The Effect of Temperature and Humidity on Yellow Tea Volatile Compounds during Yellowing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tea Leaves
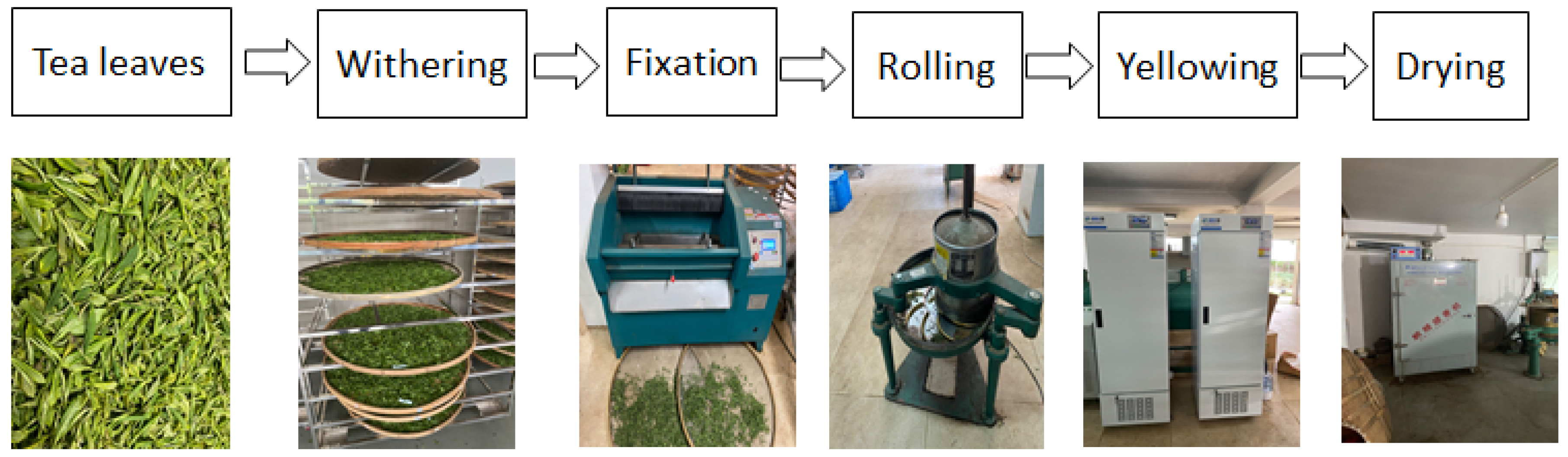
2.2. Yellow Tea Processing and Samplings
- Withering: the fresh tea leaves (5 kg) were spread on a bamboo mat (thickness was 1–3 cm) for 16 h at room temperature until the moisture level reached 70–72%.
- Fixation: the withering tea leaves were subjected to a fixation machine (6CCB-45 type)
- Rolling: the fixation tea leaves were rolled for 30 min in a rolling machine (6CR-25 type).
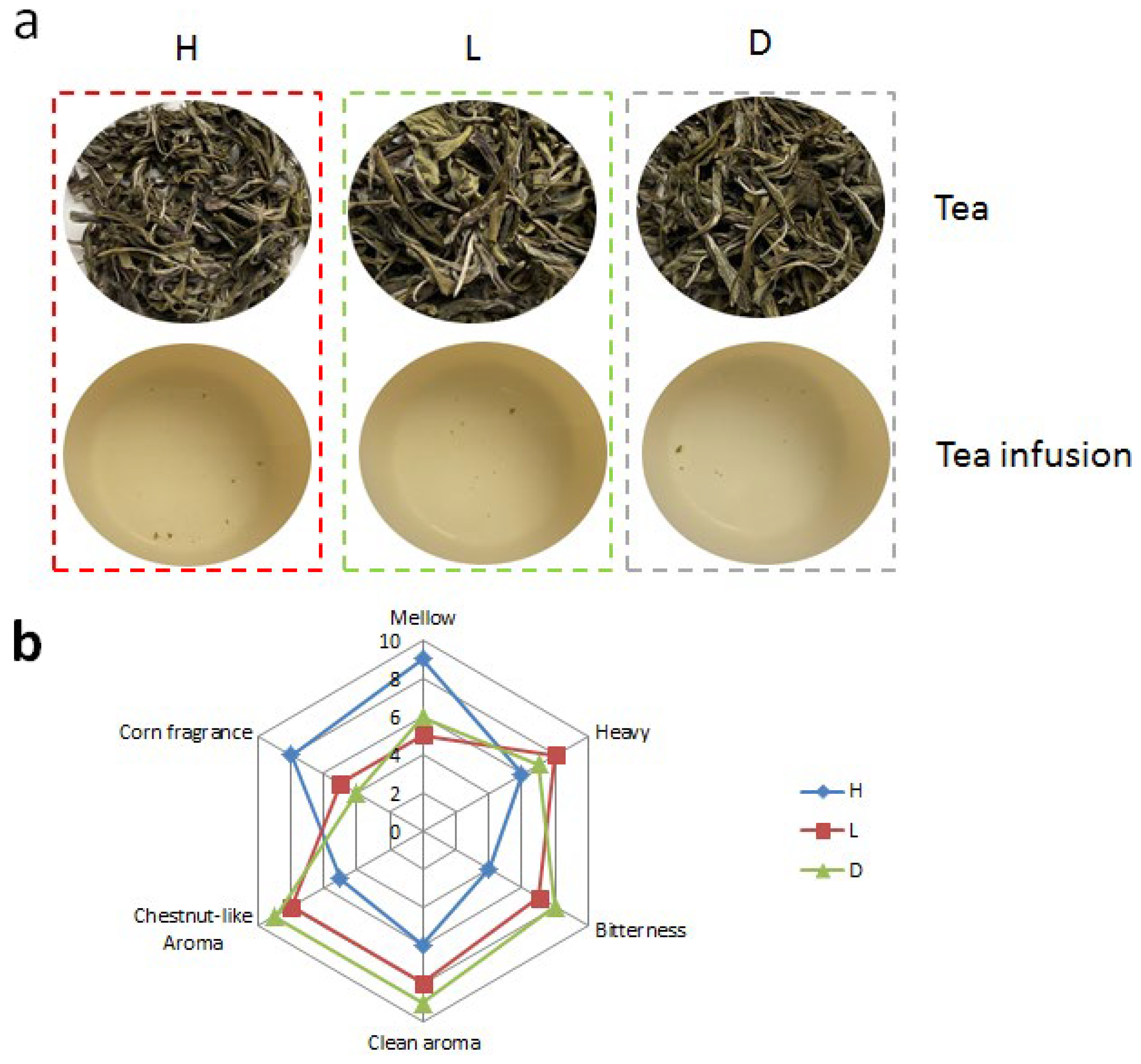
- Yellowing: The rolling tea leaves were divided into three equal portions, then we put two of them into an artificial climate box, which was purchased from Ningbo prandt instrument Co., Ltd., Ningbo, China, with the yellowing environment temperatures and humidities of 40 °C with 90% simultaneously (H) and 50 °C with 50% simultaneously (L), using natural treatment (30 °C with 70% simultaneously) as control (D).
- Samplings: A total of 100 g of each treatment of yellowing for 2 h, 4 h, 6 h, and 8 h were dried at 120 °C and collected as the tea samples.
- Yellow tea processing flowchart: The fresh leaves, processing equipment were shown in Figure 1.
