Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’
Abstract
:1. Introduction
2. Materials and Methods
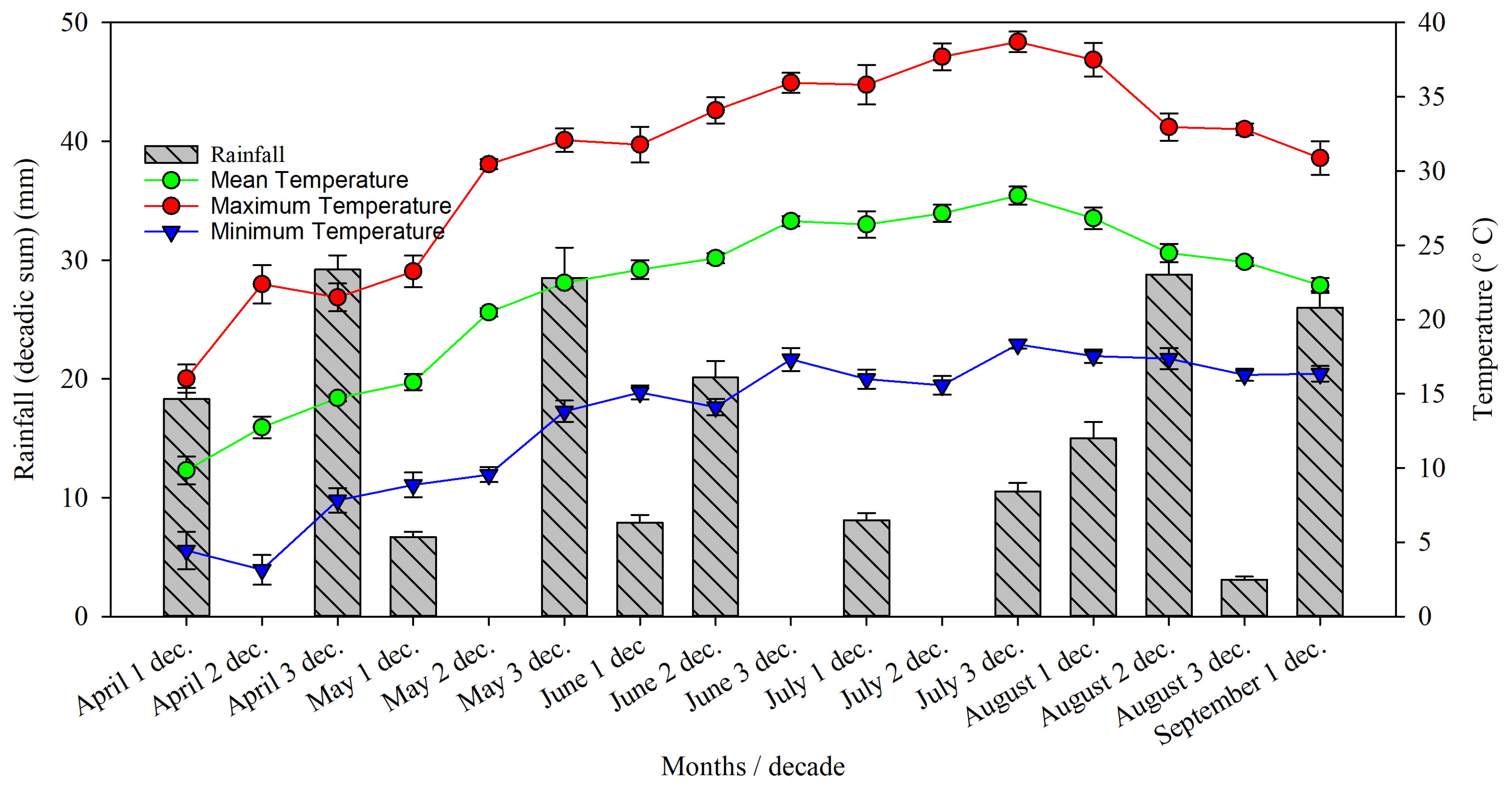
2.1. Orchard Characteristics and Weather Data
2.2. Canopy Volume, Yield and Light Penetration in the Canopy
2.3. Fruit Protein, Fat Content and Non-Structural Carbohydrates (NCS) Analysis
2.4. Tocoferol Determination
2.5. Fatty Acid (FA) Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Experimental Orchard and Weather Data
3.2. Canopy Volume, Yield and Light Penetration in the Canopy
3.3. Fat, Protein and NSC Contents
3.4. FA Composition
3.5. Tocopherol Content
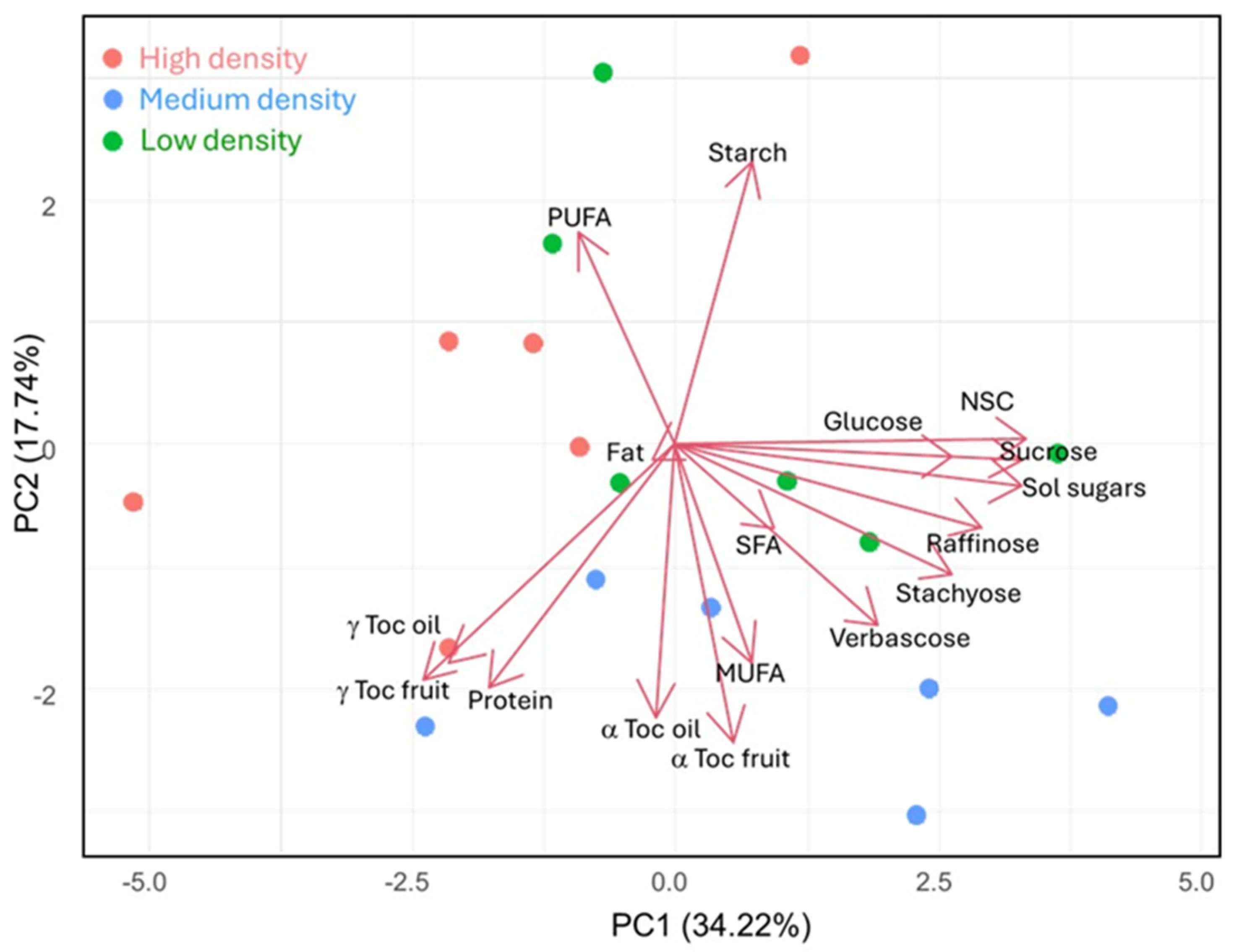
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in Cultivar Choice, Hazelnut Orchard Management, and Nut Storage to Enhance Product Quality and Safety: An Overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Orem, A.; Yucesan, F.B.; Orem, C.; Akcan, B.; Kural, B.V.; Alasalvar, C.; Shahidi, F. Hazelnut-Enriched Diet Improves Cardiovascular Risk Biomarkers beyond a Lipid-Lowering Effect in Hypercholesterolemic Subjects. J. Clin. Lipidol. 2013, 7, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.; Casal, S.; Seabra, R.M.; Oliveira, B.P.P. Effects of Roasting on Hazelnut Lipids. J. Agric. Food Chem. 2006, 54, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, L.; Aramini, M.; Zini, A.; Di Giammatteo, V.; Spera, D.; Drogoudi, P.; Rovira, M.; Silva, A.P.; Solar, A.; Botta, R. Fatty Acids and Alpha-Tocopherol Composition in Hazelnut (Corylus avellana L.): A Chemometric Approach to Emphasize the Quality of European Germplasm. Euphytica 2013, 191, 57–73. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mehrpour, G.R. Health Benefits of Hazelnut. EC Nutr. 2017, 8, 101–105. [Google Scholar]
- Snelgar, W.P.; Manson, P.J.; Martin, P.J. Influence of time of shading on flowering and yield of kiwifruit vines. J. Hortic. Sci. 1992, 67, 481–487. [Google Scholar] [CrossRef]
