Prediction and Visualization of Total Volatile Basic Nitrogen in Yellow Croaker (Larimichthys polyactis) Using Shortwave Infrared Hyperspectral Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. TVB-N Measurement
2.3. Hyperspectral Image Acquisition
2.4. Data Analysis
2.4.1. Chemometrics
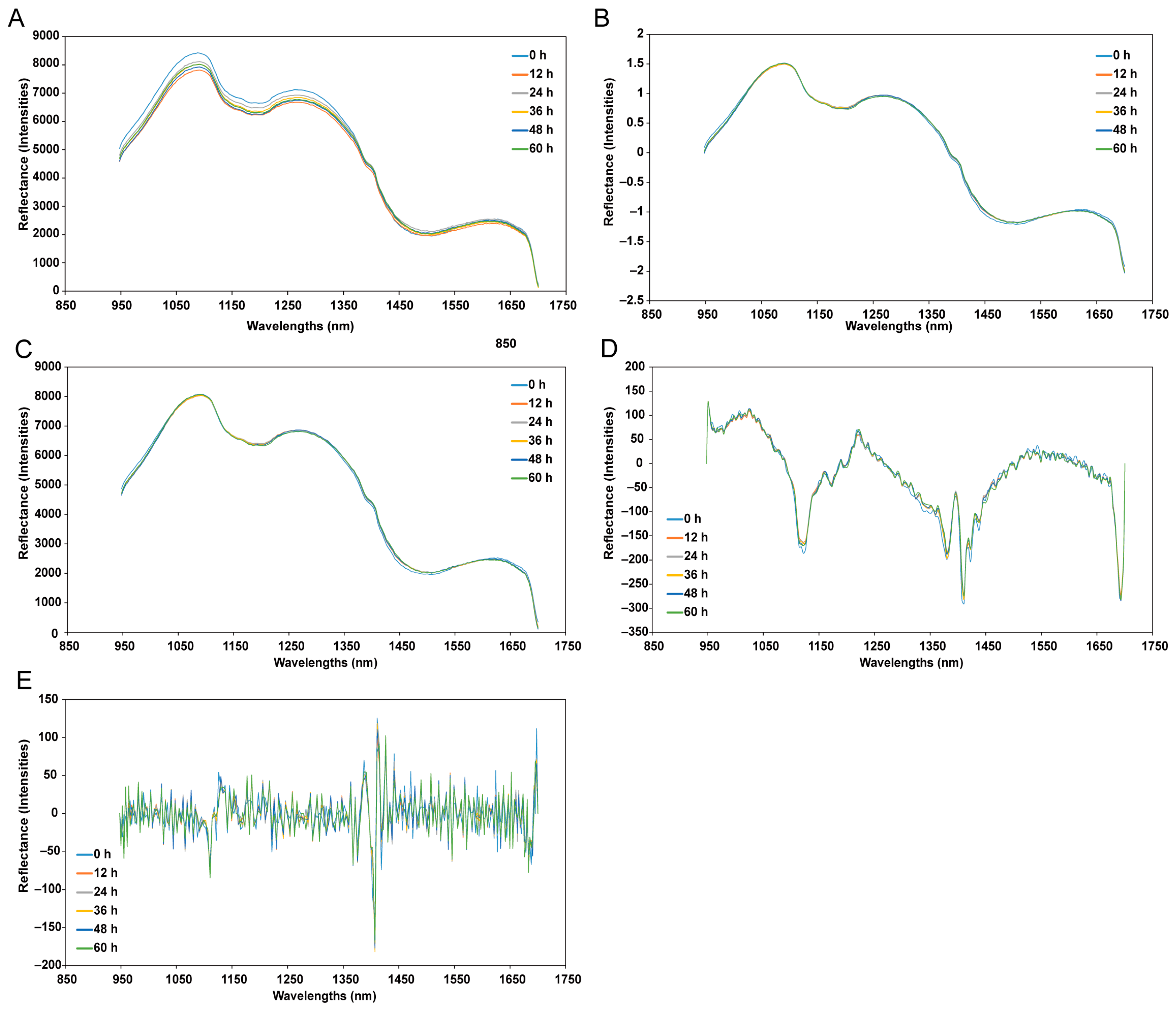
2.4.2. Spectrum Pre-Processing
2.4.3. Statistical Analysis
2.4.4. Visualization for Chemical Imaging
3. Results and Discussion
3.1. Spectral Features of Mean Spectra of the Yellow Croaker
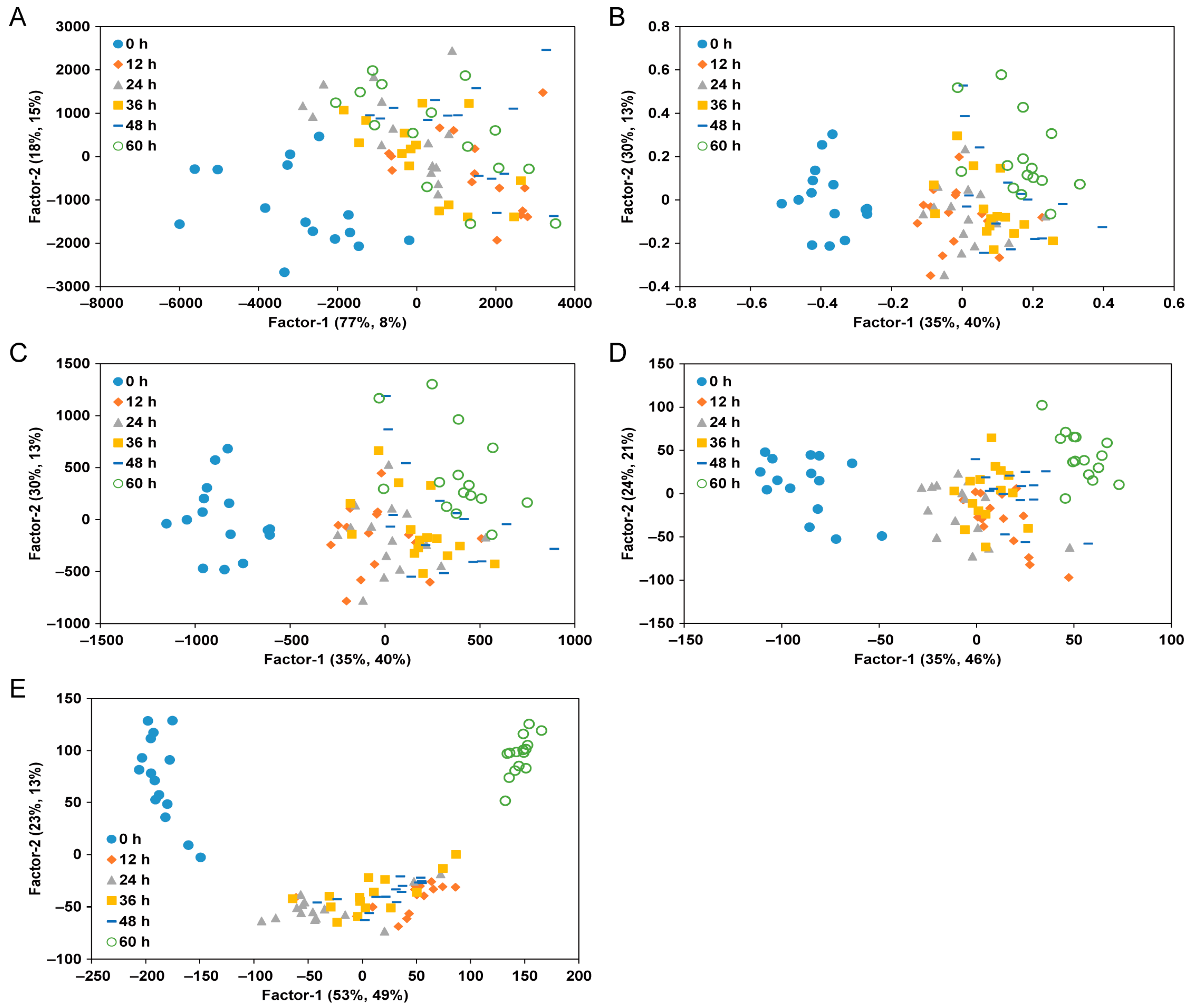
3.2. PLS Score Plots for Classifying the Freshness of Yellow Croaker According to Its Storage Periods
3.3. Changes in TVB-N of Yellow Croaker According to Freshness
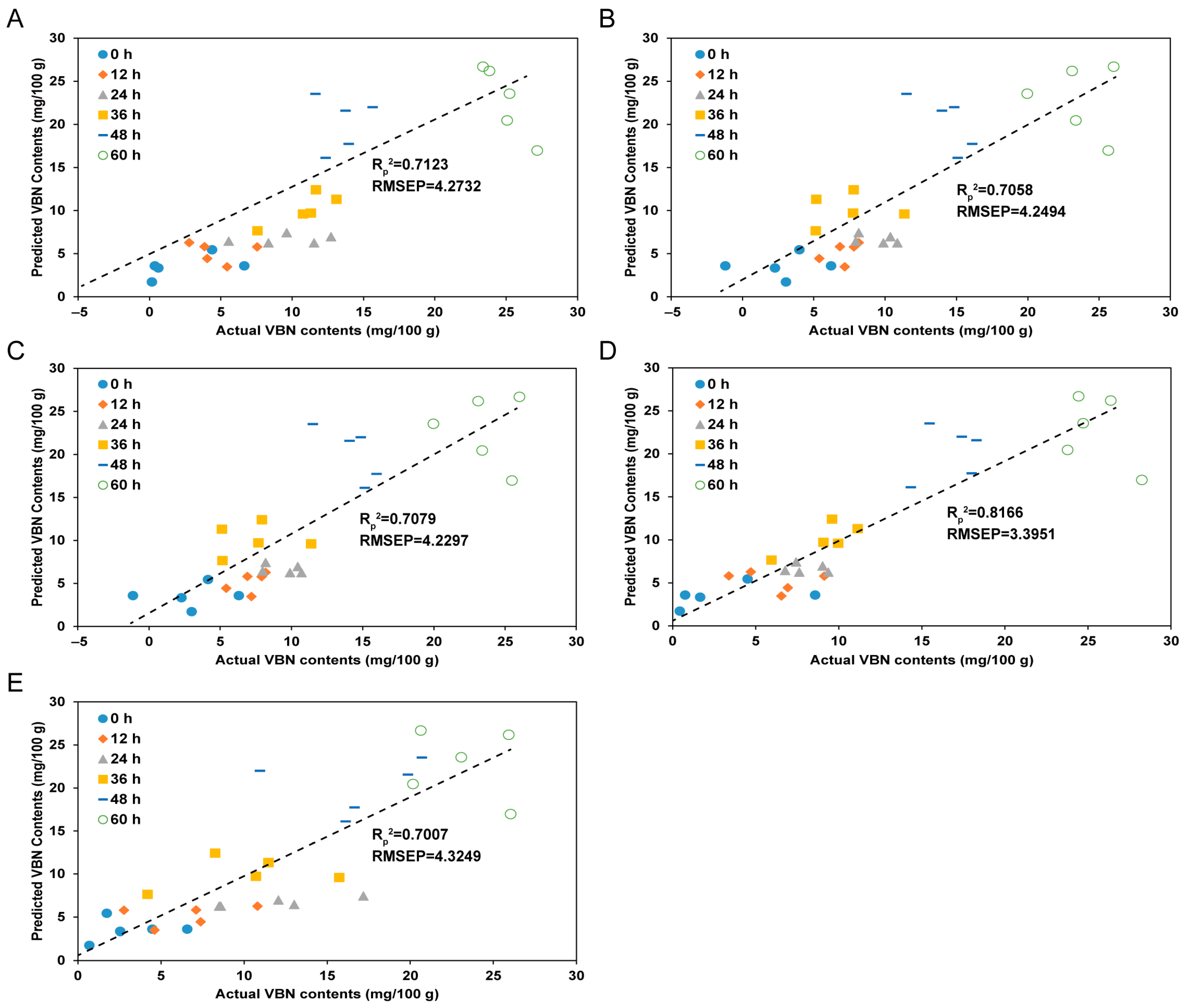
3.4. Comparison of Prediction Models Using Different Spectral Pre-Processing Methods
3.5. Prediction Results for TVB-N Contents of Yellow Croakers According to the Spectral Pre-Processing Methods
3.6. Chemical Imaging of Freshness Information of Yellow Croakers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.S.; Jin, X.S.; Yang, T.; Kooijman, S.A.L.M.; Shan, X.J. A dynamic energy budget model for small yellow croaker: Parameterisation and application in its main geographic distribution waters. Ecol. Model. 2020, 427, 109017. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Shi, Y.K.; Wang, K.L.; Li, F.F.; Zhou, G.Y.; Xuan, G.T. Detection of small yellow croaker freshness by hyperspectral imaging. J. Food Compos. Anal. 2023, 115, 104772. [Google Scholar] [CrossRef]