2.3. Preparation of Tea Broth Solution and Internal Standard
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation of Yellow Teas
3.2. Analysis of Volatile Compounds
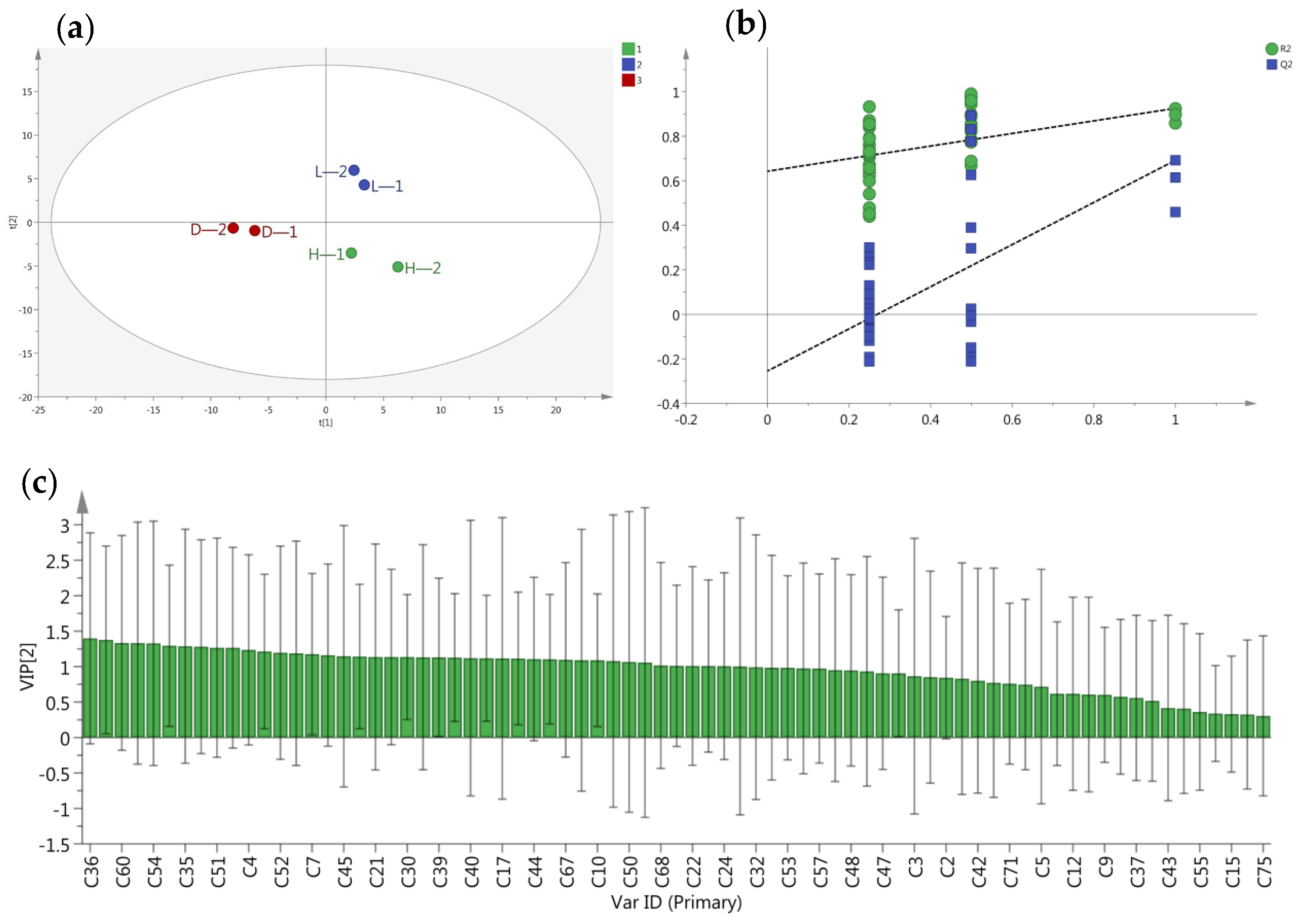
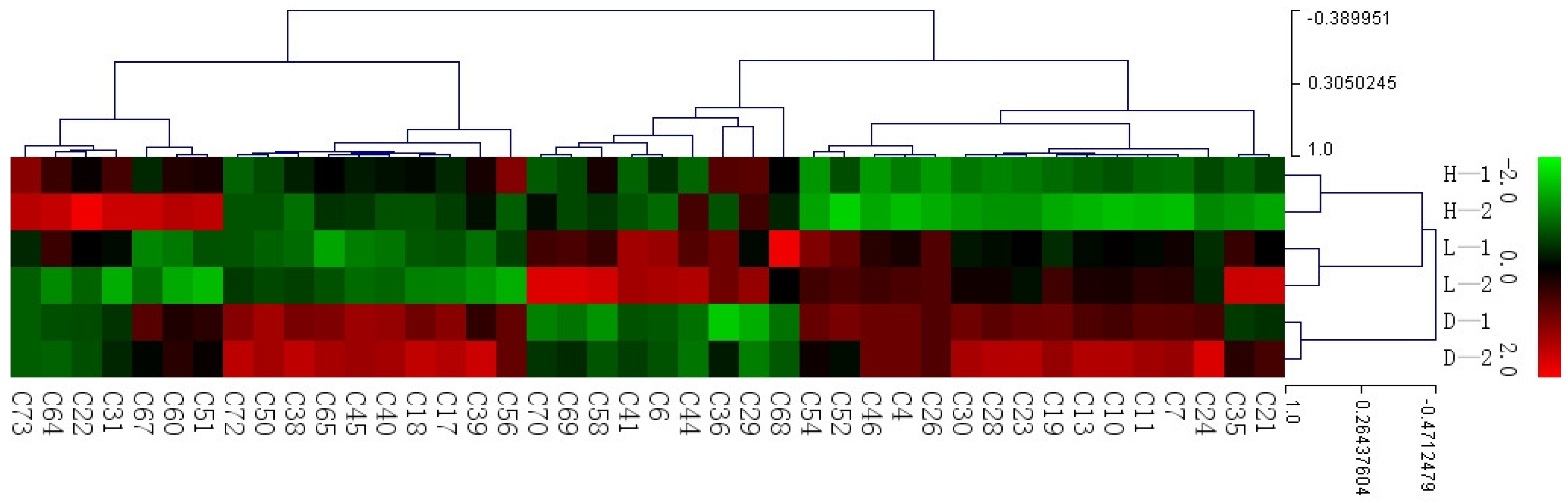
3.3. Differential Volatile Compounds Analysis of Different Treatments Yellow Tea
3.4. The Influence of Temperature and Humidity on Yellow Tea
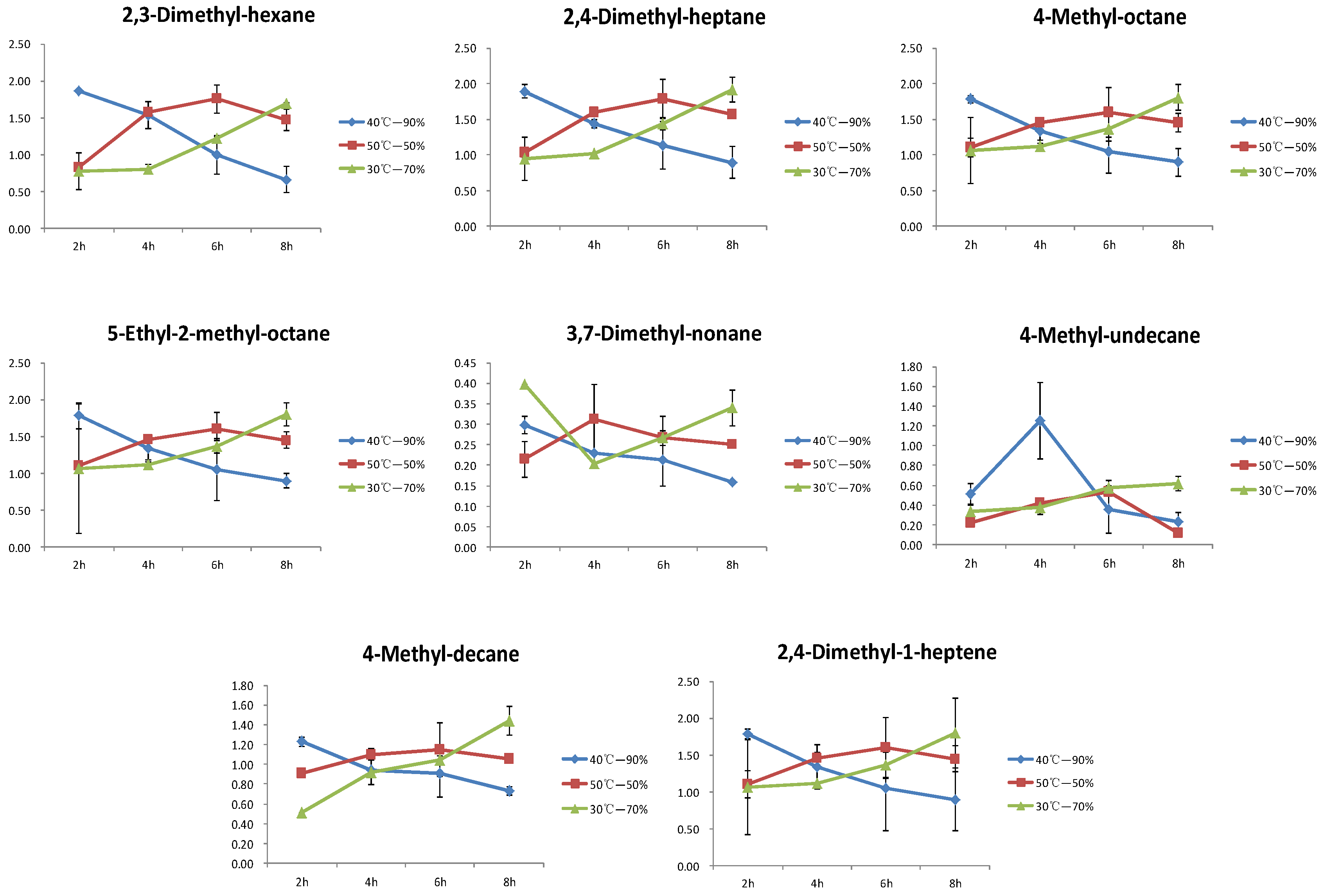
3.5. Changes in the Differential Compounds of Hydrocarbons during the Yellowing Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gramza-Michalowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, R.-L.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Zhang, C.; Suen, C.; Yang, C.; Quek, S. Antioxidant capacity and major polyphenol composition of teas as affected by geographical location, plantation elevation and leaf grade. Food Chem. 2018, 244, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Wang, M.; Zhao, J.-P.; Wang, Y.-H.; Tang, Q.; Khan, I. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.-X.; Song, C.-K. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Li, T.-H.; Xu, S.-S.; Ni, T.-C.; Deng, W.-W.; Ning, J.-M. The profile of dynamic changes in yellow tea quality and chemical composition during yellowing process. LWT-Food Sci. Technol. 2021, 139, 110792. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Zhou, S.-J.; Qian, H.; Zong, B.-Z.; Huang, C.-S.; Zhu, R.-L.; Guo, H.-W.; Gong, S.-Y. Effect of yellowing duration on the chemical profile of yellow tea and the associations with sensory traits. Molecules 2022, 27, 27030940. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-M.; Yin, X.-C.; Zhao, H.-J.; Zhang, J.-X.; Li, T.-H.; Zhang, Y.-Y.; Wang, Y.-J.; Ning, J.