Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry and Reagents
2.2. Sample Collection
2.3. Samples Extraction
2.3.1. EVOO
2.3.2. OMWW
2.4. HPLC Characterization
2.4.1. EVOO
2.4.2. OMWW
2.5. LC-MS/MS Instrumental Layout and Parameters
2.6. Determination of Total Phenolic Content
2.7. Antiradical Activity
2.7.1. DPPH Assay
2.7.2. ABTS Assay
2.8. Reactive Oxygen Species Scavenging Capacity
2.8.1. Superoxide Radical Scavenging Assay
2.8.2. Hypochlorous Acid Scavenging Assay
2.9. Cell Viability Assay
2.10. Intestinal Permeability Assay
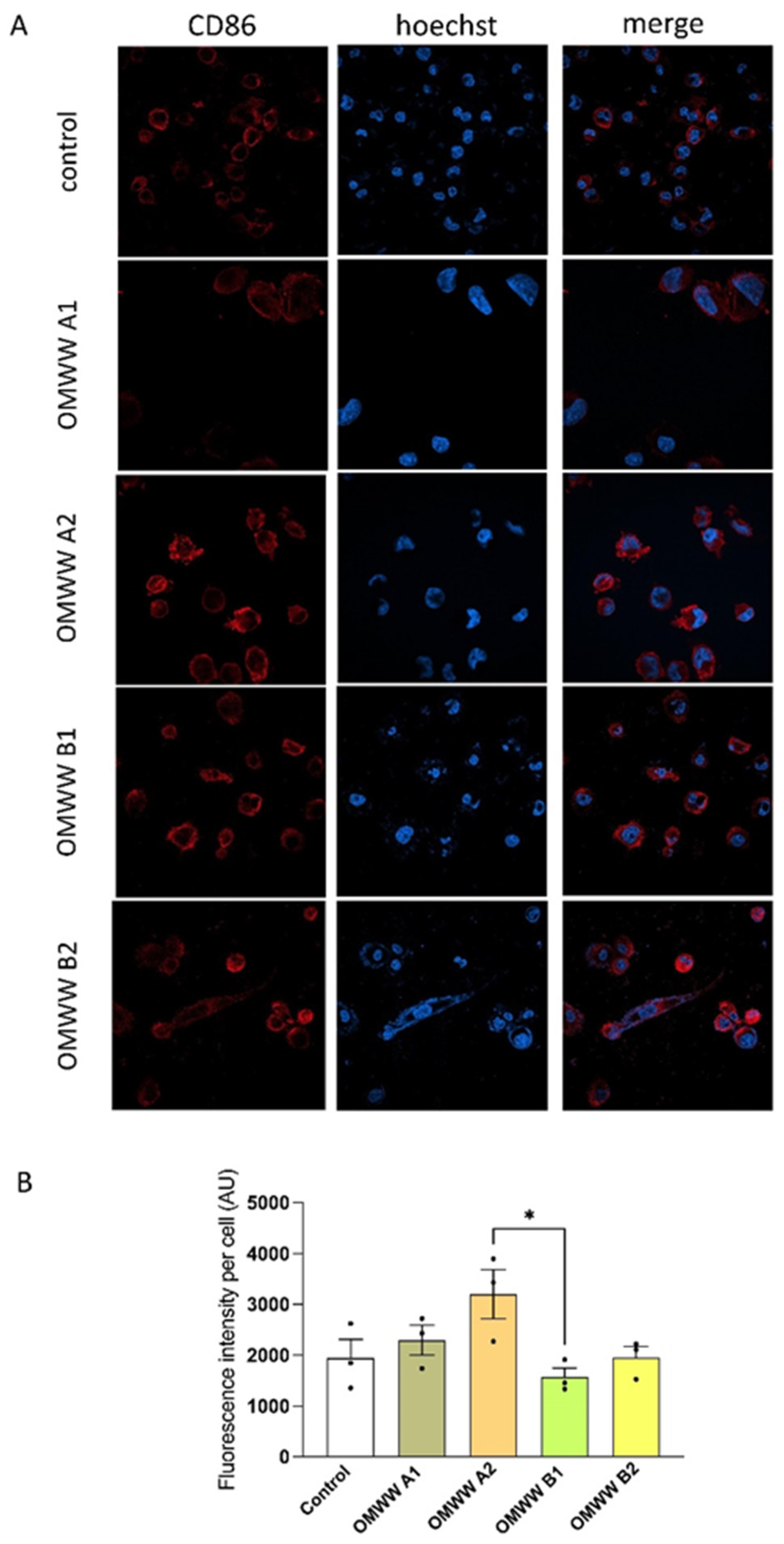
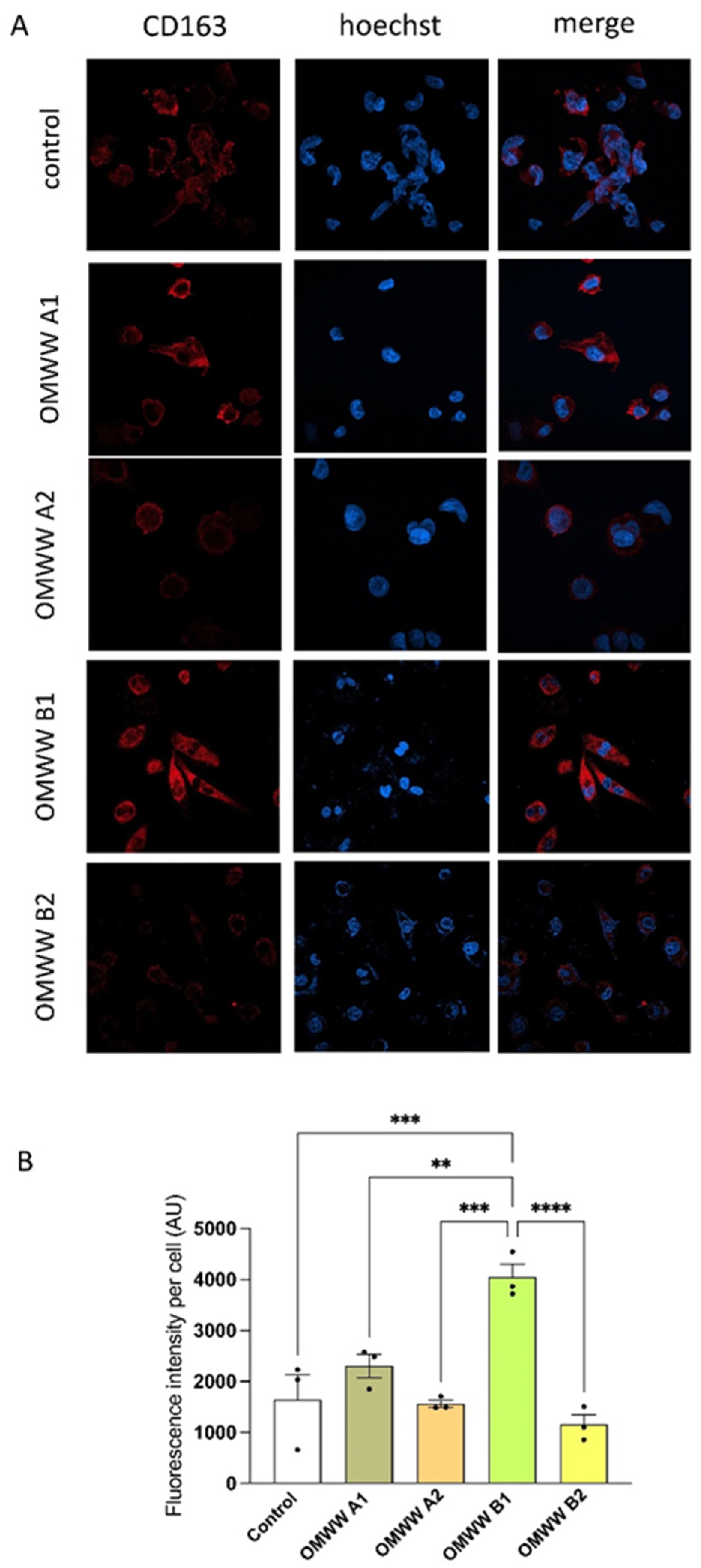
