HPLC-MS/MS and ICP-MS for Evaluation of Mycotoxins and Heavy Metals in Edible Insects and Their Defatted Cakes Resulting from Supercritical Fluid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Samples
2.3. Supercritical Fluid Extraction (SFE)
2.4. Mycotoxins Extraction
2.5. UHPLC-MS/MS Analysis
2.6. Method Optimization
2.7. Heavy Metals Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Occurrence of Mycotoxins in Insects
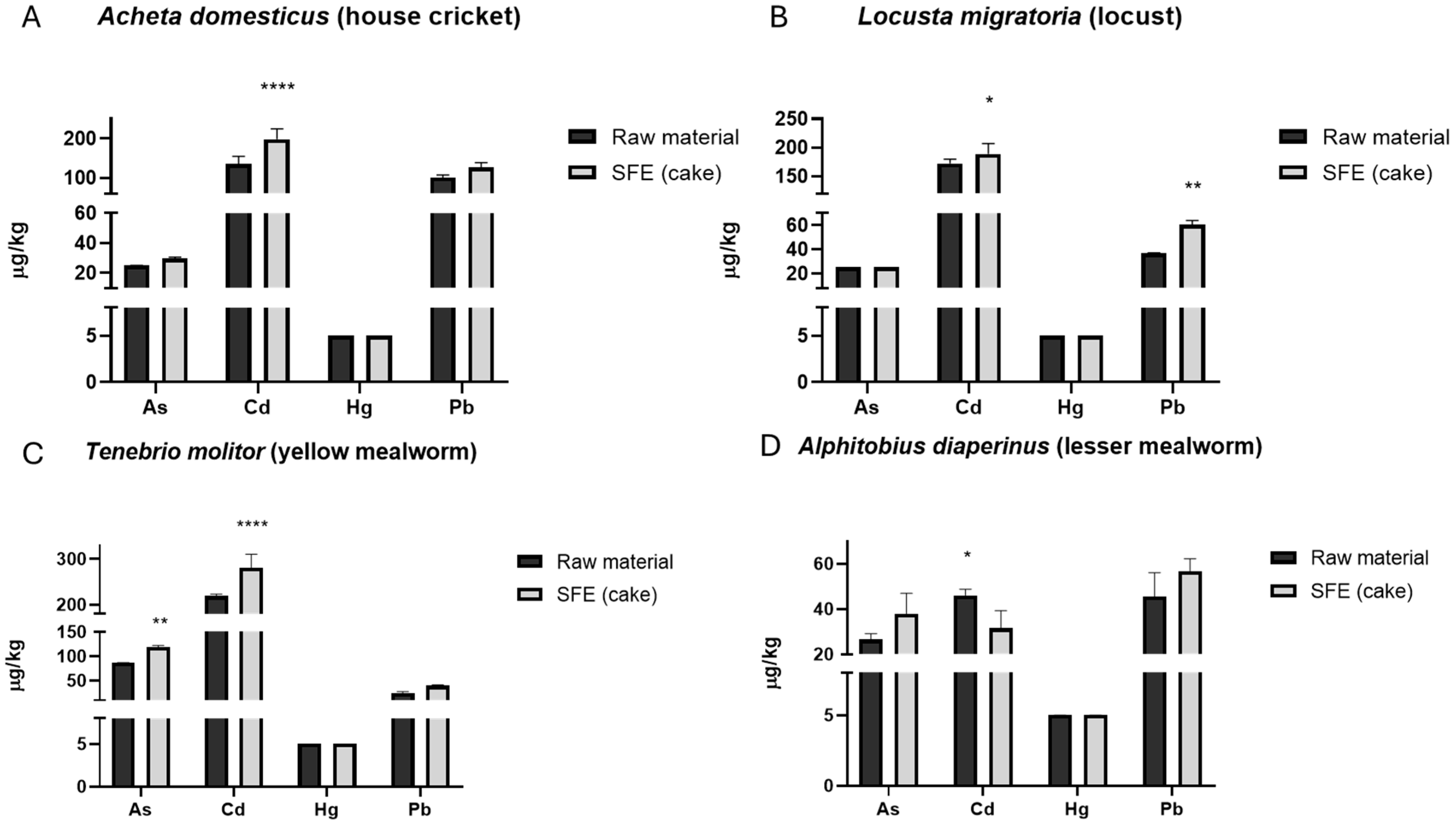
3.2. Occurrence of Heavy Metals in Insects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, T.; Zhao, Y.; Jiang, L.; Sui, X. Structural, Extraction and Safety Aspects of Novel Alternative Proteins from Different Sources. Food Chem. 2024, 436, 137712. [Google Scholar] [CrossRef] [PubMed]
- Can Karaca, A.; Nickerson, M.; Caggia, C.; Randazzo, C.L.; Balange, A.K.; Carrillo, C.; Gallego, M.; Sharifi-Rad, J.; Kamiloglu, S.; Capanoglu, E. Nutritional and Functional Properties of Novel Protein Sources. Food Rev. Int. 2023, 39, 6045–6077. [Google Scholar] [CrossRef]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as Valuable Sources of Protein and Peptides: Production, Functional Properties, and Challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ramírez, J.; Mondragón-Portocarrero, A.C.; Rodríguez, J.A.; Lorenzo, J.M.; Santos, E.M. Algae as a Potential Source of Protein Meat Alternatives. Front. Nutr. 2023, 10, 1254300. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Munialo, C.D.; Stewart, D.; Campbell, L.; Euston, S.R. Extraction, Characterisation and Functional Applications of Sustainable Alternative Protein Sources for Future Foods: A Review. Future Foods 2022, 6, 100152. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on Future Protein Production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Lombardi, A.; Vecchio, R.; Borrello, M.; Caracciolo, F.; Cembalo, L. Willingness to Pay for Insect-Based Food: The Role of Information and Carrier. Food Qual. Prefer. 2019, 72, 177–187. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Gałęcki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Gołaszewski, J. Edible Insect Farming in the Context of the EU Regulations and Marketing—An Overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef]