- Tombesi, S.; Palliotti, A.; Poni, S.; Farinelli, D. Influence of Light and Shoot Development Stage on Leaf Photosynthesis and Carbohydrate Status during the Adventitious Root Formation in Cuttings of Corylus avellana L. Front. Plant Sci. 2015, 6, 973. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Karaosmanoğlu, H. Lipid Characteristics, Bioactive Properties, and Mineral Content in Hazelnut Grown under Different Cultivation Systems. J. Food Process. Preserv. 2022, 46, e16717. [Google Scholar] [CrossRef]
- Ellena, M.; González, A.; Sandoval, P.; Marchant, F. Advantages of High Density Hazelnut Orchards in South Chile. Acta Hortic. 2018, 1226, 243–250. [Google Scholar] [CrossRef]
- Loreti, F.; Morini, S.; Muleo, R.; Masetti, C.; Vitagliano, C. Effect of solar radiation on some growth paremeters of peach fruits. Acta Hortic. 1993, 349, 117–121. [Google Scholar] [CrossRef]
- Beyhan, N. Effects of planting density on yield and quality characteristics of hazelnut (cv. Palaz) in a hedgerow training system. Can. J. Plant Sci. 2007, 87, 595–597. [Google Scholar] [CrossRef]
- Cristofori, V.; Bertazza, G.; Bignami, C. Changes in kernel chemical composition during nut development of three Italian hazelnut cultivars. Fruits 2015, 70, 311–322. [Google Scholar] [CrossRef]
- Hampson, C.R.; Azarenko, A.N.; Potter, J.R. Photosynthetic rate, flowering, and yield component alteration in hazelnut in response to different light environments. J. Am. Soc. Hortic. Sci. 1996, 121, 1103–1111. [Google Scholar] [CrossRef]
- Bignami, C.; Bertazza, G.; Bizzarri, S.; Bruziches, A.; Cammilli, C.; Cristofori, V. Effect of High Density and Dynamic Tree Spacing on Yield and Quality of the Hazelnut Cultivar ‘tonda Gentile Romana’. Acta Hortic. 2005, 686, 263–270. [Google Scholar] [CrossRef]
- Tombesi, A. Effect of Light Penetration on High Density Filbert Planting; Estratto dagli Annali della Facoltà di Agraria dell’Università di Perugia: Perugia, Italy, 1978; Volume 22–23, pp. 301–310. [Google Scholar]
- Fideghelli, C.; De Salvador, F.R. World Hazelnut Situation and Perspectives. Acta Hortic. 2009, 845, 39–52. [Google Scholar] [CrossRef]