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT Food Sci. Technol. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H.S. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021, 354, 129559. [Google Scholar] [CrossRef]
- Fazial, F.F.; Tan, L.L. Phenylalanine-responsive fluorescent biosensor based on graphene oxide-chitosan nanocomposites catalytic film for non-destructive fish freshness grading. Food Control 2021, 125, 107995. [Google Scholar] [CrossRef]
- Song, Y.J.; Lyu, C.C.; Guo, W. On-line monitoring research of seafood quality safety based on image monitoring. J. Coast. Res. 2018, 85, 627–631. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Prediction of various freshness indicators in fish fillets by one multispectral imaging system. Sci. Rep. 2019, 9, 12419. [Google Scholar] [CrossRef]
- Özoğul, F.; Özoğul, Y. Comparison of methods used for determination of total volatile basic nitrogen (TVB-N) in rainbow trout (Oncorhynchus mykiss). Turk. J. Zool. 2000, 24, 113–120. [Google Scholar]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.H.; Feng, L. Sensitivity enhancement of pH indicator and its application in the evaluation of fish freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.W.; Kim, M.S.; Chao, K.L.; Chan, D.E.; Delwiche, S.R.; Cho, B.K. Line-scan hyperspectral imaging techniques for food safety and quality applications. Appl. Sci. 2017, 7, 125. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Hyperspectral imaging as an effective tool for quality analysis and control of fish and other seafoods: Current research and potential applications. Trends Food Sci. Technol. 