- Ros-Baró, M.; Casas-Agustench, P.; Díaz-Rizzolo, D.A.; Batlle-Bayer, L.; Adrià-Acosta, F.; Aguilar-Martínez, A.; Medina, F.-X.; Pujolà, M.; Bach-Faig, A. Edible Insect Consumption for Human and Planetary Health: A Systematic Review. Int. J. Env. Res. Public. Health 2022, 19, 11653. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Edible Insects: An Overview on Nutritional Characteristics, Safety, Farming, Production Technologies, Regulatory Framework, and Socio-Economic and Ethical Implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Niermans, K.; Meyer, A.M.; den Hil, E.F.H.-V.; van Loon, J.J.A.; van der Fels-Klerx, H.J. A Systematic Literature Review on the Effects of Mycotoxin Exposure on Insects and on Mycotoxin Accumulation and Biotransformation. Mycotoxin Res. 2021, 37, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of Hazardous Chemicals in Edible Insects and Insect-Based Food Intended for Human Consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Evans, N.M.; Shao, S. Mycotoxin Metabolism by Edible Insects. Toxins 2022, 14, 217. [Google Scholar] [CrossRef]
- The Commission of the European Communities Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L 364/5. Available online: http://data.europa.eu/eli/reg/2006/1881/oj (accessed on 5 October 2024).
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Viñas, P. High-Resolution Mass Spectrometry for the Determination of Mycotoxins in Biological Samples. A Review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Gacem, M.A.; Ould El Hadj-Khelil, A.; Boudjemaa, B.; Gacem, H. Mycotoxins Occurrence, Toxicity and Detection Methods. Sustain. Agric. Rev. 2020, 40, 1–42. [Google Scholar]
- Liu, L.; Wu, Q.; Miao, X.; Fan, T.; Meng, Z.; Chen, X.; Zhu, W. Study on Toxicity Effects of Environmental Pollutants Based on Metabolomics: A Review. Chemosphere 2022, 286, 131815. [Google Scholar] [CrossRef]
- Malematja, E.; Manyelo, T.G.; Sebola, N.A.; Kolobe, S.D.; Mabelebele, M. The Accumulation of Heavy Metals in Feeder Insects and Their Impact on Animal Production. Sci. Total Environ. 2023, 885, 163716. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Sajid Hamid Akash, M. Prevalence of Exposure of Heavy Metals and Their Impact on Health Consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Meijer, N.; van den Hil, E.F.H.; van der Fels-Klerx, H.J. Chemical Food Safety Hazards of Insects Reared for Food and Feed. J. Insects Food Feed. 2021, 7, 823–831. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Cravotto, G.; Moreno, A.; Barba, F.J. Sequential Extraction of Almond Hull Biomass with Pulsed Electric Fields (PEF) and Supercritical CO2 for the Recovery of Lipids, Carbohydrates and Antioxidants. Food Bioprod. Process. 2023, 139, 216–226. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2002/657/EC. Off. J. Eur. Communities 2002, 8, L 221/8. Available online: http://data.europa.eu/eli/dec/2002/657/oj (accessed on 5 October 2024).