- Rovira, M. Advances in Hazelnut (Corylus avellana L.) Rootstocks Worldwide. Horticulturae 2021, 7, 267. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Brugnoli, E.; Battistelli, A.; Proietti, S.; Moscatello, S.; Famiani, F.; Tombesi, S.; Zadra, C.; Farinelli, D. Carbon Allocation Strategies and Water Uptake in Young Grafted and Own-Rooted Hazelnut (Corylus avellana L.) Cultivars. Tree Physiol. 2022, 42, 939–957. [Google Scholar] [CrossRef]
- Me, G.; Valentini, N.; Caviglione, M.; Lovisolo, C. Effect of Shade on Flowering and Yield for Two Different Hazelnut Training Systems. Acta Hortic. 2005, 686, 187–192. [Google Scholar] [CrossRef]
- Anthony, B.M.; Minas, I.S. Optimizing peach tree canopy architecture for efficient light use, increased productivity and improved fruit quality. Agronomy 2021, 11, 1961. [Google Scholar] [CrossRef]
- Pannico, A.; Cirillo, C.; Giaccone, M.; Scognamiglio, P.; Romano, R.; Caporaso, N.; Sacchi, R.; Basile, B. Fruit Position within the Canopy Affects Kernel Lipid Composition of Hazelnuts. J. Sci. Food Agric. 2017, 97, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Vinci, A.; Traini, C.; Portarena, S.; Farinelli, D. Assessment of the Midseason Crop Coefficient for the Evaluation of the Water Demand of Young, Grafted Hazelnut Trees in High-Density Orchards. Water 2023, 15, 1683. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca vesicaria (L.) Cav. in Controlled Environment With Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef] [PubMed]
- Mitsikaris, P.D.; Kokokiris, L.; Pritsa, A.; Papadopoulos, A.N.; Kalogiouri, N.P. Investigating the Tocopherol Contents of Walnut Seed Oils Produced in Different European Countries Analyzed by HPLC-UV: A Comparative Study on the Basis of Geographical Origin. Foods 2022, 11, 3719. [Google Scholar] [CrossRef]
- Peacock, A.G.; Mullen, M.D.; Ringelberg, D.B.; Tyler, D.D.; Hedrick, D.B.; Gale, P.M.; White, D.C. Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol. Biochem. 2001, 33, 1011–1019. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philosophical transactions of the royal society A: Mathematical. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar]
