Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Cells and Viruses
2.4. Cytotoxicity Test
2.5. Nitric Oxide Concentration Determination
2.6. Western Blotting
2.7. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
2.8. Transcriptome Analysis
2.9. In-Cell Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Plaque Reduction Neutralization Test (PRNT)
2.11. HPLC-QTOF-MS Characterization of Compounds in EA Fraction
2.12. Data Analysis
3. Results and Discussion
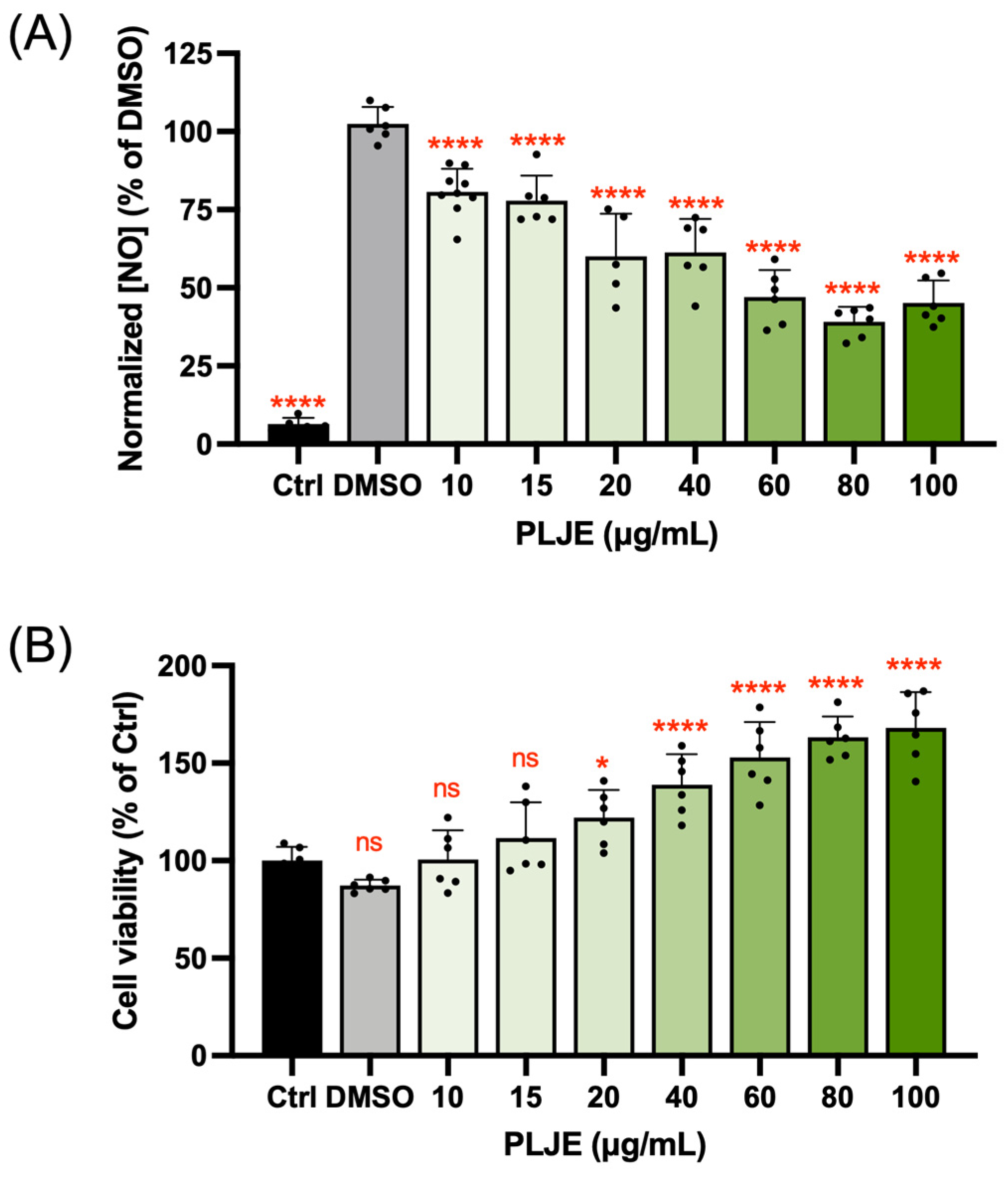
3.1. Nitric Oxide Inhibitory Activity of Papaya Leaf Juice Extract
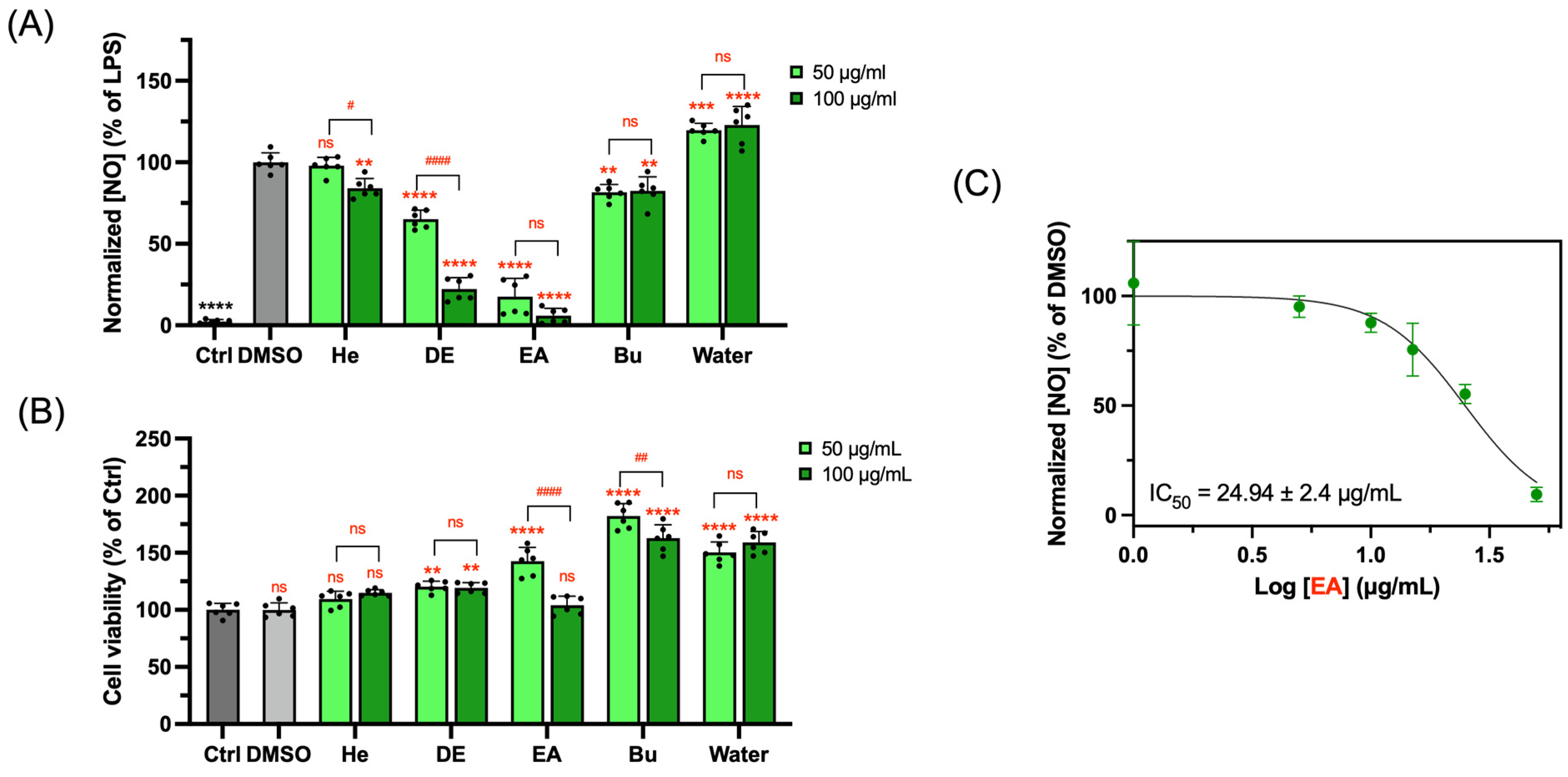
3.2. NO Inhibitory Activity of Subfractions Extracted from Papaya Leaf Juice
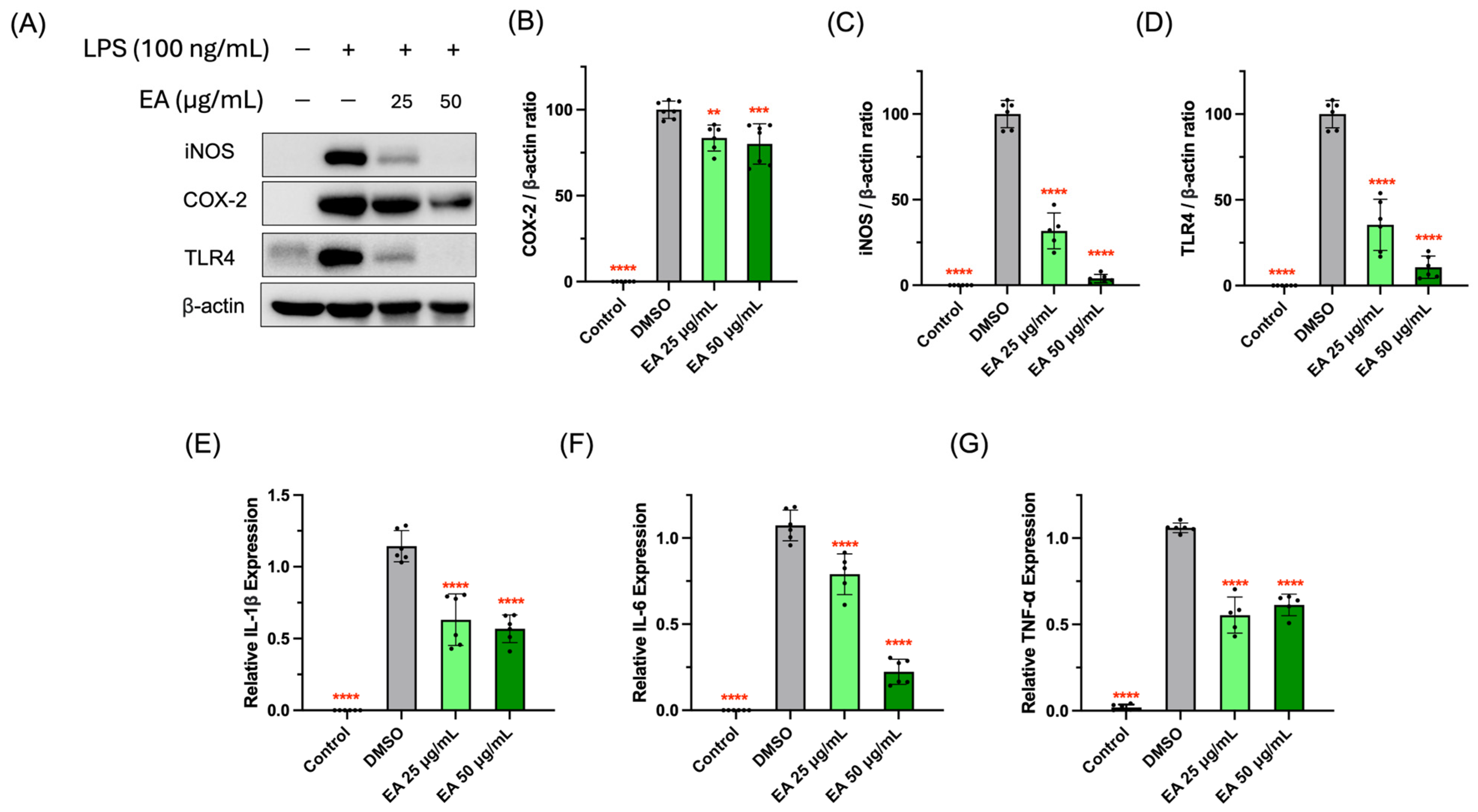
3.3. Suppressive Activity of EA Fraction on Pro-Inflammatory Protein and Cytokines
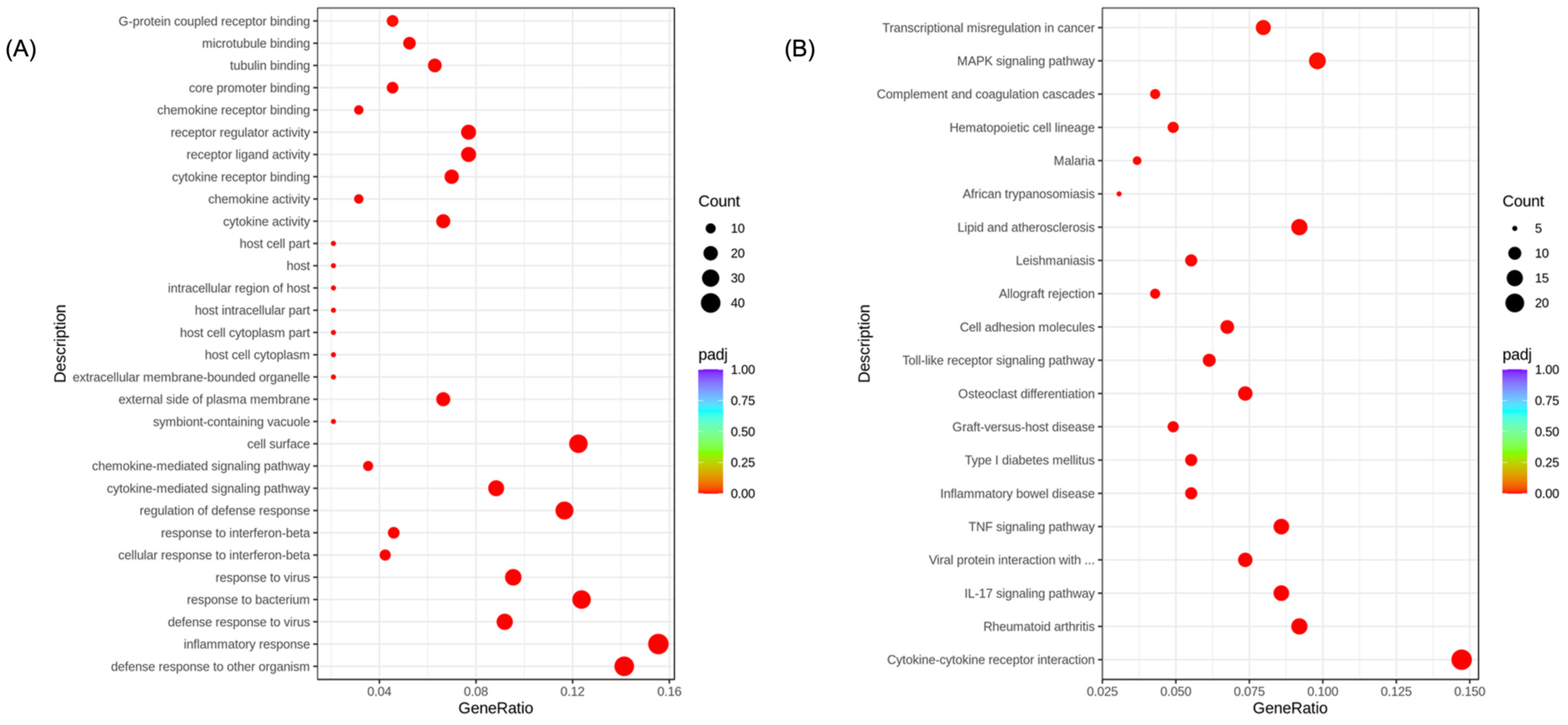
3.4. Transcriptome Analysis for the Potential Anti-Inflammatory Mechanisms of EA Fraction
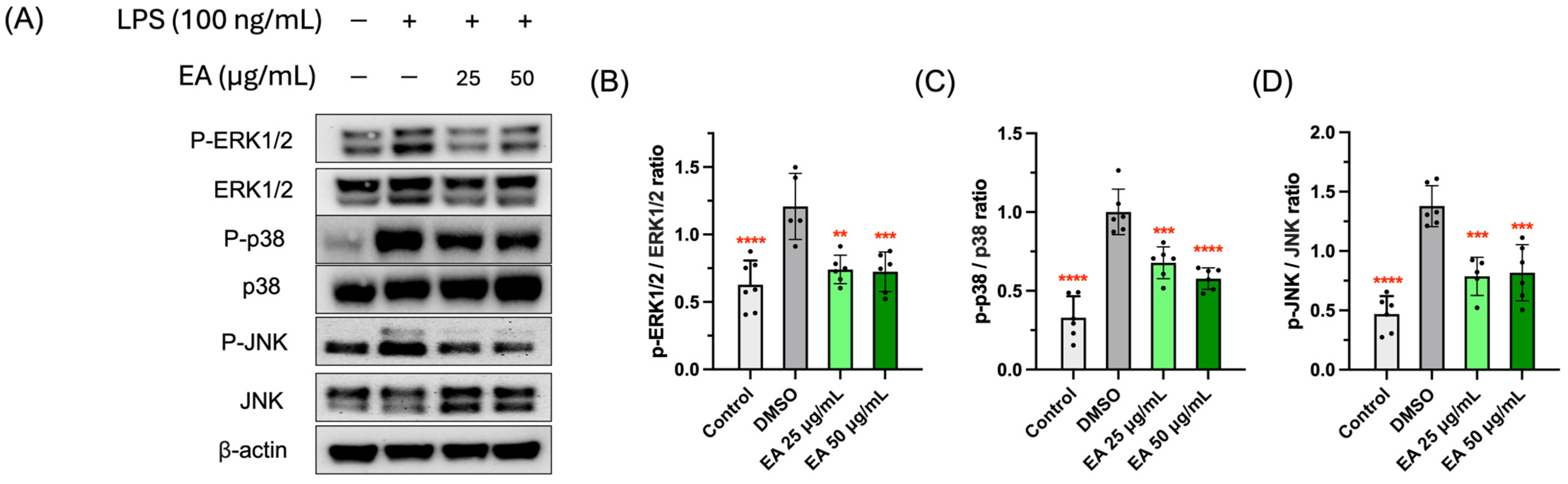
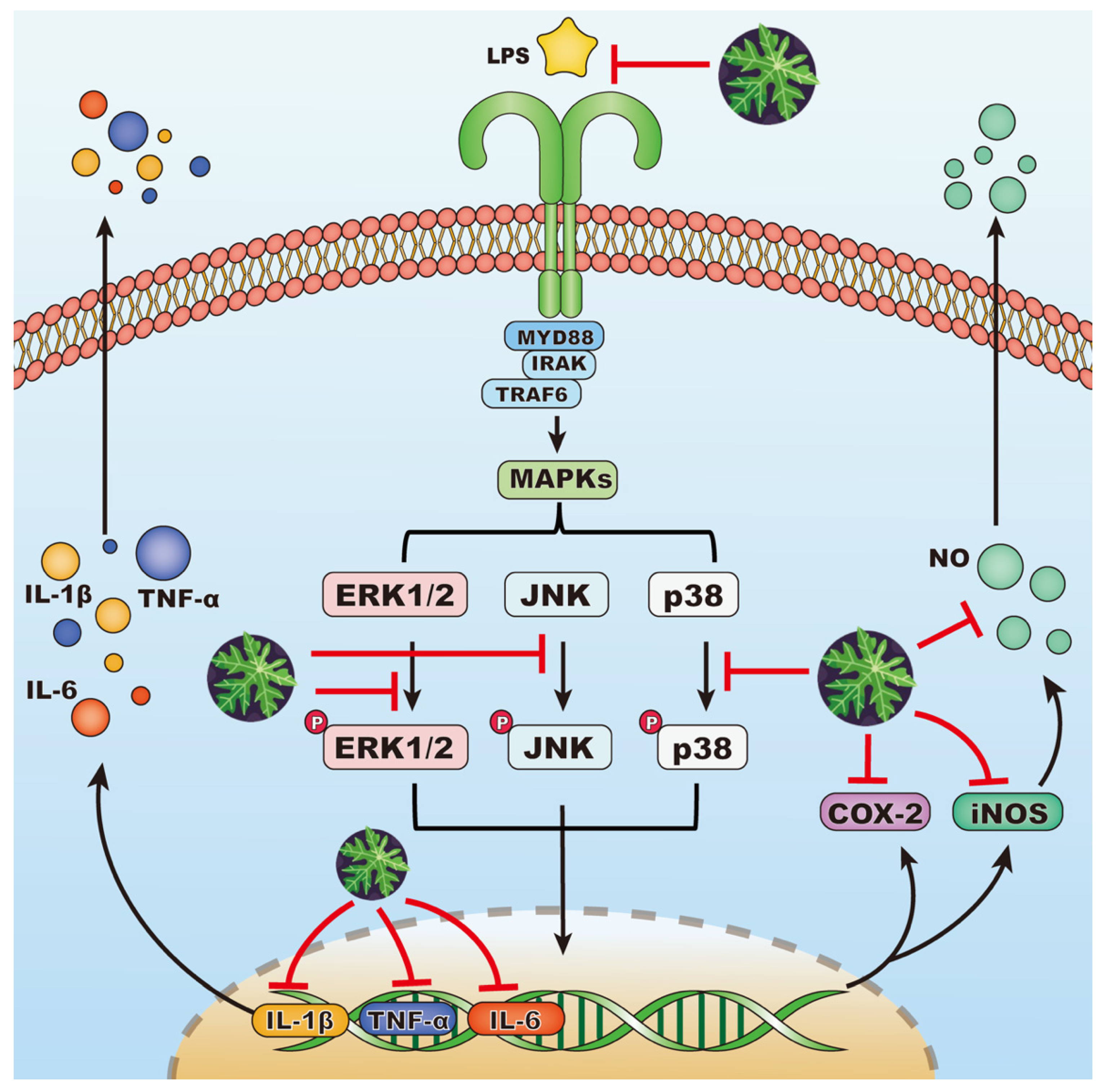
3.5. Effects of EA Fraction on Suppressing MAPK Signaling Pathway
3.6. Anti-Coronavirus Activity of EA Fraction
3.7. HPLC-QTOF-MS Analysis of Phytochemicals in EA Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Antunes Carvalho, F.; Renner, S.S., IV. A Dated Phylogeny of the Papaya Family (Caricaceae) Reveals the Crop’s Closest