2014, 37, 78–91. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Rapid quantification analysis and visualization of loads in grass carp fish flesh by hyperspectral imaging method. Food Bioprocess Technol. 2015, 8, 951–959. [Google Scholar] [CrossRef]
- Sun, D.W. Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: London, UK, 2010. [Google Scholar]
- Ghidini, S.; Varrà, M.O.; Zanardi, E. Approaching authenticity issues in fish and seafood products by qualitative spectroscopy and chemometrics. Molecules 2019, 24, 1818. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Wei, Q.Y. Enhancing visible and near-infrared hyperspectral imaging prediction of TVB-N level for fish fillet freshness evaluation by filtering optimal variables. Food Anal. Methods 2017, 10, 1888–1898. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Zeng, X.A.; Pu, H.B. Non-destructive and rapid determination of TVB-N content for freshness evaluation of grass carp (Ctenopharyngodon idella) by hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2014, 21, 179–187. [Google Scholar] [CrossRef]
- Dai, Q.; Cheng, J.H.; Sun, D.W.; Zeng, X.A. Advances in feature selection methods for hyperspectral image processing in food industry applications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1368–1382. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Hyperspectral imaging (TS-HSI) for non-invasive determination of microbial spoilage of salmon flesh Potential of Time Series. Talanta 2013, 111, 39–46. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Rapid and non-invasive detection of fish microbial spoilage by visible and near infrared hyperspectral imaging and multivariate analysis. LWT Food Sci. Technol. 2015, 62, 1060–1068. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M.; Nassiri, S.M.; Azimifar, Z. Determination of total viable count in rainbow-trout fish fillets based on hyperspectral imaging system and different variable selection and extraction of reference data methods. Food Anal. Methods 2018, 11, 3481–3494. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Data fusion and hyperspectral imaging in tandem with least squares-support vector machine for prediction of sensory quality index scores of fish fillet. LWT Food Sci. Technol. 2015, 63, 892–898. [Google Scholar] [CrossRef]
- Wang, X.; Shan, J.J.; Han, S.Q.; Zhao, J.B.; Zhang, Y.T. Optimization of fish quality by evaluation of total volatile basic nitrogen (TVB-N) and texture profile analysis (TPA) by near-infrared (NIR) hyperspectral imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Conway, E.J.; O’Malley, E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10 mug. N) (revised methods for ranges greater than 10 μg. N). Biochem. J. 1942, 36, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, S.; Sung, S.; Park, D. A study on the prediction of CNC tool wear using machine learning technique. J. Korea Converg. Soc. 2019, 10, 792–795. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, C.H.; Choi, T.H.; Kim, J.H.; Kwon, G.H.; Oh, S.I.; Kim, H.; Kim, Y.J. The analysis of oat chemical properties using visible-near infrared spectroscopy. Korean J. Agric. Sci. 2016, 43, 715–722. [Google Scholar] [CrossRef]
