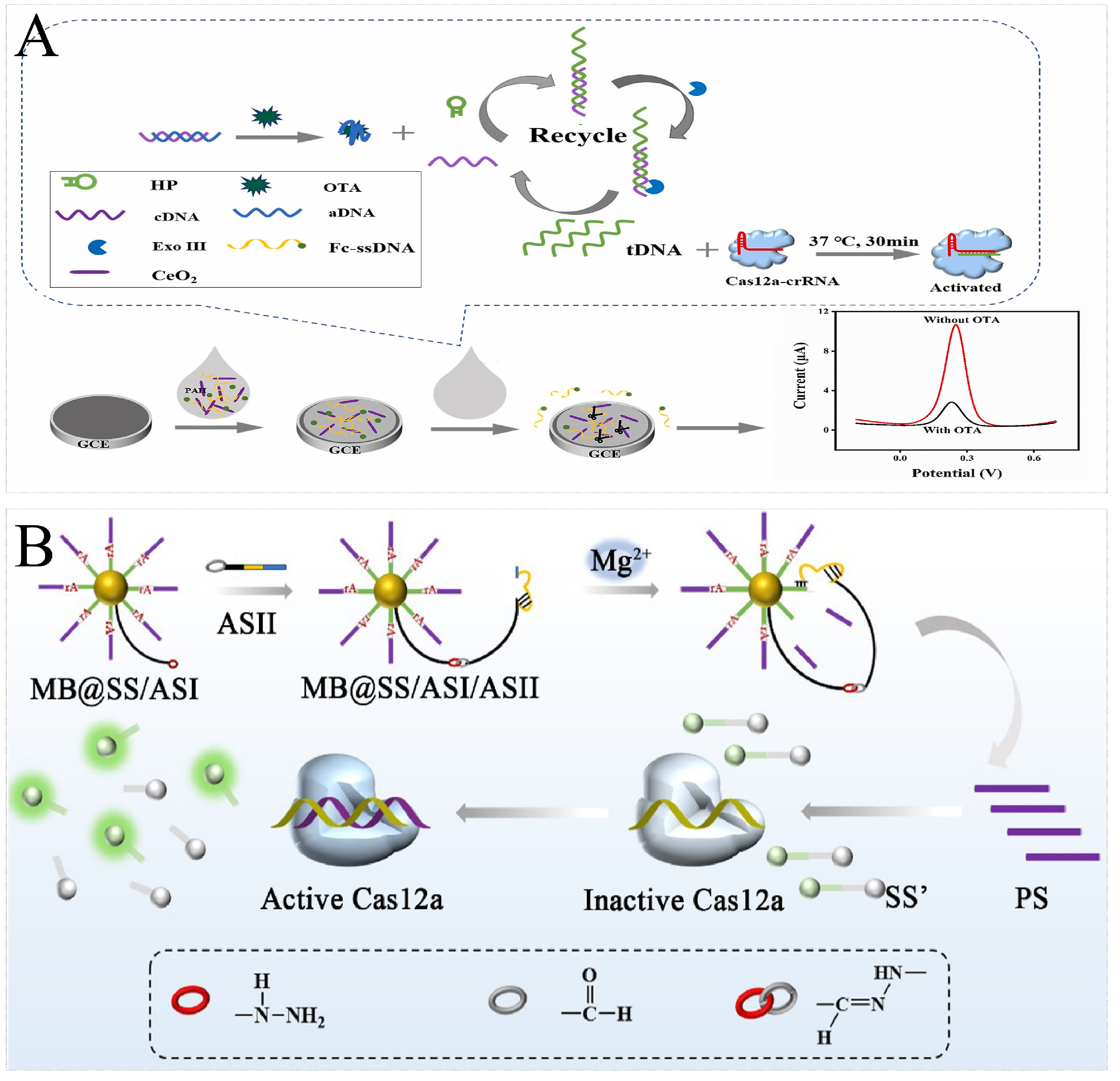
- Xu, S.; Wang, S.; Guo, L.; Tong, Y.; Wu, L.; Huang, X. Nanozyme-catalysed CRISPR-Cas12a system for the preamplification-free colorimetric detection of lead ion. Anal. Chim. Acta 2023, 1243, 340827. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, S.; Wang, X.; Li, J.; Pan, W.; Li, N.; Tang, B. Strand displacement amplification assisted CRISPR-Cas12a strategy for colorimetric analysis of viral nucleic acid. Anal. Chem. 2021, 93, 15216–15223. [Google Scholar] [CrossRef]
- Zhao, R.; Tang, Y.; Song, D.; Liu, M.; Li, B. CRISPR/Cas12a-Responsive Hydrogels for Conjugation-Free and Universal Indicator Release in Colorimetric Detection. Anal. Chem. 2023, 95, 18522–18529. [Google Scholar] [CrossRef]
- Li, F.; Ye, Q.; Chen, M.; Zhou, B.; Zhang, J.; Pang, R.; Xue, L.; Wang, J.; Zeng, H.; Wu, S. An ultrasensitive CRISPR/Cas12a based electrochemical biosensor for Listeria monocytogenes detection. Biosens. Bioelectron. 2021, 179, 113073. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Howes, P.D. DeMello, A.J. An amplification-free ultra-sensitive electrochemical CRISPR/Cas biosensor for drug-resistant bacteria detection. Chem. Sci. 2021, 12, 12733–12743. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, W.; Huang, Y.; Zhang, X.; Yuan, X. A label-free electrochemical sensor for the detection of two kinds of targets based on CRISPR/Cas12a system. Sens. Actuators B Chem. 2024, 406, 135406. [Google Scholar] [CrossRef]
- Lin, X.; Li, C.; Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Wu, S. CRISPR-Cas12a-mediated luminescence resonance energy transfer aptasensing platform for deoxynivalenol using gold nanoparticle-decorated Ti3C2Tx MXene as the enhanced quencher. J. Hazard. Mater. 2022, 433, 128750. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Kim, D.-e.; Kweon, J.; Jin, C.E.; Kim, S.-H.; Kim, Y.; Shin, Y. CRISPR/dCas9-mediated biosensor for detection of tick-borne diseases. Sens. Actuators B Chem. 2018, 273, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhou, J.; Yin, L.; Man, S.; Ma, L. Integration of logic gates to CRISPR/Cas12a system for rapid and sensitive detection of pathogenic bacterial genes. Anal. Chim. Acta 2020, 1125, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ye, Q.; Li, F.; Xiang, X.; Shang, Y.; Wang, C.; Shao, Y.; Xue, L.; Zhang, J.; Wang, J. CRISPR/Cas12a based fluorescence-enhanced lateral flow biosensor for detection of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 351, 130906. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; He, F.; Chen, G.; Bai, L.; He, K.; Zhang, F.; Xu, X. Label-free colorimetric method for detection of Vibrio parahaemolyticus by trimming the G-quadruplex DNAzyme with CRISPR/Cas12a. Anal. Chem. 2021, 93, 14300–14306. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Liu, R.; Wang, Y.; Li, A.; Tian, S.; Cheng, W.; Ding, S.; Li, W.; Zhao, M. A novel CRISPR/Cas14a-based electrochemical biosensor for ultrasensitive detection of Burkholderia pseudomallei with PtPd@PCN-224 nanoenzymes for signal amplification. Biosens. Bioelectron. 2023, 225, 115098. [Google Scholar] [CrossRef]
- Ma, P.; Guo, H.; Zhang, Y.; Wang, Z. A novel CRISPR/Cas14a1-Exo III aptasensor for melamine detection coupled with systematically studied binding mechanism of truncated aptamer. Sens. Actuators B Chem. 2023, 374, 132847. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, L.; Bu, S.; Zhang, W.; Chen, J.; Li, Z.; Hao, Z.; Wan, J. CRISPR/Cas12a and immuno-RCA based electrochemical biosensor for detecting pathogenic bacteria. Electroanal. Chem. 2021, 901, 115755. [Google Scholar] [CrossRef]
- Duan, M.; Li, B.; Zhao, Y.; Liu, Y.; Liu, Y.; Dai, R.; Li, X.; Jia, F. A CRISPR/Cas12a-mediated, DNA extraction and amplification-free, highly direct and rapid biosensor for Salmonella Typhimurium. Biosens. Bioelectron. 2023, 219, 114823. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Chen, Z.; Wang, H.; Xu, W.; Chang, J.; Liang, C.; Liu, X.; Xu, S. Identification of transgenic crops by CRISPR/Cas12a-assisted magnetic surface-enhanced resonance Raman spectroscopy. Sens. Actuators B Chem. 2024, 405, 135345. [Google Scholar] [CrossRef]
- Pataer, P.; Gao, K.; Zhang, P.; Wang, X.; Li, Z. A simple, portable and multiplex LAMP-based CRISPR/Cas12a assay for visually screening genetically modified crops. Sens. Actuators B Chem. 2024, 403, 135124. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qian, C.; Wu, C.; Wang, Z.; Wang, D.; Ye, Z.; Ping, J.; Wu, J.; Ji, F. End-point dual specific detection of nucleic acids using CRISPR/Cas12a based portable biosensor. Biosens. Bioelectron. 2020, 157, 112153. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Chen, X.; Wang, X.; Ding, L.; Xu, X.; Wei, W.; Yang, L.; Wu, J.; Sun, M. A localized CRISPR assay that detects short nucleic acid fragments in unamplified genetically modified samples. ACS Sens. 2023, 8, 1054–1063. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Wei, H.; Luo, Q.; Yang, P.; Liu, M.; Wang, Z.; Zou, X.; Zhu, H.; Zha, G. Design and development of a rapid meat detection system based on RPA-CRISPR/Cas12a-LFD. Curr. Res. Food Sci. 2023, 7, 100609. [Google Scholar] [CrossRef]