Relatives and the Family’s Biogeographic History §. In Molecular Phylogeny, Biogeography and an e-Monograph of the Papaya Family (Caricaceae) as an Example of Taxonomy in the Electronic Age; Springer: Berlin/Heidelberg, Germany, 2015; pp. 49–81. [Google Scholar]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Ikram, E.H.K.; Stanley, R.; Netzel, M.; Fanning, K. Phytochemicals of papaya and its traditional health and culinary uses—A review. J. Food Compos. Anal. 2015, 41, 201–211. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Shaw, P.N.; Parat, M.O.; Hewavitharana, A.K. Anticancer activity of C arica papaya: A review. Mol. Nutr. Food Res. 2013, 57, 153–164. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Ayaz, M.; Abbasi, B.H.; Mohammad, I.; Fazal, L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop. Biomed. 2011, 1, 330–333. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Shaw, P.N.; Parat, M.-O.; Pandey, S.; Falconer, J.R. Compound identification and in vitro cytotoxicity of the supercritical carbon dioxide extract of papaya freeze-dried leaf juice. Processes 2020, 8, 610. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-inflammation activity of flavones and their structure–activity relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Salim, E.; Kumolosasi, E.; Jantan, I. Inhibitory effect of selected medicinal plants on the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated human peripheral blood mononuclear cells. J. Nat. Med. 2014, 68, 647–653. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Adebukola, O.M.; Funmilayo, A.A.; Soladoye, A.O. Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 2008, 16, 168–173. [Google Scholar] [CrossRef]
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef]
- Ng, K.T.; Mohd-Ismail, N.K.; Tan, Y.J. Spike S2 subunit: The dark horse in the race for prophylactic and therapeutic interventions against SARS-CoV-2. Vaccines 2021, 9, 178. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Rahimi, B.; Panahi, M.; Abkhiz, S.; Saraygord-Afshari, N.; Milani, M.; Alizadeh, E. An overview of Betacoronaviruses-associated severe respiratory syndromes, focusing on sex-type-specific immune responses. Int. Immunopharmacol. 2021, 92, 107365. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.L.; Wark, P.A.; Esneau, C.; Nichol, K.S.; Hsu, A.C.; Bartlett, N.W. Human coronaviruses 229E and OC43 replicate and induce distinct antiviral responses in differentiated primary human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L926–L931. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- García, L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020, 11, 556786. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Sheida, A.; Taghizadieh, M.; Memar, M.Y.; Hamblin, M.R.; Baghi, H.B.; Nahand, J.S.; Asemi, Z.; Mirzaei, H. Paxlovid (Nirmatrelvir/Ritonavir): A new approach to COVID-19 therapy? Biomed. Pharmacother. 2023, 162, 114367. [Google Scholar] [CrossRef]
- Taghavi, M.R.; Tamanaei, T.T.; Oghazian, M.B.; Tavana, E.; Mollazadeh, S.; Niloofar, P.; Oghazian, S.; Hoseinzadeh, A.; Hesari, A.; Mohseni, M.A. Effectiveness of Fortified Garlic Extract Oral Capsules as Adjuvant Therapy in Hospitalized Patients with Coronavirus Disease 2019: A Triple-Blind Randomized Controlled Clinical Trial. Curr. Ther. Res. 2023, 98, 100699. [Google Scholar] [CrossRef]
- Adel, A.; Elnaggar, M.S.; Albohy, A.; Elrashedy, A.A.; Mostafa, A.; Kutkat, O.; Abdelmohsen, U.R.; Al-Sayed, E.; Rabeh, M.A. Evaluation of antiviral activity of Carica papaya leaves against SARS-CoV-2 assisted by metabolomic profiling. RSC Adv. 2022, 12, 32844–32852. [Google Scholar] [CrossRef]
- Patil, P.; Alagarasu, K.; Chowdhury, D.; Kakade, M.; Cherian, S.; Kaushik, S.; Yadav, J.; Parashar, D. In-vitro antiviral activity of Carica papaya formulations against dengue virus type 2 and chikungunya virus. Heliyon 2022, 8, e11879. [Google Scholar] [CrossRef]
- Lee, K.; Padzil, A.; Syahida, A.; Abdullah, N.; Zuhainis, S.; Maziah, M.; Sulaiman, M.; Israf, D.; Shaari, K.; Lajis, N. Evaluation of anti-inflammatory, antioxidant and antinociceptive activities of six Malaysian medicinal plants. J. Med. Plant Res. 2011, 5, 5555–5563. [Google Scholar] [CrossRef]