- Lewis-Beck, M.S.; Bryman, A.; Liao, T.F. The SAGE Encyclopedia of Social Science Research Methods. Encyclopedia of Social Science Research Methods; Sage Publications: Thousand Oaks, CA, USA, 2004; pp. 792–795. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Chen, Y.; Wang, P.; Wei, X.; Tao, F.L. Dynamic variability of the heading-flowering stages of single rice in China based on field observations and NDVI estimations. Int. J. Biometeorol. 2015, 59, 643–655. [Google Scholar] [CrossRef]
- Choudhry, P. High-throughput method for automated colony and cell counting by digital image analysis based on edge detection. PLoS ONE 2016, 11, e0148469. [Google Scholar] [CrossRef]
- Cui, C.; Fearn, T. Modern practical convolutional neural networks for multivariate regression: Applications to NIR calibration. Chemom. Intell. Lab. Syst. 2018, 182, 9–20. [Google Scholar] [CrossRef]
- Rinnan, Å.; Berg, F.v.d.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Riccioli, C.; Sun, D.W. Efficient integration of particle analysis in hyperspectral imaging for rapid assessment of oxidative degradation in salmon fillet. J. Food Eng. 2016, 169, 259–271. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.W.; Allen, P. Application of NIR hyperspectral imaging for discrimination of lamb muscles. J. Food Eng. 2011, 104, 332–340. [Google Scholar] [CrossRef]
- Sánchez-Parra, M.; Fernández Pierna, J.A.; Baeten, V.; Muñoz-Redondo, J.M.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M. Rapid screening of tuna samples for food safety issues related to histamine content using fourier-transform mid-infrared (FT-MIR) and chemometrics. J. Food Eng. 2024, 379, 112129. [Google Scholar] [CrossRef]