- Pan, R.; Liu, J.; Wang, P.; Wu, D.; Chen, J.; Wu, Y.; Li, G. Ultrasensitive CRISPR/Cas12a-driven SERS biosensor for on-site nucleic acid detection and its application to milk authenticity testing. J. Agric. Food Chem. 2022, 70, 4484–4491. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Yao, C.; Xie, P.; Li, X.; Xu, Z.; Xian, Y.; Lei, H.; Shen, X. Alkaline lysis-recombinase polymerase amplification combined with CRISPR/Cas12a assay for the ultrafast visual identification of pork in meat products. Food Chem. 2022, 383, 132318. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Wang, X.; Hong, L.; Yin, X.; Zhang, Y.; Hu, B.; Zheng, Q.; Cao, J. CRISPR/Cas12a integrated electrochemiluminescence biosensor for pufferfish authenticity detection based on NiCo2O4 NCs@Au as a coreaction accelerator. Food Chem. 2024, 445, 138781. [Google Scholar] [CrossRef]
- Chen, S.; Fang, R.; Li, Y.; Deng, F.; Liu, X.; Yang, D. CRISPR/Cas12a-powered CLASA towards OTA ultrasensitive detection in cereal samples. Microchem. J. 2024, 196, 109691. [Google Scholar] [CrossRef]
- Xiang, X.; Lu, J.; Tao, M.; Xu, X.; Wu, Y.; Sun, Y.; Zhang, S.; Niu, H.; Ding, Y.; Shang, Y. High-throughput identification of meat ingredients in adulterated foods based on centrifugal integrated purification-CRISPR array. Food Chem. 2024, 443, 138507. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Wang, X.; Chen, R.; Zhou, Z.; Ren, S.; Liang, J.; Gao, Z. Upconversion-mediated CRISPR-Cas12a biosensing for sensitive detection of ochratoxin A. Talanta 2022, 242, 123232. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, X.; Guo, L.; Huang, X.; Wu, L.; Huang, H. An electrochemical aptasensor based on exonuclease III-assisted signal amplification coupled with CRISPR-Cas12a for ochratoxin A detection. Food Control 2023, 148, 109631. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Yu, Y.; Zhang, Y.; Peng, J.; Niu, L.; Zhang, J. Hydrazone ligation assisted DNAzyme walking nanomachine coupled with CRISPR-Cas12a for lipopolysaccharide analysis. Anal. Chim. Acta 2021, 1174, 338747. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Nameghi, M.A.; Zavvar, T.S.; Taghdisi, S.M. A novel colorimetric aptasensor for ultrasensitive detection of aflatoxin M1 based on the combination of CRISPR-Cas12a, rolling circle amplification and catalytic activity of gold nanoparticles. Anal. Chim. Acta 2021, 1165, 338549. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Qian, S.; Yu, X.; Chen, H.; Wu, J. Applying CRISPR/Cas system as a signal enhancer for DNAzyme-based lead ion detection. Anal. Chim. Acta 2022, 1192, 339356. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Zuo, C.; Dai, L.; Guo, Y.; Xie, G. Applying CRISPR-Cas12a as a signal amplifier to construct biosensors for non-DNA targets in ultralow concentrations. ACS Sens. 2020, 5, 970–977. [Google Scholar] [CrossRef]
- Wen, J.; Deng, H.; He, D.; Yuan, Y. Dual-functional DNAzyme powered CRISPR-Cas12a sensor for ultrasensitive and high-throughput detection of Pb2+ in freshwater. Sci. Total Environ. 2024, 911, 168708. [Google Scholar] [CrossRef]
- Yu, Y.; Li, W.; Gu, X.; Yang, X.; Han, Y.; Ma, Y.; Wang, Z.; Zhang, J. Inhibition of CRISPR-Cas12a trans-cleavage by lead (II)-induced G-quadruplex and its analytical application. Food Chem. 2022, 378, 131802. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J. Am. Chem. Soc. 2019, 142, 207–213. [Google Scholar] [CrossRef]
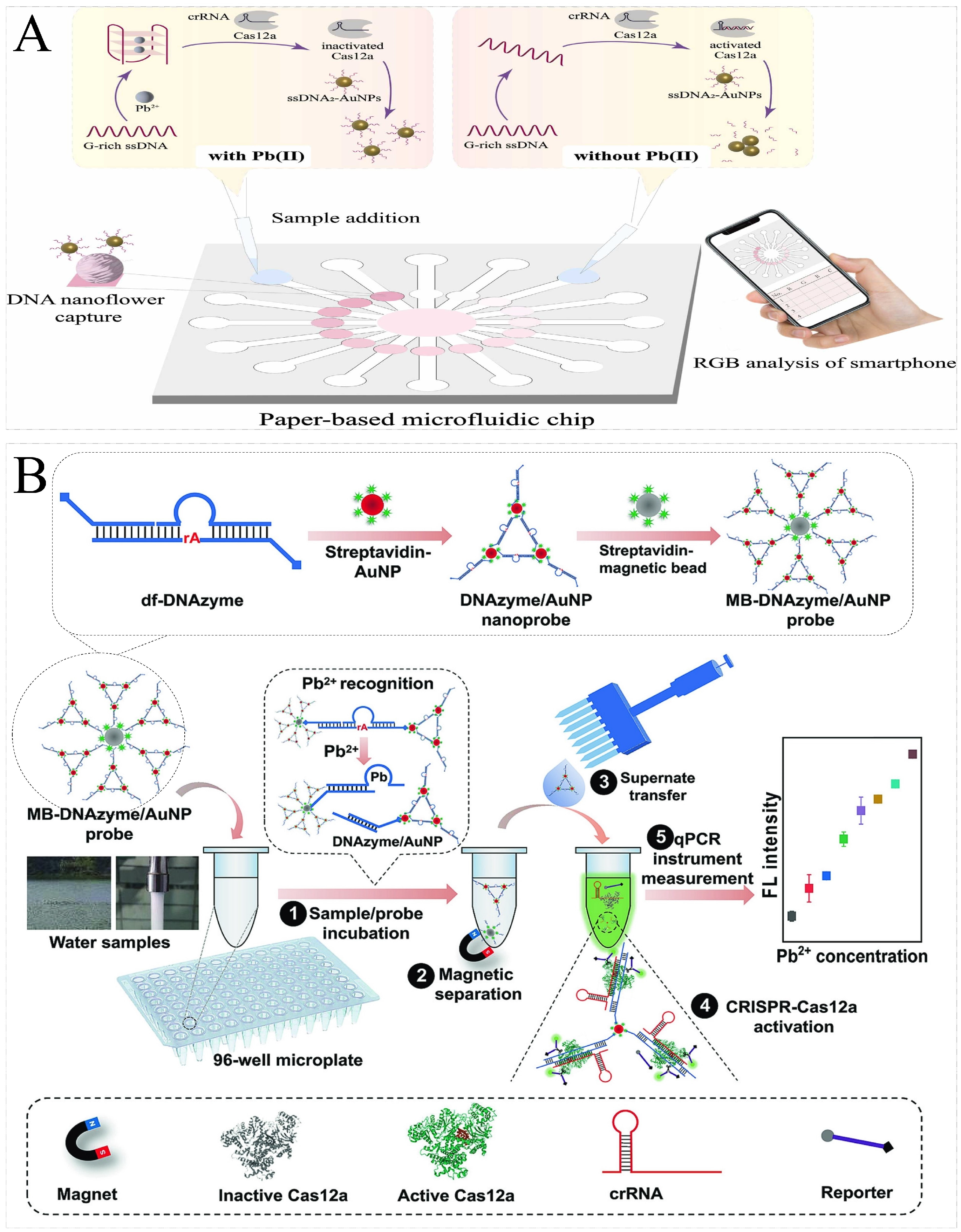
- Zhang, Y.; Yu, Y.; Yang, X.; Yuan, X.; Zhang, J. Pb (II) inhibits CRISPR/Cas12a activation and application for paper-based microfluidic biosensor assisted by smartphone. Sens. Actuators B Chem. 2024, 398, 134732. [Google Scholar] [CrossRef]
- Yee, B.J.; Zakaria, S.N.A.; Chandrawati, R.; Ahmed, M.U. Detection of Tetracycline with a CRISPR/Cas12a Aptasensor Using a Highly Efficient Fluorescent Polystyrene Microsphere Reporter System. ACS Synth. Biol. 2024, 13, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Sun, Y.; Zhou, Y.; Khan, I.M.; Niazi, S.; Yue, L.; Zhang, Y.; Wang, Z. Cascade DNA Circuits Mediated CRISPR-Cas12a Fluorescent Aptasensor based on Multifunctional Fe3O4@hollow-TiO2@MoS2 Nanochains for Tetracycline Determination. Small 2023, 19, 2206105. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Y.; Liu, Y.; Liu, H.; Zhao, Q.; Zhang, Y.; Wang, C.; Li, Z.; Jiao, B.; He, Y. CRISPR-Cas12a based fluorescence assay for organophosphorus pesticides in agricultural products. Food Chem. 2022, 387, 132919. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Yuan, R.; Zhong, X.; Zhuo, Y. Electrochemiluminescence covalent organic framework coupling with CRISPR/Cas12a-mediated biosensor for pesticide residue detection. Food Chem. 2022, 389, 133049. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Shi, G.; Chen, J. Fluorescent detection of two pesticides based on CRISPR-Cas12a and its application for the construction of four molecular logic gates. J. Agric. Food Chem. 2022, 70, 12700–12707. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Jiang, L.; Wang, Z.; Peng, L.; Zhang, Z.; Huang, Y. Mn2+-activated CRISPR-Cas12a strategy for fluorescence detection of the insecticide carbaryl. Sens. Actuators B Chem. 2024, 398, 134695. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Shao, S.; Ci, Q.; Chen, L.; Lu, X.; Liu, Q.; Chen, J. CRISPR/Cas14 and G-Quadruplex DNAzyme-Driven Biosensor for Paper-Based Colorimetric Detection of African Swine Fever Virus. ACS Sens. 2024, 9, 2413–2420. [Google Scholar] [CrossRef]
- Wang, L.; Pu, G.; Liu, T.; Chen, G.; Li, H.; Amuda, T.O.; Cao, S.; Yan, H.; Yin, H.; Fu, B. Parasite-derived microRNA let-7-5p detection for metacestodiasis based on rolling circular amplification-assisted CRISPR/Cas9. FASEB J. 2024, 38, e23708. [Google Scholar] [CrossRef]

| Cas9 | Cas12a (Cpf1) | Cas12b (C2c1) | Cas13a (C2c2) | Cas14 (Cas12f) | |
|---|---|---|---|---|---|
| Classification | Class II Type II | Class II Type V | Class II Type V | Class II Type VI | Class II Type V |
| Nuclease Domains | HNH, RuvC | RuvC only | RuvC only | HEPN × 2 | RuvC only |
| Guide RNA | crRNA, tracrRNA | crRNA only | crRNA, tracrRNA | crRNA only | crRNA, tracrRNA |
| Spacer Length | 18–24 nt | 16–30 nt (commonly, 20 nt) | 20 nt | 22–28 nt | 20–25 nt |
| Cis-Cleavage Target | dsDNA with PAM | dsDNA with PAM or ssDNA | dsDNA with PAM or ssDNA | ssRNA | ssDNA |
| PAM Sequence | NGG | (T)TTN | TTN | No PAM but PFS | / |
| Cis-Cleavage Site | 3 bases 5′ of the PAM | dsDNA: ~14/18–23 bp 3′ of the PAM ssDNA: ~22 nt from the first 3′ guide-complementary base | dsDNA: ~17–24 bp 3′ of the PAM ssDNA: / | Many cleavage sites, U and A | Beyond the guide-complementary region |
| Trans-Cleavage Target | / | ssDNA | ssDNA | ssRNA | ssDNA |
| Targets | Recognition Elements | Effector Protein | Signal Amplification | Signal Reporter | Signal Output | Sensitivity | Results Evaluation | References |
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Genome | Cas12a | PCR | F/Q-ssDNA | Fluorescence | 103 CFU/mL | Can be finished within 2 H, but requires pre-culture and genome extraction. | [85] |
| Staphylococcus aureus | Genome | Cas12a | RAA | QDs-SA-Bio-ssDNA | Colorimetric lateral flow strip | 5.4 × 102 CFU/mL | RAA shortens the assay time but requires pre-culture and genome extraction. | [86] |