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, 180, 84–109. [Google Scholar]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo Salar) Side Streams as a Bioresource to Obtain Potential Antioxidant Peptides after Applying Pressurized Liquid Extraction (Ple). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- Gallardo, J.A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Occurrence and Dietary Exposure Assessment to Enniatin B through Consumption of Cereal-Based Products in Spain and the Catalonia Region. Toxins 2023, 15, 24. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Natural Occurrence of Emerging Fusarium Mycotoxins in Feed and Fish from Aquaculture. J. Agric. Food Chem. 2014, 62, 12462–12470. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2021/1975 of 12 November 2021 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Locusta Migratoria as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2021, 402, 10–16. [Google Scholar]
- Kachapulula, P.W.; Akello, J.; Bandyopadhyay, R.; Cotty, P.J. Aflatoxin Contamination of Dried Insects and Fish in Zambia. J. Food Prot. 2018, 81, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, E.; Wauters, J.; Van Der Borght, M.; Claes, J.; Huysman, S.; Croubels, S.; Vanhaecke, L. Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole Orbitrap High-Resolution Mass Spectrometry for Multi-Residue Screening of Pesticides, (Veterinary) Drugs and Mycotoxins in Edible Insects. Food Chem. 2019, 293, 187–196. [Google Scholar] [CrossRef]
- Camenzuli, L.; Van Dam, R.; de Rijk, T.; Andriessen, R.; Van Schelt, J.; Van der Fels-Klerx, H. Tolerance and Excretion of the Mycotoxins Aflatoxin B1, Zearalenone, Deoxynivalenol, and Ochratoxin A by Alphitobius Diaperinus and Hermetia Illucens from Contaminated Substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef]
- Bisconsin-Junior, A.; Feitosa, B.F.; Silva, F.L.; Mariutti, L.R.B. Mycotoxins on Edible Insects: Should We Be Worried? Food Chem. Toxicol. 2023, 177, 113845. [Google Scholar] [CrossRef]
- Lopes, S.J.S.; Sant’Ana, A.S.; Freire, L. Non-Thermal Emerging Processing Technologies: Mitigation of Microorganisms and Mycotoxins, Sensory and Nutritional Properties Maintenance in Clean Label Fruit Juices. Food Res. Int. 2023, 168, 112727. [Google Scholar] [CrossRef]