-M. Metabolomics analysis reveals the mechanism underlying the improvement in the color and taste of yellow tea after optimized yellowing. Food Chem. 2023, 428, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Hong, X.; Wang, C.; Jiang, R.-G.; Hu, T.-F.; Zheng, X.-X.; Huang, J.; Liu, Z.-H.; Li, Q. Characterization of the key aroma compounds in different aroma types of Chinese yellow tea. Foods 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-Y.; Yang, S.-Y.; Pan, Y.-N.; Zhou, S.; Ma, S.-C.; Ou, C.-S.; Fan, F.-Y.; Gong, S.-Y.; Chen, P.; Chu, Q. Yellow tea: More than turning green leaves to yellow. Crit. Rev. Food Sci. 2023, 64, 7836–7853. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.-J.; Sheng, X.-H.; Xie, Q.-T.; Huang, X.-L.; Yan, F.-J.; Liu, S.-B. Aroma formation and transformation during sealed yellowing process of Pingyang yellow tea. Food Res. Int. 2023, 165, 112535. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-H.; Ryan, E.-P.; Huang, J.-A.; Liu, Z.-H.; Weir, T.-L.; Snook, R.-L.; Ryan, T.-P. Fermented camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Ho, C.-T.; Schwab, W.; Song, C.; Wan, X.-C. Aroma compositions of large-leaf yellow tea and potential effect of theanine on volatile formation in tea. Food Chem. 2019, 280, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wan, X. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Shi, Y.-L.; Wang, M.-G.; Dong, Z.-B.; Zhu, Y.; Shi, J.; Ma, W.-J.; Lin, Z.; Lv, H.-P. Volatile components and key odorants of Chinese yellow tea (Camellia sinensis). LWT-Food Sci. Technol. 2021, 146, 111512. [Google Scholar] [CrossRef]
- Zheng, C.-Y.; Gong, L.-T.; Huang, Z.-Q.; Liu, Z.-B.; Zhang, W.; Ni, L. Analysis of the key volatile aroma compounds in sweet hongqu glutious rice wine. J. Chin. Ins. Food Sci. Technol. 2014, 14, 209–217. [Google Scholar]