| Vibrio parahaemolyticus | Genome | Cas12a | LAMP | G4 ssDNA | Colorimetric | 6.1 × 102 CFU/mL | Visual analysis requires pre-culture and genome extraction. | [87] |
| Burkholderia pseudomallei | Genome | Cas14a | / | PtPd@PCN-224-ssDNA | Electrochemical | 12.8 aM of DNA | Ultra-sensitive analysis of DNA. | [88] |
| Staphylococcus aureus Pseudomonas aeruginosa | Genome | Cas13a and Cas13b | Multiple RPA | F/Q-poly U F/Q-poly A | Fluorescence | As low as the attomolar range | Multiple analyses but poor generalizability. | [48] |
| Escherichia coli O157:H7 | Aptamer | Cas12a | RCA | MB-ssDNA | Electrochemical | 10 CFU/mL | Avoids genome extraction and improves assay sensitivity. | [89] |
| Salmonella Typhimurium | Aptamer | Cas12a | / | F/Q-ssDNA | Fluorescence | 6.31 × 103 CFU/mL | Easy to operate but lacking in sensitivity. | [90] |
| GMO species | P-35S and T-nos | Cas12a | RPA | F/Q-ssDNA | Fluorescence | 10 copies | Simple to operate and finished within 15 min at room temperature. | [91] |
| GMO species | P-35S and T-nos | Cas12a | RPA | FeAuG-MB and G4 ssDNA | SERS | At the pM level | Significant increase in sensitivity with the aid of SERS. | [92] |
| GMO species | P-35S | Cas12a | / | F/Q-ssDNA | Fluorescence | 0.1% | Direct detection of short fragments using multiple crRNAs expands application in further processed products. | [93] |
| GM maize | P-35S, GUS, and T-nos | Cas12a | Multiplex LAMP | F/Q-ssDNA | Fluorescence | 100 aM | Multiple detections but realized by system separation. | [94] |
| GM soybeans | P-35S and lectin | Cas12a | Dual LAMP | F/Q-ssDNA | Fluorescence | 0.1% | Multiple detections but realized by system separation. | [95] |
| Pork, beef, and duck | Genome | Cas12a | RPA | F/Q-ssDNA | Fluorescence | 100, 10, and 10 copies | Simple to operate and finished within 15 min at room temperature. | [91] |
| Chicken, duck, beef, pork, and lamb | Genome | Cas12a | RPA | F/Q-ssDNA and AuNPs | Fluorescence and colorimetric lateral flow strip | 1 × 100 copies/μL | Suitable for on-site identification of large quantities of meat. | [96] |
| Goat milk | Genome | Cas12a | LAMP | PB-ssDNA | SERS | 224 aM | Ultra-sensitive detection with the help of Raman signals. | [97] |
| Pufferfish | Genome | Cas12a | PCR | NiCo2O4 NCs@Au-ABEI-ssDNA | Electrochemical | 0.1% | Provides a simple, low-cost, and sensitive approach to trace pufferfish adulteration. | [98] |
| Pork | Genome | Cas12a | RPA | F/Q-ssDNA | Fluorescence | 10−3 ng | Simplified genome extraction process and the detection could be completed within 30 min. | [99] |