- Lip, K.M.; Shen, S.; Yang, X.; Keng, C.T.; Zhang, A.; Oh, H.L.J.; Li, Z.H.; Hwang, L.-A.; Chou, C.-F.; Fielding, B.C. Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J. Virol. 2006, 80, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Monteil, V.M.; Maurer-Stroh, S.; Yew, C.W.; Leong, C.; Mohd-Ismail, N.K.; Arularasu, S.C.; Chow, V.T.K.; Lin, R.T.P.; Mirazimi, A. Monoclonal antibodies for the S2 subunit of spike of SARS-CoV-1 cross-react with the newly-emerged SARS-CoV-2. Eurosurveillance 2020, 25, 2000291. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Yang, X.; Huang, D. Structure–activity relationship (SAR) of flavones on their anti-inflammatory activity in murine macrophages in culture through the NF-κB pathway and c-Src kinase receptor. J. Agric. Food Chem. 2022, 70, 8788–8798. [Google Scholar] [CrossRef]
- Amanat, F.; White, K.M.; Miorin, L.; Strohmeier, S.; McMahon, M.; Meade, P.; Liu, W.C.; Albrecht, R.A.; Simon, V.; Martinez-Sobrido, L. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr. Protoc. Microbiol. 2020, 58, e108. [Google Scholar] [CrossRef]