- Jahirul, M.I.; Rasul, M.G.; Brown, R.J.; Senadeera, W.; Hosen, M.A.; Haque, R.; Saha, S.C.; Mahlia, T.M.I. Investigation of correlation between chemical composition and properties of biodiesel using principal component analysis (PCA) and artificial neural network (ANN). Renew. Energy 2021, 168, 632–646. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Brugnoli, E.; Battistelli, A.; Proietti, S.; Moscatello, S.; Famiani, F.; Zadra, C.; Tombesi, S.; Farinelli, D. Grafted Hazelnut: A Sustainable Agricultural Practice to Face Summer Stressful Conditions. Acta Hortic. 2023, 1379, 303–308. [Google Scholar] [CrossRef]
- Ryugo, K.; Marangoni, B.; Ramos, D.E. Light intensity and fruiting effects on carbohydrate contents, spur development, and return bloom of ‘Hartley’ walnut. J. Am. Soc. Hortic. Sci. 1980, 105, 223–227. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Pathania, V. Effect of shading and plant density on growth, yield and oil composition of clary sage (Salvia sclarea L.) in north western Himalaya. J. Essent. Oil Res. 2013, 25, 23–32. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, E.D.; Türkhan, A.; Bulut, M.; Koyuncu, M.; Güler, E.; Sayın, F.; Muradoğlu, F. The Effect of Altitude on Phenolic, Antioxidant and Fatty Acid Compositions of Some Turkish Hazelnut (Coryllus avellana L.) Cultivars. Molecules 2023, 28, 5067. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Momchilova, S.M.; Taneva, S.P.; Zlatanov, M.D.; Antova, G.A.; Angelova-Romova, M.J.; Blagoeva, E. Fatty Acids, Tocopherols and Oxidative Stability of Hazelnuts during Storage. Bulg. Chem. Commun. 2017, 49, 65–70. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Ljubic, A.; Holdt, S.L.; Jakobsen, J.; Bysted, A.; Jacobsen, C. Fatty acids, carotenoids, and tocopherols from microalgae: Targeting the accumulation by manipulating the light during growth. J. Appl. Phycol. 2021, 33, 2783–2793. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
| Plant Density | Canopy Volume (m3) | Light Penetration (%) | Yield (kg/Tree) | Yield Efficiency (kg/m3) | Yield (kg/ha) |
|---|---|---|---|---|---|
| high | 3.5 b | 19.2 b | 1.16 b | 0.34 a | 2898 a |
| medium | 4.4 a | 46.9 a | 1.67 a | 0.39 a | 2089 b |
| low | 3.7 ab | 53.0 a | 1.32 b | 0.36 a | 822 c |