- Wei, Y.-M.; Zhang, J.-X.; Li, T.-H.; Zhao, M.-J.; Song, Z.-S.; Wang, Y.-J.; Ning, J.-M. GC–MS, GC–O, and sensomics analysis reveals the key odorants underlying the improvement of yellow tea aroma after optimized yellowing. Food Chem. 2024, 431, 137139. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Sun, L.-L.; Zhang, S.-W.; Chen, Z.-Z.; Chen, R.-H.; Li, Z.-G.; Lai, X.-F.; Zhang, Z.-B.; Cao, J.-X.; Li, Q.; et al. The formation mechanism of aroma quality of green and yellow teas based on GC-MS/MS metabolomics. Food Res. Int. 2023, 172, 113137. [Google Scholar] [CrossRef] [PubMed]
| No. | RT | CAS | RI | Volatile Compounds | Mean Content (μg/kg) | Content Range |
|---|---|---|---|---|---|---|
| C1 | 4.260 | 591-93-5 | 686 | 1,4-Pentadiene | 1.00 | 0.78–1.28 |
| C2 | 4.435 | 74-93-1 | 706 | Methanethiol | 0.25 | 0.21–0.29 |
| C3 | 4.584 | 109-92-2 | 713 | Ethoxyethene | 0.47 | 0.40–0.61 |
| C4 | 5.042 | 584-94-1 | 740 | 2,3-Dimethyl-hexane | 1.28 | 0.66–1.70 |
| C5 | 5.492 | 926-56-7 | 813 | 4-Methyl-1,3-pentadiene | 1.60 | 1.08–1.88 |
| C6 | 5.549 | 110-00-9 | 816 | Furan | 0.77 | 0.35–1.57 |
| C7 | 5.610 | 2213-23-2 | 819 | 2,4-Dimethyl-heptane | 1.46 | 0.89–1.92 |
| C8 | 5.762 | 78-84-2 | 827 | 2-Methyl propanal | 6.42 | 5.30–7.82 |
| C9 | 6.055 | 79-20-9 | 840 | Acetic acid, methyl ester | 2.62 | 2.42–2.89 |
| C10 | 6.378 | 619-99-8 | 855 | 3-Ethyl-hexane | 0.19 | 0.19–0.24 |
| C11 | 6.484 | 2216-34-4 | 859 | 4-Methyl-octane | 1.38 | 0.90–1.80 |
| C12 | 6.900 | 534-22-5 | 877 | 2-Methyl-furan | 0.86 | 0.78–0.91 |
| C13 | 7.036 | 19549-87-2 | 883 | 2,4-Dimethyl-1-heptene | 3.26 | 2.33–4.12 |
| C14 | 7.284 | 141-78-6 | 893 | Ethyl acetate | 1.32 | 1.09–1.68 |
| C15 | 7.505 | 534-22-5 | 901 | 2-Methyl-furan | 0.46 | 0.44–0.48 |
| C16 | 7.608 | 78-93-3 | 905 | 2-Butanone | 1.22 | 1.13–1.29 |