2.11. Immunocytochemistry on THP-1-Derived M0 Macrophages to Assess CD86 and CD163 Expression
2.12. Statistical Analysis
3. Results and Discussion
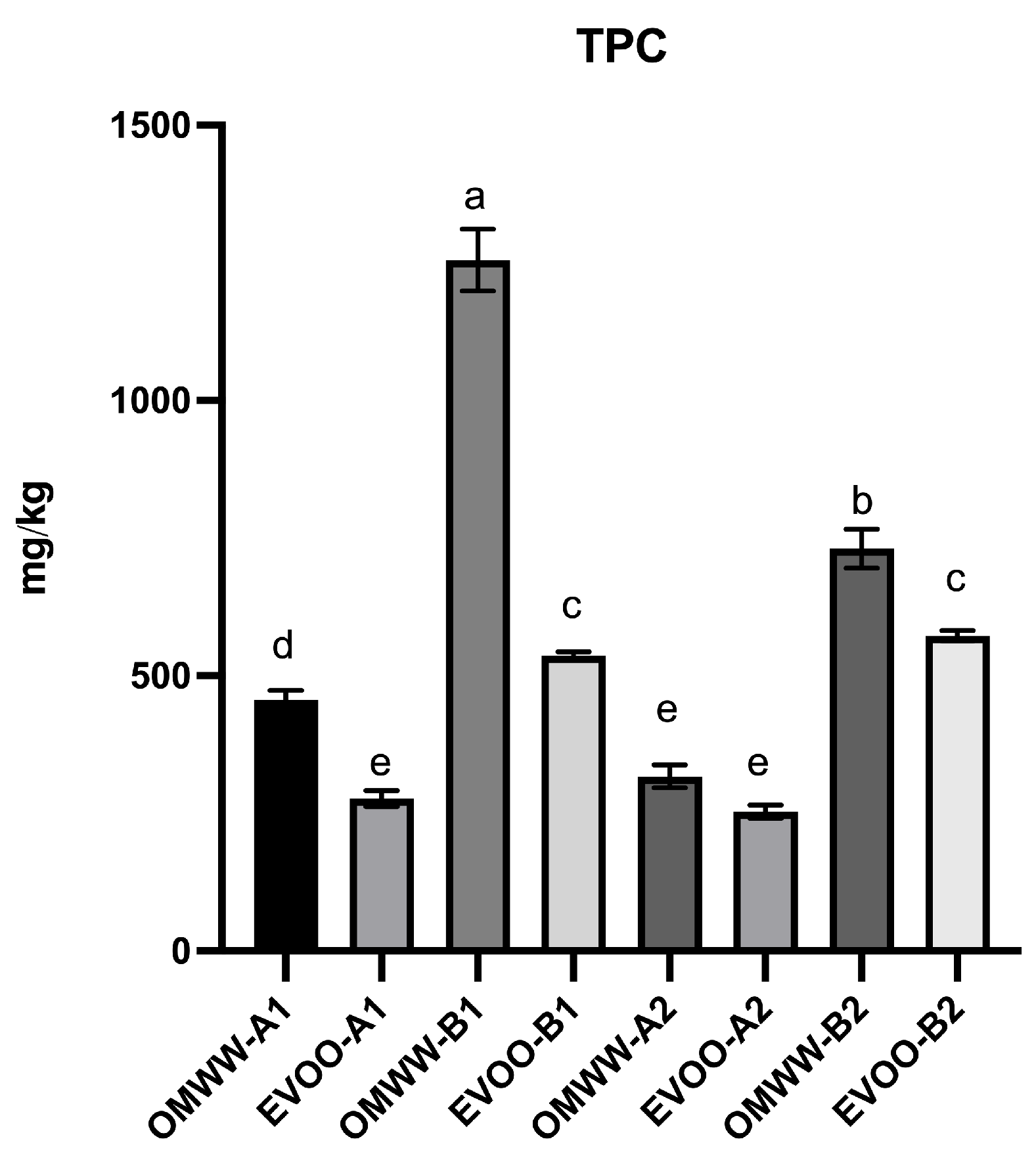
3.1. Analysis of Polyphenolic Contents
3.2. Antiradical Properties
3.3. In Vitro Reactive Oxygen Species Scavenging Capacity
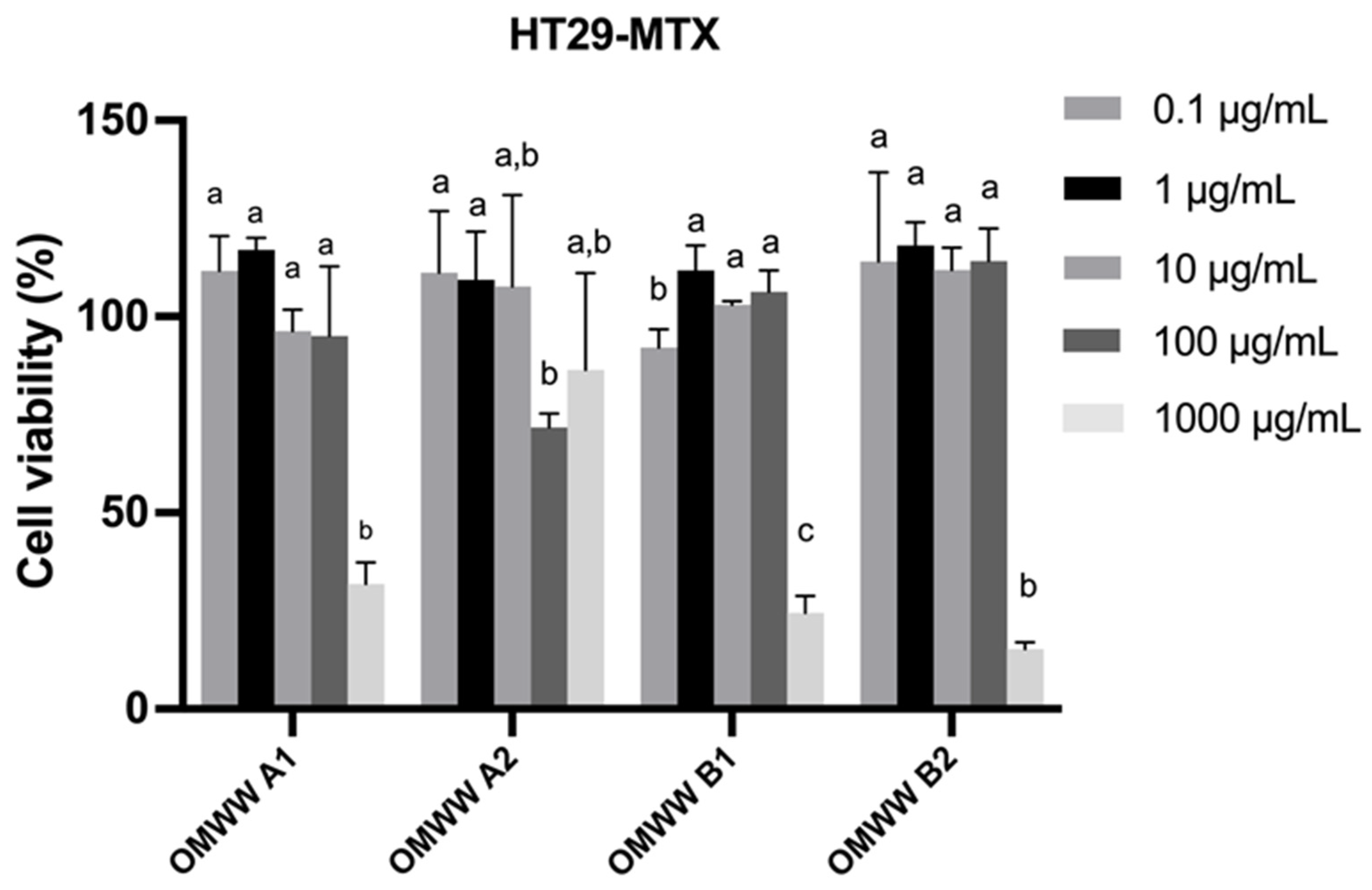
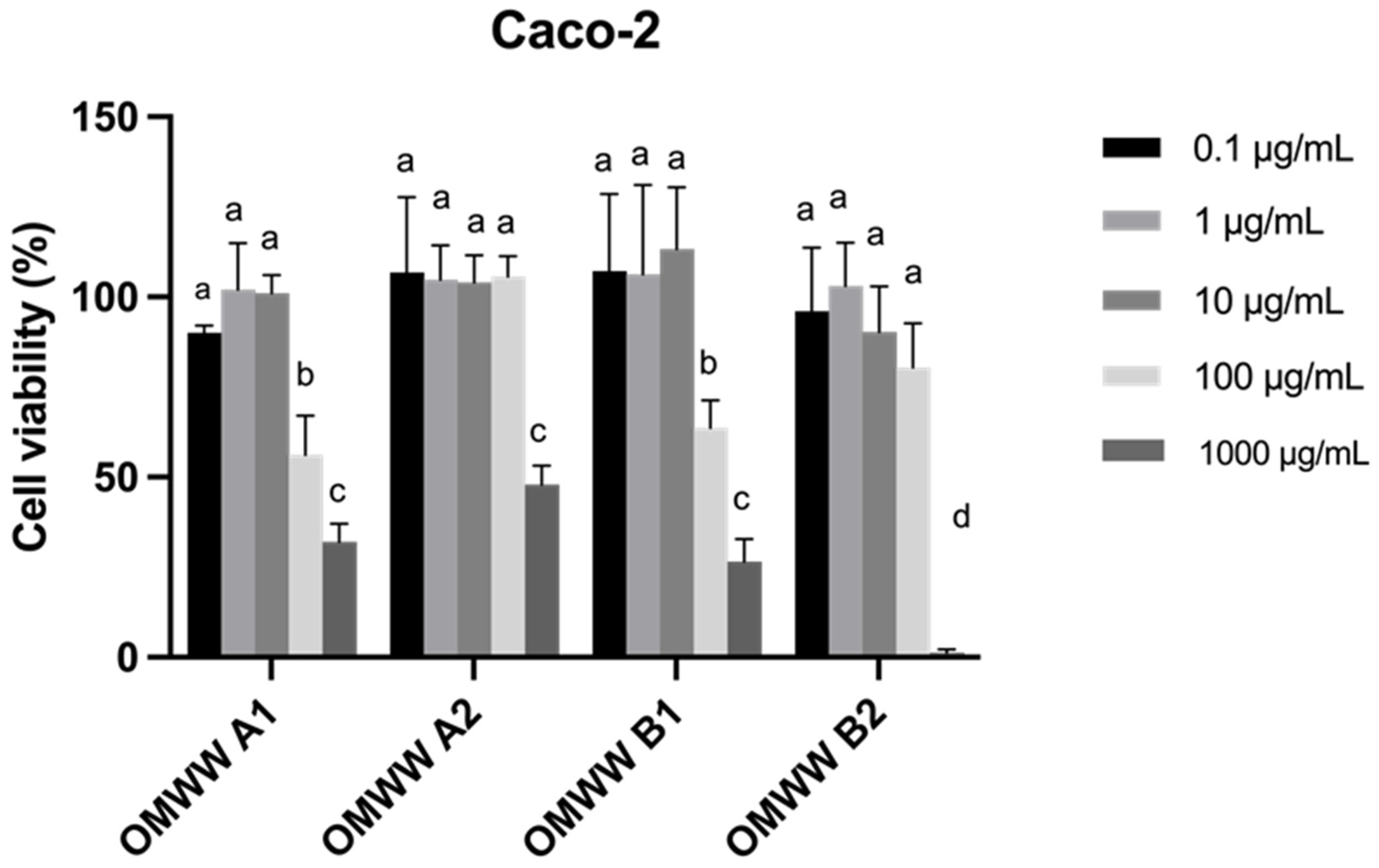
3.4. In Vitro Cell Studies of OMWW Extracts Toward Caco-2 and HT29-MTX Intestinal Cell Lines
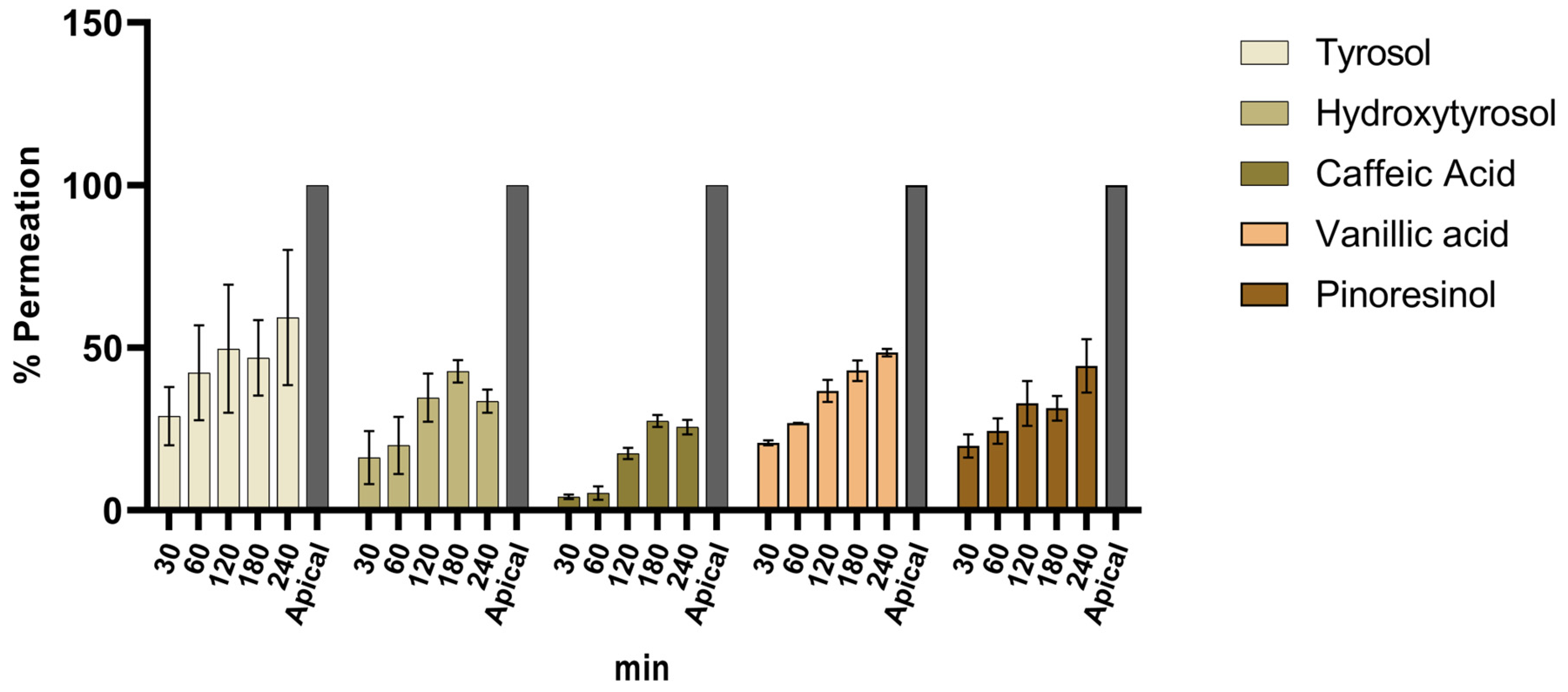
3.5. Intestinal Co-Culture Model Permeation Assay