| Pig, chicken, duck, and lamb | Genome | Cas12a | RAA | F/Q-ssDNA | Fluorescence | 0.1% | Integration of amplification analysis with the help of centrifugal microfluidic chip. | [100] |
| Ochratoxin A | Aptamer | Cas12a | / | F/Q-ssDNA | Fluorescence | 171 fg/mL | Lower price and easier preparation. | [101] |
| Ochratoxin A | Aptamer | Cas12a | / | UCNP-ssDNA-Fe3O4 | Upconversion fluorescence | 1.5641 ng/mL | Rapid and stable, but slightly lacking in sensitivity. | [102] |
| Ochratoxin A | Aptamer | Cas12a | Exo III-assisted recycling system | Fc-ssDNA | Electrochemical | 0.74 fg/mL | Enhances sensitivity through nucleic acid amplification. | [103] |
| Lipopolysaccharide | Aptamer and Mg2+-independent DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 7.31 fg/mL | Ingenious DNA enzyme-walking nanomachines with high sensitivity. | [104] |
| Aflatoxin M1 | Aptamer | Cas12a | RCA | AuNPs-ssDNA and 4-nitrophenol | Colorimetric | 0.05 ng/L | RCA adds operational complexity without a significant increase in sensitivity. | [105] |
| Pb2+ | Pb2+-independent DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 0.48 nM | Simplicity and high sensitivity; also with designed 3D-printing equipment to assist fluorescence analysis. | [106] |
| Pb2+ | Pb2+-independent DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 0.053 nM | Reduced system interference and increased sensitivity with the help of magnetic separation. | [107] |
| Pb2+ | MB-DNAzyme/AuNP | Cas12a | / | F/Q-ssDNA | Fluorescence | 1 pg/L | Ultrasensitive and high-throughput detection. | [108] |
| Pb2+ | Na+/Pb2+-induced G-quadruplex | Cas12a | / | F/Q-G4 ssDNA | Fluorescence | 2.6 nM | First provides a new method for Pb2+ detection based on induced G-quadruplex inhibition on CRISPR-Cas12a trans-cleavage. | [109] |
| Pb2+ | Pb2+-induced G-quadruplex | Cas12a | / | AuNPs-ssDNA | Colorimetric | 18.3 nM | A chip-scale paper microfluidic smart analysis system that has great potential for the in situ detection of Pb2+. | [110] |
| Na+ | Na+-independent DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 0.10 mM | Simple and fast inspection workflow—two steps in less than 15 min. | [111] |
| Acetamiprid | Aptamer | Cas12a | / | Fc-ssDNA | Electrochemical | 2.7 pM | Designed PTCA-COF shows strong and stable ECL emission signals for enhanced detection sensitivity. | [112] |
| Acetamiprid and atrazine | Aptamer | Cas12a | Toehold-mediated strand displacement reaction | F/Q-ssDNA | Fluorescence | 2.5 pM 0.2 pM | With high sensitivity, the realization of multiple analyses relies on logic gate design. | [113] |