- Tokuşoǧlu, Ö.; Alpas, H.; Bozoǧlu, F. High Hydrostatic Pressure Effects on Mold Flora, Citrinin Mycotoxin, Hydroxytyrosol, Oleuropein Phenolics and Antioxidant Activity of Black Table Olives. Innov. Food Sci. Emerg. Technol. 2010, 11, 250–258. [Google Scholar] [CrossRef]
- Hao, H.; Zhou, T.; Koutchma, T.; Wu, F.; Warriner, K. High Hydrostatic Pressure Assisted Degradation of Patulin in Fruit and Vegetable Juice Blends. Food Control 2016, 62, 237–242. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Acheta Domesticus as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022, 30, 108–114. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Yellow Mealworm (Tenebrio Molitor Larva) as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Off. J. Eur. Union 2022, 2016, 48–119. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder Forms of Alphitobius Diaperinus Larvae (Lesser Mealworm) as a Novel Food and Amending Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2023, 58, 10–15. [Google Scholar]
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Prot. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- Vijver, M.; Jager, T.; Posthuma, L.; Peijnenburg, W. Metal Uptake from Soils and Soil–Sediment Mixtures by Larvae of Tenebrio molitor (L.) (Coleoptera). Ecotoxicol. Env. Saf. 2003, 54, 277–289. [Google Scholar] [CrossRef]
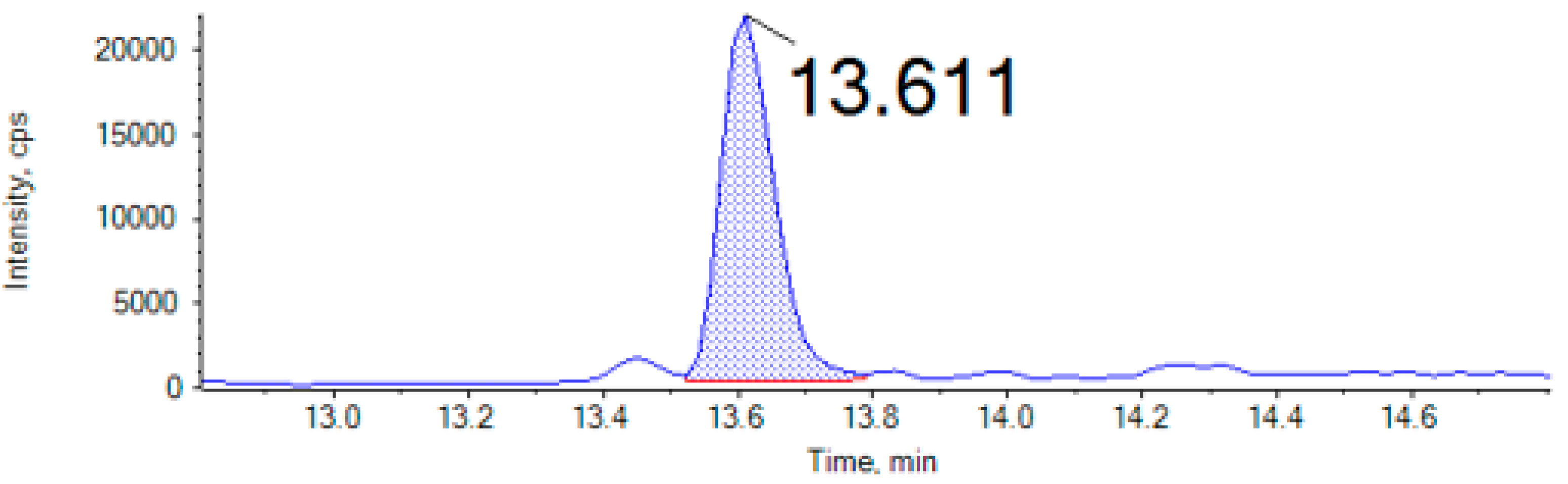
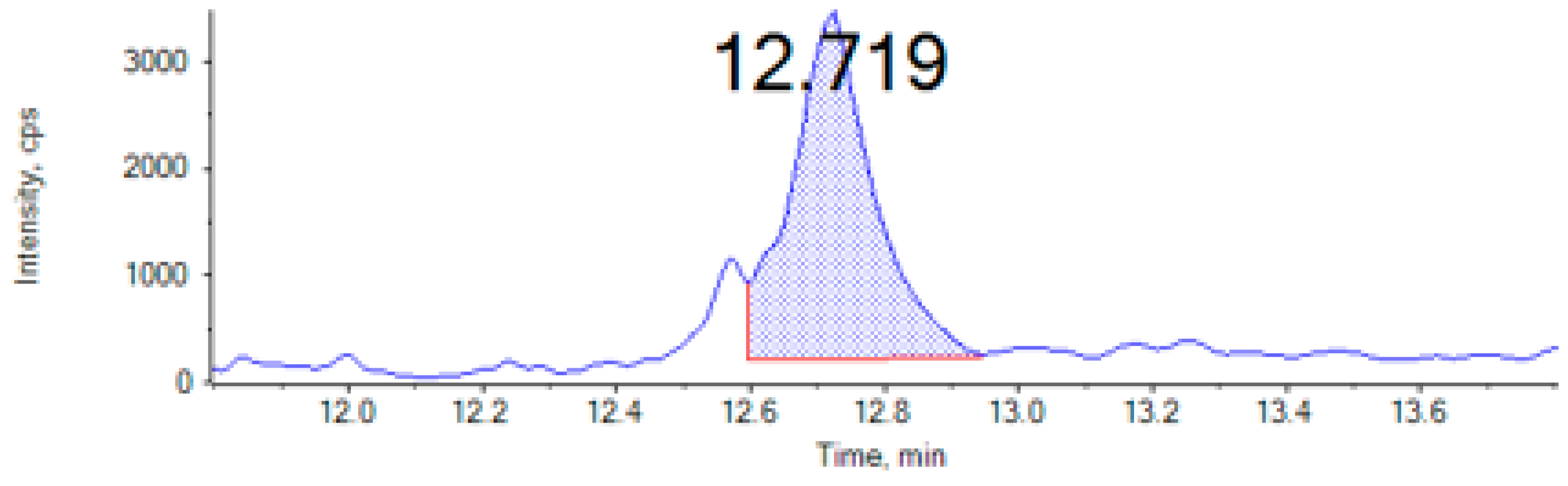
| Mycotoxin | tR (min) | DP | Precursor Ion (m/z) | Quantification Ion (Q) | Confirmation Ion (q) | SSE% | Recovery | LOQ µg/Kg | LOD µg/Kg | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE | Product Ion (m/z) | CXP | CE | Precursor Ion (m/z) | CXP | ||||||||
| AFB1 | 8.1 | 106 | 313.1 | 33 | 285.2 | 16 | 91 | 128.1 | 10 | 66 | 114 | 1 | 0.3 |
| AFB2 | 7.7 | 96 | 315.1 | 37 | 287.2 | 18 | 43 | 259.2 | 18 | 64 | 93 | 0.1 | 0.03 |
| AFG1 | 7.5 | 86 | 329.1 | 39 | 243.1 | 14 | 59 | 200 | 12 | 63 | 119 | 0.1 | 0.03 |
| AFG2 | 7.2 | 111 | 331.1 | 35 | 313.2 | 18 | 43 | 245.2 | 14 | 65 | 106 | 1 | 0.3 |
| OTA | 10.1 | 91 | 404 | 37 | 239 | 16 | 105 | 102 | 14 | 52 | 118 | 0.1 | 0.03 |
| ENNA | 12.6 | 106 | 699.4 | 43 | 210.1 | 12 | 47 | 228 | 18 | 84 | 64 | 0.1 | 0.03 |
| ENNA1 | 12.4 | 96 | 685.4 | 46 | 210.1 | 8 | 49 | 228.2 | 20 | 76 | 67 | 0.1 | 0.03 |
| ENNB | 12 | 81 | 657.5 | 45 | 196.3 | 18 | 47 | 214.1 | 18 | 73 | 65 | 0.1 | 0.03 |