| C17 | 7.950 | 96-17-3 | 918 | 2-Methyl-butanal | 58.73 | 50.93–70.27 |
| C18 | 8.091 | 590-86-3 | 923 | 3-Methyl-butanal | 19.80 | 17.68–22.82 |
| C19 | 9.137 | 75-97-8 | 959 | 3,3-Dimethyl-2-butanone | 0.19 | 0.12–0.25 |
| C20 | 9.272 | 3208-16-0 | 963 | 2-Ethyl-furan | 0.76 | 0.70–0.86 |
| C21 | 10.075 | 3710-43-8 | 988 | 2,4-Dimethylfuran | 0.35 | 0.28–0.41 |
| C22 | 10.162 | 110-62-3 | 990 | Pentanal | 9.65 | 8.20–12.05 |
| C23 | 10.582 | 2847-72-5 | 1002 | 4-Methyl-decane | 1.07 | 0.73–1.44 |
| C24 | 12.409 | 2801-84-5 | 1049 | 2,4-Dimethyl-decane | 0.36 | 0.21–0.57 |
| C25 | 12.684 | 4170-30-3 | 1055 | 2-Butenal | 0.16 | 0.14–0.17 |
| C26 | 13.577 | 600-14-6 | 1075 | 2,3-Pentanedione | 0.16 | 0.00–0.48 |
| C27 | 13.732 | 25044-01-3 | 1079 | 2-Methyl-1-penten-3-one | 0.79 | 0.73–0.89 |
| C28 | 13.952 | 17302-32-8 | 1083 | 3,7-Dimethyl-nonane | 0.25 | 0.16–0.34 |
| C29 | 14.052 | 624-92-0 | 1086 | Dimethyl-disulfide | 0.42 | 0.29–0.48 |
| C30 | 14.177 | 62016-18-6 | 1088 | 5-Ethyl-2-methyl-octane | 1.46 | 0.93–2.01 |
| C31 | 14.406 | 66-25-1 | 1093 | Hexanal | 3.11 | 2.72–4.33 |
| C32 | 14.798 | 3744-02-3 | 1101 | 4-Methyl-4-penten-2-one | 1.51 | 1.25–1.91 |
| C33 | 16.578 | 79-46-9 | 1137 | 2-Nitro-propane | 1.58 | 1.31–1.72 |
| C34 | 16.798 | 141-79-7 | 1141 | 4-Methyl-3-penten-2-one | 11.05 | 10.40–12.26 |
| C35 | 17.026 | 108-38-3 | 1145 | 1,3-Dimethyl-benzene | 1.42 | 0.99–1.88 |
| C36 | 18.166 | 616-25-1 | 1167 | 1-Penten-3-ol | 0.54 | 0.00–0.88 |
| C37 | 19.230 | 617-92-5 | 1188 | 1-Ethyl-1-H-pyrrole | 2.95 | 2.56–3.53 |
| C38 | 19.554 | 96-04-8 | 1194 | Acetyl valeryl | 0.29 | 0.13–0.58 |
| C39 | 20.338 | 123-51-3 | 1209 | 3-Methyl-1-butanol | 1.04 | 0.36–1.70 |
| C40 | 20.589 | 2980-69-0 | 1214 | 4-Methyl-undecane | 0.32 | 0.11–0.62 |