- Liu, L.; Wen, K.; Li, J.; Hu, D.; Huang, Y.; Qiu, L.; Cai, J.; Che, X. Comparison of plaque-and enzyme-linked immunospot-based assays to measure the neutralizing activities of monoclonal antibodies specific to domain III of dengue virus envelope protein. Clin. Vaccine Immunol. 2012, 19, 73–78. [Google Scholar] [CrossRef]
- Oche, O.; John, O.; Chidi, E.; Rebecca, S.; Vincent, U. Chemical constituents and nutrient composition of Carica papaya and vernonia amygdalina leaf extracts. J. Complement. Altern. Med. 2017, 2, 5. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; Diniz, M.d.F.F.M.; de Athayde-Filho, P.F.; Filho, J.M.B. Anti-inflammatory activity of alkaloids: An update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Funk, C.D.; FitzGerald, G.A. COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 2007, 50, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Tatro, J.M.; Lowe, R.; Goldberg, V.M.; Greenfield, E.M. Comparison of the roles of IL-1, IL-6, and TNFα in cell culture and murine models of aseptic loosening. Bone 2007, 40, 1276–1283. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Neamatallah, T. Mitogen-activated protein kinase pathway: A critical regulator in tumor-associated macrophage polarization. J. Microsc. Ultrastruct. 2019, 7, 53. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Food and Drug Administration. Ritonavir-Boosed Nirmatrelvir (Paxlovid) [Package Insert]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf (accessed on 20 November 2023).