| ENNB1 | 12.2 | 111 | 671.4 | 43 | 196 | 12 | 41 | 210 | 12 | 80 | 71 | 0.1 | 0.03 |
| Mycotoxins | ENNA (µg/kg) | ENNA1 (µg/kg) | ENNB (µg/kg) | ENNB1 (µg/kg) | AFG2 (µg/kg) | OTA (µg/kg) | |
|---|---|---|---|---|---|---|---|
| Insect Samples | |||||||
| Cricket | 1.4 ± 0.02 | 1.4 ± 0.03 | 2.2 ± 0.26 | 1.7 ± 0.14 | nd | nd | |
| Locust | nd | 0.7 ± 0.04 | 1.2 ± 0.01 | 0.8 ± 0.04 | 115.5 ± 1.7 | nd | |
| Mealworm | nd | nd | nd | nd | nd | 58.1 ± 0.4 | |
| Buffalo worm | nd | 0.9 ± 0.03 | 1.3 ± 0.03 | 1.0 ± 0.05 | nd | nd | |
| Insects Species | As (µg/Kg) | Cd (µg/Kg) | Hg (µg/Kg) | Pb (µg/Kg) |
|---|---|---|---|---|
| Cricket | 25.00 ± 0.1 | 134.33 ± 20 | <5 | 100.33 ± 7 |
| Locust | <25 | 172 ± 8 | <5 | 36.70 ± 0.4 |
| Mealworm | 86.67 ± 0.6 | 219 ± 4.2 | <5 | 24 ± 4 |
| Buffalo worm | 26.5 ± 2.6 | 46.05 ± 2.8 | <5 | 45.50 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, L.C.; Rodríguez-García, A.; Castagnini, J.M.; Salgado-Ramos, M.; Martínez-Culebras, P.V.; Barba, F.J.; Pallarés, N. HPLC-MS/MS and ICP-MS for Evaluation of Mycotoxins and Heavy Metals in Edible Insects and Their Defatted Cakes Resulting from Supercritical Fluid Extraction. Foods 2024, 13, 3233. https://doi.org/10.3390/foods13203233
Ramos LC, Rodríguez-García A, Castagnini JM, Salgado-Ramos M, Martínez-Culebras PV, Barba FJ, Pallarés N. HPLC-MS/MS and ICP-MS for Evaluation of Mycotoxins and Heavy Metals in Edible Insects and Their Defatted Cakes Resulting from Supercritical Fluid Extraction. Foods. 2024; 13(20):3233. https://doi.org/10.3390/foods13203233
Chicago/Turabian StyleRamos, Lucia Cuesta, Aroa Rodríguez-García, Juan M. Castagnini, Manuel Salgado-Ramos, Pedro V. Martínez-Culebras, Francisco J. Barba, and Noelia Pallarés. 2024. "HPLC-MS/MS and ICP-MS for Evaluation of Mycotoxins and Heavy Metals in Edible Insects and Their Defatted Cakes Resulting from Supercritical Fluid Extraction" Foods 13, no. 20: 3233. https://doi.org/10.3390/foods13203233
APA StyleRamos, L. C., Rodríguez-García, A., Castagnini, J. M., Salgado-Ramos, M., Martínez-Culebras, P. V., Barba, F. J., & Pallarés, N. (2024). HPLC-MS/MS and ICP-MS for Evaluation of Mycotoxins and Heavy Metals in Edible Insects and Their Defatted Cakes Resulting from Supercritical Fluid Extraction. Foods, 13(20), 3233. https://doi.org/10.3390/foods13203233