| C41 | 22.172 | 2051-78-7 | 1246 | 2-Propenyl ester butanoic acid | 0.09 | 0.00–0.17 |
| C42 | 22.342 | 71-41-0 | 1249 | 1-Pentanol | 1.45 | 1.29–1.70 |
| C43 | 22.783 | 100-42-5 | 1259 | Styrene | 1.13 | 1.07–1.18 |
| C44 | 24.195 | 124-13-0 | 1289 | Octanal | 0.86 | 0.38–1.40 |
| C45 | 24.306 | 17301-32-5 | 1291 | 4,7-Dimethyl-undecane | 0.68 | 0.24–1.28 |
| C46 | 25.404 | 2408-37-9 | 1316 | 2,2,6-Trimethyl-cyclohexanone | 0.38 | 0.00–0.85 |
| C47 | 25.875 | 585-25-1 | 1327 | 2,3-Octanedione | 0.66 | 0.35–0.88 |
| C48 | 26.417 | 110-93-0 | 1340 | 6-Methyl-5-hepten-2-one | 2.14 | 0.92–3.01 |
| C49 | 26.953 | 111-27-3 | 1352 | 1-Hexanol | 0.21 | 0.19–0.24 |
| C50 | 28.260 | 544-12-7 | 1385 | 3-Hexen-1-ol | 0.14 | 0.00–0.22 |
| C51 | 28.626 | 124-19-6 | 1394 | Nonanal | 1.66 | 0.97–2.22 |
| C52 | 29.373 | 15726-15-5 | 1414 | 3-Methyl-4-heptanone | 0.15 | 0.09–0.19 |
| C53 | 29.821 | 1014-60-4 | 1426 | 1,3-Bis(1,1-dimethylethyl)-benzene | 1.18 | 0.99–1.44 |
| C54 | 30.175 | 62108-21-8 | 1435 | 6-Ethyl-2-methyl-decane | 0.24 | 0.11–0.32 |
| C55 | 30.579 | 996-12-3 | 1446 | 2,2-Dimethyl-hexanal | 0.47 | 0.43–0.53 |
| C56 | 30.732 | 818-49-5 | 1451 | 4-Methyl-1-hexanol | 0.16 | 0.09–0.22 |
| C57 | 31.368 | 98-01-1 | 1469 | Furfural | 0.37 | 0.30–0.44 |
| C58 | 31.752 | 56606-79-2 | 1479 | 1-Decen-3-one | 0.27 | 0.20–0.36 |
| C59 | 31.872 | 104-76-7 | 1483 | 2-Ethyl-1-hexanol | 4.00 | 2.75–4.89 |
| C60 | 32.256 | 112-31-2 | 1494 | Decanal | 3.85 | 0.99–5.99 |
| C61 | 33.014 | 109-97-7 | 1516 | Pyrrole | 0.36 | 0.34–0.37 |
| C62 | 33.260 | 100-52-7 | 1524 | Benzaldehyde | 1.19 | 0.96–1.40 |
| C63 | 33.649 | 78-70-6 | 1536 | Linalool | 0.41 | 0.24–0.48 |
| C64 | 33.918 | 111-87-5 | 1544 | 1-Octanol | 0.20 | 0.16–0.26 |
| C65 | 34.428 | 79-31-2 | 1560 | 2-Methyl-propanoic acid | 0.40 | 0.28–0.54 |