- Song, H.N.; Lee, D.G.; Han, S.W.; Yoon, H.K.; Hwang, I.K. Quality changes of salted and semi-dried mackerel fillets by UV treatment during refrigerated storage. Korean J. Food Cook Sci. 2005, 21, 662–668. [Google Scholar]
- Mishra, P.; Biancolillo, A.; Roger, J.M.; Marini, F.; Rutledge, D.N. New data preprocessing trends based on ensemble of multiple preprocessing techniques. TrAC Trends Anal. Chem. 2020, 132, 116045. [Google Scholar] [CrossRef]
- Kim, S.S.; Choi, J.Y.; Lim, J.H.; Cho, J.S. Non-destructive quality prediction of domestic, commercial red pepper powder using hyperspectral imaging. Korean J. Food Preserv. 2023, 30, 224–234. [Google Scholar] [CrossRef]
- Wang, L.; Pang, L.; Yan, L.; Zhang, J. Nondestructive rapid identification of soybean varieties using hyperspectral imaging technology. J. Appl. Spectrosc. 2022, 89, 84–91. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, J.; Wu, W.; Luo, J.; Long, T.; Wu, Q.; Wang, Q.; Zhen, J.; Zhao, Y.; Wang, Y.; et al. Detection of peanut seed vigor based on hyperspectral imaging and chemometrics. Front. Plant Sci. 2023, 14, 1127108. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Tian, X.; Yu, S.; Zhang, X.; Zareef, M. Application of hyperspectral imaging and colorimetric sensor array coupled with multivariate analysis for quality detection during salted duck eggs processing. J. Food Process Eng. 2024, 47, e14589. [Google Scholar] [CrossRef]
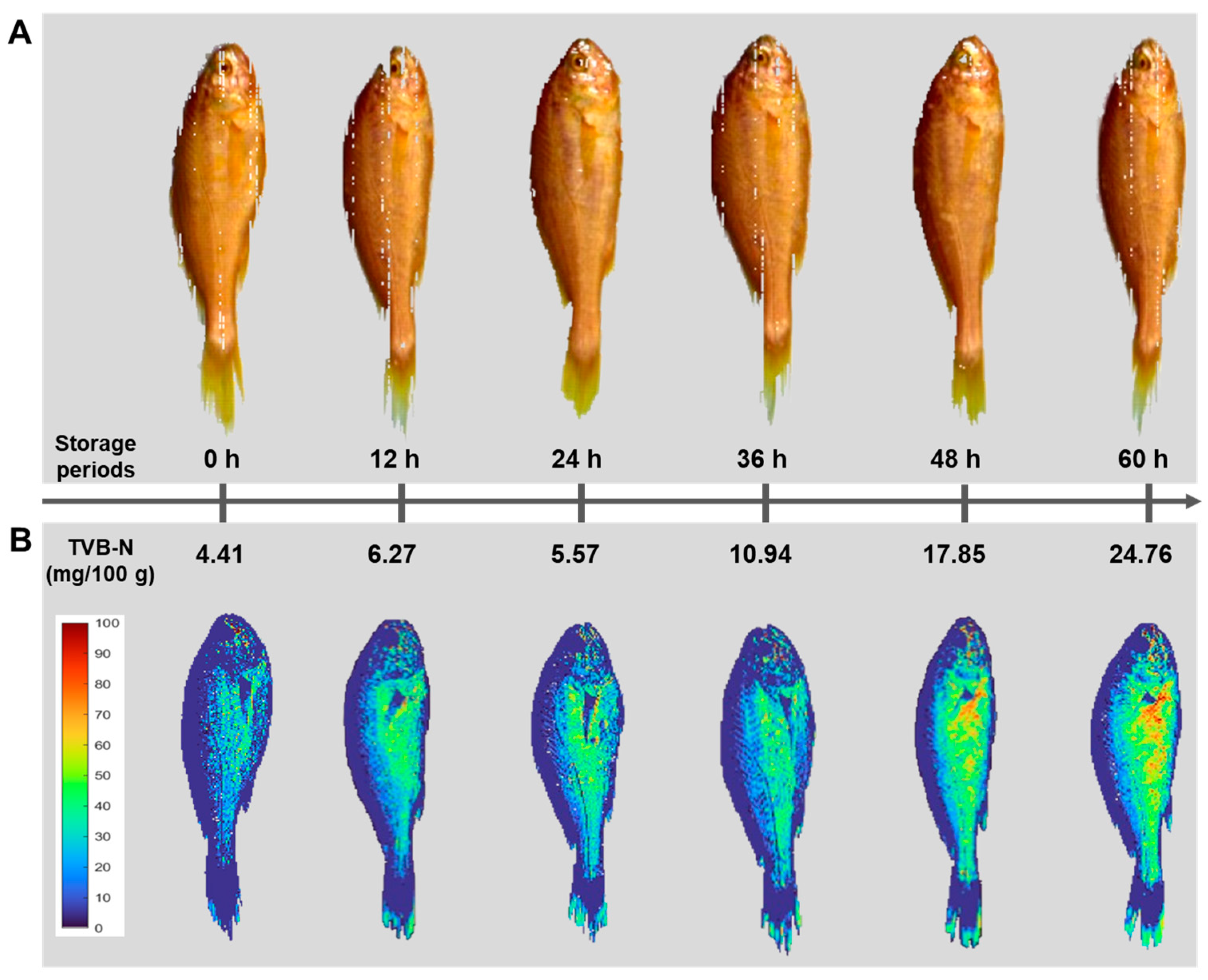