- Weil, T.; Lawrenz, J.; Seidel, A.; Münch, J.; Müller, J.A. Immunodetection assays for the quantification of seasonal common cold coronaviruses OC43, NL63, or 229E infection confirm nirmatrelvir as broad coronavirus inhibitor. Antiviral Res. 2022, 203, 105343. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef]
- Norahmad, N.A.; Mohd Abd Razak, M.R.; Mohmad Misnan, N.; Md Jelas, N.H.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement. Altern. Med. 2019, 19, 44. [Google Scholar] [CrossRef]
- Hamed, A.N.; Abouelela, M.E.; El Zowalaty, A.E.; Badr, M.M.; Abdelkader, M.S. Chemical constituents from Carica papaya Linn. leaves as potential cytotoxic, EGFR wt and aromatase (CYP19A) inhibitors; a study supported by molecular docking. RSC Adv. 2022, 12, 9154–9162. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, W.R.; Lin, H.-Y.; Huang, H.C.; Ko, C.H.; Yang, L.L.; Chen, Y.C. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E2 production. Eur. J. Pharmacol. 2002, 446, 187–194. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Q.; Jia, T.; Zhang, X.; Guo, D.; Jia, Y.; Li, J.; Sun, J. Mechanism of action of nicotiflorin from Tricyrtis maculata in the treatment of acute myocardial infarction: From network pharmacology to experimental pharmacology. Drug Des. Devel. Ther. 2021, 15, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, I.; Timotius, K.H. Phytochemical Analysis, Antimutagenic and antiviral activity of Moringa oleifera L. leaf infusion: In Vitro and in silico studies. Molecules 2022, 27, 4017. [Google Scholar] [CrossRef] [PubMed]
- Fouedjou, R.T.; Chtita, S.; Bakhouch, M.; Belaidi, S.; Ouassaf, M.; Djoumbissie, L.A.; Tapondjou, L.A.; Abul Qais, F. Cameroonian medicinal plants as potential candidates of SARS-CoV-2 inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 8615–8629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Scholle, F.; Kisthardt, S.C.; Xie, D.Y. Flavonols and dihydroflavonols inhibit the main protease activity of SARS-CoV-2 and the replication of human coronavirus 229E. Virology 2022, 571, 21–33. [Google Scholar] [CrossRef]
- Geng, P.; Yang, Y.; Gao, Z.; Yu, Y.; Shi, Q.; Bai, G. Combined effect of total alkaloids from Feculae Bombycis and natural flavonoids on diabetes. J. Pharm. Pharmacol. 2007, 59, 1145–1150. [Google Scholar] [CrossRef]
- Raafat, K.; Breitinger, U.; Mahran, L.; Ayoub, N.; Breitinger, H.G. Synergistic inhibition of glycinergic transmission in vitro and in vivo by flavonoids and strychnine. Toxicol. Sci. 2010, 118, 171–182. [Google Scholar] [CrossRef]
- Tavadyan, L.A.; Minasyan, S.H. Synergistic and antagonistic co-antioxidant effects of flavonoids with trolox or ascorbic acid in a binary mixture. Chem. Sci. J. 2019, 131, 40. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Fernandez, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombo, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, L.; Liu, Z.; Li, Y.; He, X.; Wu, X.; Pehrsson, P.; Sun, J.; Xie, Z.; Slavin, M. Chemical composition of honeysuckle (Lonicerae japonicae) extracts and their potential in inhibiting the SARS-CoV-2 spike protein and ACE2 binding, suppressing ACE2, and scavenging radicals. J. Agric. Food Chem. 2023, 71, 6133–6143. [Google Scholar] [CrossRef]