| C66 | 34.704 | 620-02-0 | 1568 | 5-Methyl-2-furancarboxaldehyde | 0.12 | 0.12–0.13 |
| C67 | 35.307 | 112-44-7 | 1588 | Undecanal | 0.20 | 0.09–0.26 |
| C68 | 35.460 | 29957-43-5 | 1593 | 3,7-Dimethyl-1,5,7-octatrien-3-ol | 1.34 | 0.58–2.25 |
| C69 | 35.727 | 2835-98-5 | 1601 | 2-Amino-5-methyl-phenol | 0.28 | 0.22–0.38 |
| C70 | 35.937 | 432-25-7 | 1608 | 2,6,6-Trimethyl-1-cyclohexene-1-carboxaldehyde | 0.12 | 0.10–0.16 |
| C71 | 36.664 | 122-78-1 | 1632 | Benzeneacetaldehyde | 1.31 | 0.89–1.63 |
| C72 | 37.141 | 116-53-0 | 1648 | 2-Methyl-butanoic acid | 0.15 | 0.09–0.27 |
| C73 | 41.460 | 3879-26-3 | 1806 | 6,10-Dimethyl-,(Z)-5,9-undecadien-2-one | 5.14 | 1.00–12.20 |
| C74 | 41.800 | 120-50-3 | 1820 | 2-Methylpropyl ester benzoic acid | 0.19 | 0.16–0.23 |
| C75 | 41.877 | 74367-30-9 | 1823 | 2-Methyl-, 2-ethyl-1-propyl-1,3-propanediyl ester-propanoic acid | 11.76 | 11.05–12.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Feng, Z.; Min, R.; Yin, J.; Jiang, H. The Effect of Temperature and Humidity on Yellow Tea Volatile Compounds during Yellowing Process. Foods 2024, 13, 3283. https://doi.org/10.3390/foods13203283
Wang W, Feng Z, Min R, Yin J, Jiang H. The Effect of Temperature and Humidity on Yellow Tea Volatile Compounds during Yellowing Process. Foods. 2024; 13(20):3283. https://doi.org/10.3390/foods13203283
Chicago/Turabian StyleWang, Weiwei, Zhihui Feng, Rui Min, Junfeng Yin, and Heyuan Jiang. 2024. "The Effect of Temperature and Humidity on Yellow Tea Volatile Compounds during Yellowing Process" Foods 13, no. 20: 3283. https://doi.org/10.3390/foods13203283
APA StyleWang, W., Feng, Z., Min, R., Yin, J., & Jiang, H. (2024). The Effect of Temperature and Humidity on Yellow Tea Volatile Compounds during Yellowing Process. Foods, 13(20), 3283. https://doi.org/10.3390/foods13203283