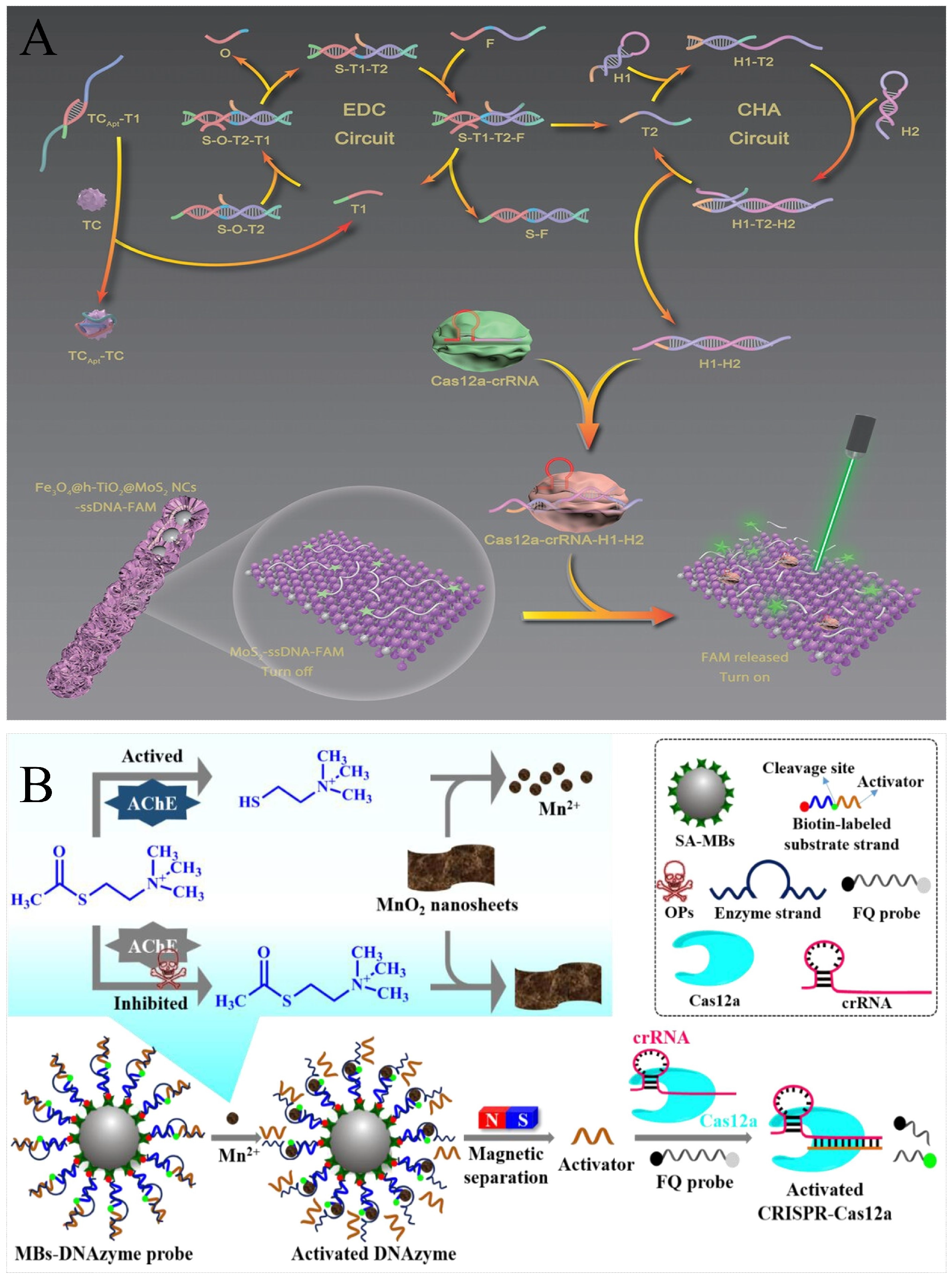
| Paraoxon, dichlorvos, and demeton | Acetylcholinesterase and Mn2+ dependence of the DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 270 pg/mL, 406 pg/mL, 218 pg/mL | Great versatility for detecting OPs. | [114] |
| Carbaryl | Acetylcholinesterase and Mn2+ dependence of the DNAzyme | Cas12a | / | F/Q-ssDNA | Fluorescence | 29 nM | Can be extended for rapid screening of pesticide residues. | [115] |
| Tetracycline | Aptamer | Cas12a | / | Polystyrene microsphere/Q-ssDNA | Fluorescence | 0.1 μM | The problems of being time-consuming and costly, giving false positive signals, and having a complex primer design and difficult optimization phase are solved. | [116] |
| Tetracycline | Aptamer | Cas12a | Cascade amplification circuits | FAM-ssDNA-Fe3O4@hollow-TiO2@MoS2 | Fluorescence | 0.384 pg/mL | The introduction of nanomaterials significantly improves detection sensitivity. | [117] |
| Parasite | Cestode-derived miRNA let-7-5p | Cas9 | RCA | RCA products complement F/Q-ssDNA | Fluorescence | 10 aM | RCA enables signal amplification but increases operational complexity for Cas9. | [118] |
| African swine fever virus | Genome | Cas14a | PCR | G4 ssDNA | Colorimetric | 5 copies/μL | Paper-based microfluidic device significantly improves assay portability. | [119] |
| Melamine | Aptamer | Cas14a1 | / | F/Q-ssDNA | Fluorescence | 3.2 nM | Enhancement of affinity by aptamer modification to improve assay specificity and sensitivity. | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wen, T.; Zhang, P.; Wang, M.; Xu, Y. Recent Advances in the CRISPR/Cas-Based Nucleic Acid Biosensor for Food Analysis: A Review. Foods 2024, 13, 3222. https://doi.org/10.3390/foods13203222
Sun Y, Wen T, Zhang P, Wang M, Xu Y. Recent Advances in the CRISPR/Cas-Based Nucleic Acid Biosensor for Food Analysis: A Review. Foods. 2024; 13(20):3222. https://doi.org/10.3390/foods13203222
Chicago/Turabian StyleSun, Yanan, Tianjian Wen, Ping Zhang, Minglian Wang, and Yuancong Xu. 2024. "Recent Advances in the CRISPR/Cas-Based Nucleic Acid Biosensor for Food Analysis: A Review" Foods 13, no. 20: 3222. https://doi.org/10.3390/foods13203222
APA StyleSun, Y., Wen, T., Zhang, P., Wang, M., & Xu, Y. (2024). Recent Advances in the CRISPR/Cas-Based Nucleic Acid Biosensor for Food Analysis: A Review. Foods, 13(20), 3222. https://doi.org/10.3390/foods13203222