| Storage Periods | 0 h | 12 h | 24 h | 36 h | 48 h | 60 h |
|---|---|---|---|---|---|---|
| TVB-N (mg/100 g) | 4.41 ± 2.04 a,1 | 6.27 ± 1.81 a | 5.57 ± 2.28 a | 10.94 ± 1.31 b | 17.85 ± 3.21 c | 24.76 ± 7.17 d |
| Spectrum Pre-Processing 1 | Calibration (n = 90) Parameters 2 | |||
|---|---|---|---|---|
| Rc2 | RMSEC | Rcv2 | RMSECV | |
| Raw | 0.8395 | 3.4496 | 0.7991 | 3.8525 |
| SNV | 0.8544 | 3.2857 | 0.7975 | 3.9497 |
| MSC | 0.8534 | 3.2963 | 0.7912 | 4.0116 |
| SG-1 | 0.9028 | 2.6842 | 0.8265 | 3.6853 |
| SG-2 | 0.8603 | 3.2182 | 0.7699 | 4.1698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.S.; Yun, D.-Y.; Lee, G.; Park, S.-K.; Lim, J.-H.; Choi, J.-H.; Park, K.-J.; Cho, J.-S. Prediction and Visualization of Total Volatile Basic Nitrogen in Yellow Croaker (Larimichthys polyactis) Using Shortwave Infrared Hyperspectral Imaging. Foods 2024, 13, 3228. https://doi.org/10.3390/foods13203228
Kim SS, Yun D-Y, Lee G, Park S-K, Lim J-H, Choi J-H, Park K-J, Cho J-S. Prediction and Visualization of Total Volatile Basic Nitrogen in Yellow Croaker (Larimichthys polyactis) Using Shortwave Infrared Hyperspectral Imaging. Foods. 2024; 13(20):3228. https://doi.org/10.3390/foods13203228
Chicago/Turabian StyleKim, Sang Seop, Dae-Yong Yun, Gyuseok Lee, Seul-Ki Park, Jeong-Ho Lim, Jeong-Hee Choi, Kee-Jai Park, and Jeong-Seok Cho. 2024. "Prediction and Visualization of Total Volatile Basic Nitrogen in Yellow Croaker (Larimichthys polyactis) Using Shortwave Infrared Hyperspectral Imaging" Foods 13, no. 20: 3228. https://doi.org/10.3390/foods13203228