| Samples (μg/mL) | IC50 (μg/mL) | CC50 (μg/mL) | SI | |||
|---|---|---|---|---|---|---|
| HCoV-OC43 on H1299 (MOI = 0.1) | SARS-CoV-2 on Vero E6 (MOI = 0.025) | H1299 | Vero E6 | HCoV-OC43 on H1299 (MOI = 0.1) | SARS-CoV-2 on Vero E6 (MOI = 0.025) | |
| EA | 0.1247 ± 0.0078 | 0.1853 ± 0.0184 | 294.7 ± 22.7 | >100 | 2363 | >539.7 |
| Nirmatrelvir | 0.03873 ± 0.00316 | 0.3416 ± 0.0477 | >25 | >25 | >645.5 | >73.19 |
| RT (min) | Main Observed m/z | Quantification Ions | MS/MS Fragmentation (m/z) | Possible Formula | Experimental Mass | Error (ppm) | Suggested Compounds |
|---|---|---|---|---|---|---|---|
| 9.12 | 479.3845 | [M + H]+ | 337.19102; 217.13337; 121.06514 | C28H50N2O4 | 478.37706 | 0.4 | Carpaine |
| 9.28 | 757.2209 | [M + H]+ | 303.05058; 121.06528 | C33H40O20 | 756.21129 | 3.0 | Manghaslin |
| 9.29 | 755.2084 | [M − H]− | 300.02432; 178.99403 | C33H40O20 | 756.21129 | 6.5 | Manghaslin |
| 9.79 | 741.2257 | [M + H]+ | 287.05555; 240.19609; 153.01867 | C33H40O19 | 740.21638 | 2.8 | Clitorin |
| 9.80 | 739.2131 | [M − H]− | 285.03526; 284.02927; 178.99423 | C33H40O19 | 740.21638 | 5.5 | Clitorin |
| 10.41 | 611.1619 | [M + H]+ | 303.05017; 229.05013; 153.01869 | C27H30O16 | 610.15338 | 2.8 | Rutin |
| 10.41 | 609.1478 | [M − H]− | 301.03157; 300.02455; 271.02135 | C27H30O16 | 610.15338 | 2.8 | Rutin |
| 10.67 | 595.1664 | [M + H]+ | 287.05510; 240.19552; 153.01845; 91.05425 | C27H30O15 | 594.15847 | 1.2 | Kaempferol-3-O-neohesperidoside |
| 10.67 | 593.1528 | [M − H]− | 285.03523; 284.02950; 225.02632 | C27H30O15 | 594.15847 | 2.8 | Kaempferol-3-O-neohesperidoside |
| 11.12 | 595.1656 | [M + H]+ | 287.05411; 153.01810 | C27H30O15 | 594.15847 | −0.2 | Nicotiflorin |
| 11.12 | 593.1519 | [M − H]− | 285.03652; 284.02909 255.02596 | C27H30O15 | 594.15847 | 1.2 | Nicotiflorin |
| 11.32 | 495.3773 | [M + H]+ | 240.19491; 222.18426 | C26H48N5O4 | 474.37063 | −1.3 | Unknown |
| 11.82 | 449.1059 | [M + H]+ | 287.05374; 153.01770 | C21H20O11 | 448.10056 | −4.4 | Kaempferol-3-O-β-D-glucopyranoside |
| 11.82 | 447.0911 | [M − H]− | 285.03526; 284.02885; 255.02570; 227.03053; 195.06156 | C21H20O11 | 448.10056 | −4.9 | Kaempferol-3-O-β-D-glucopyranoside |
| 12.02 | 428.2976 | [M + H]+ | 287.05333; 240.19400; 222.18360 | C18H41N3O8 | 427.28937 | 2.3 | Unknown |
| 12.02 | 635.1631 | [M − H]− | 301.03095; 284.02913; 119.04588 | C29H32O6 | 636.16903 | 2.1 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Lai, K.-M.; Fu, K.-C.; Kuo, C.-L.; Tan, Y.-J.; Yu, L.; Huang, D. Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism. Foods 2024, 13, 3274. https://doi.org/10.3390/foods13203274
Cao Y, Lai K-M, Fu K-C, Kuo C-L, Tan Y-J, Yu L, Huang D. Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism. Foods. 2024; 13(20):3274. https://doi.org/10.3390/foods13203274
Chicago/Turabian StyleCao, Yujia, Kah-Man Lai, Kuo-Chang Fu, Chien-Liang Kuo, Yee-Joo Tan, Liangli (Lucy) Yu, and Dejian Huang. 2024. "Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism" Foods 13, no. 20: 3274. https://doi.org/10.3390/foods13203274
APA StyleCao, Y., Lai, K.-M., Fu, K.-C., Kuo, C.-L., Tan, Y.-J., Yu, L., & Huang, D. (2024). Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism. Foods, 13(20), 3274. https://doi.org/10.3390/foods13203274