| Tree Density | Protein | Fat | Glucose | Sucrose | Raffinose | Stachyose | Verbascose | Starch | Solubles | Tot NSC |
|---|---|---|---|---|---|---|---|---|---|---|
| high | 18.9 ± 1.2 | 63.3 ± 2.2 | 0.009 b | 3.5 ± 0.4 | 0.17 ± 0.01 | 0.65 ± 0.09 | 0.009 ± 0.007 | 0.25 b | 4.4 ± 0.4 | 4.6 ± 0.5 |
| medium | 19.2 ± 0.8 | 64.8 ± 1.6 | 0.014 a | 4.4 ± 0.8 | 0.21 ± 0.03 | 0.77 ± 0.13 | 0.007 ± 0.001 | 0.24 b | 5.4 ± 0.9 | 5.7 ± 0.9 |
| low | 18.1 ± 0.9 | 64.5 ± 0.6 | 0.011 b | 4.3 ± 0.7 | 0.20 ± 0.01 | 0.77 ± 0.12 | 0.006 ± 0.001 | 0.53 a | 5.4 ± 0.8 | 5.9 ± 0.8 |
| Plant Density | SFAs (%) | MUFAs (%) | PUFAs (%) |
|---|---|---|---|
| high | 9.5 b | 80.2 b | 10.3 a |
| medium | 9.9 a | 81.4 a | 8.7 b |
| low | 9.8 a | 79.9 b | 10.1 ab |
| Plant Density | γ Tocopherol (mg/100 g Oil) | α Tocopherol (mg/100 g Oil) | α + γ Tocopherol (mg/100 g Oil) | γ Tocopherol (mg/100 g dw) | α Tocopherol (mg/100 g dw) | α + γ Tocopherol (mg/100 g dw) |
|---|---|---|---|---|---|---|
| high | 0.99 ± 0.20 | 10.5 ± 1.4 | 11.5 ± 1.5 | 0.43 ± 0.20 | 4.6 ± 1.9 | 5.1 ± 1.9 |
| medium | 0.73 ± 0.11 | 13.1 ± 1.3 | 13.8 ± 1.3 | 0.32 ± 0.14 | 5.7 ± 1.9 | 6.0 ± 1.9 |
| low | 0.64 ± 0.87 | 12.5 ± 1.5 | 13.1 ± 1.5 | 0.28 ± 0.08 | 5.5 ± 1.8 | 5.8 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portarena, S.; Proietti, S.; Moscatello, S.; Zadra, C.; Cinosi, N.; Traini, C.; Farinelli, D. Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’. Foods 2024, 13, 3307. https://doi.org/10.3390/foods13203307
Portarena S, Proietti S, Moscatello S, Zadra C, Cinosi N, Traini C, Farinelli D. Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’. Foods. 2024; 13(20):3307. https://doi.org/10.3390/foods13203307
Chicago/Turabian StylePortarena, Silvia, Simona Proietti, Stefano Moscatello, Claudia Zadra, Nicola Cinosi, Chiara Traini, and Daniela Farinelli. 2024. "Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’" Foods 13, no. 20: 3307. https://doi.org/10.3390/foods13203307
APA StylePortarena, S., Proietti, S., Moscatello, S., Zadra, C., Cinosi, N., Traini, C., & Farinelli, D. (2024). Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’. Foods, 13(20), 3307. https://doi.org/10.3390/foods13203307