3.6. In Vitro Effect of OMWWs on the Polarization of THP-1-Derived Macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Chamorro, F.; Pereira, A.G.; Carrera-Casais, A.; Fraga-Corral, M.; Carpena, M.; Simal-Gandara, J.; Prieto, M.A. Evolution of Flavors in Extra Virgin Olive Oil Shelf-Life. Antioxidants 2021, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Virruso, C.; Accardi, G.; Colonna-Romano, G.; Candore, G.; Vasto, S.; Caruso, C. Nutraceutical Properties of Extra-Virgin Olive Oil: A Natural Remedy for Age-Related Disease? Rejuvenation Res. 2014, 17, 217–220. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Freschi, M.; Rinaldi, I.; Zallocco, L.; Malaguti, M.; Manera, C.; Ortore, G.; Zuccarini, M.; Ronci, M.; Cuffaro, D.; et al. Unraveling the Protective Role of Oleocanthal and Its Oxidation Product, Oleocanthalic Acid, against Neuroinflammation. Antioxidants 2024, 13, 1074. [Google Scholar] [CrossRef]
- Artajo, L.-S.; Romero, M.-P.; Suárez, M.; Motilva, M.-J. Partition of Phenolic Compounds during the Virgin Olive Oil Industrial Extraction Process. Eur. Food Res. Technol. 2007, 225, 617–625. [Google Scholar] [CrossRef]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the Main Phenolic Compounds of Olive Mill Wastewater (OMW) on the Kinetics of Acetoclastic Methanogenesis. Process. Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Türker, L.; Yücel, M. Biological Hydrogen Production from Olive Mill Wastewater with Two-Stage Processes. Int. J. Hydrogen Energy 2006, 31, 1527–1535. [Google Scholar] [CrossRef]
- Rinaldi, M.; Rana, G.; Introna, M. Olive-Mill Wastewater Spreading in Southern Italy: Effects on a Durum Wheat Crop. Field Crops Res. 2003, 84, 319–326. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and Fractionation of Phenolic Compounds Extracted from Olive Oil Mill Wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Nakbi, A.; Dabbou, S.; Champion, S.; Fouchier, F.; Mehri, S.; Attia, N.; Leger, C.; Hammami, M. Modulation of the Superoxide Anion Production and MMP-9 Expression in PMA Stimulated THP-1 Cells by Olive Oil Minor Components: Tyrosol and Hydroxytyrosol. Food Res. Int. 2011, 44, 575–581. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Zanotto, I.; Sayaf, K.; Colognesi, M.; Polini, B.; Digiacomo, M.; Macchia, M.; Nieri, P.; et al. The Phenolic Compounds Tyrosol and Hydroxytyrosol Counteract Liver Fibrogenesis via the Transcriptional Modulation of NADPH Oxidases and Oxidative Stress-Related miRNAs. Biomed. Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol Alleviate Intestinal Inflammation, Oxidative Stress and Apoptosis Resulted in Ulcerative Colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Franceschelli, S.; De Cecco, F.; Pesce, M.; Ripari, P.; Guagnano, M.T.; Nuevo, A.B.; Grilli, A.; Sancilio, S.; Speranza, L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. Int. J. Mol. Sci. 2023, 24, 2057. [Google Scholar] [CrossRef]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Zahi, M.R.; Zam, W.; El Hattab, M. State of Knowledge on Chemical, Biological and Nutritional Properties of Olive Mill Wastewater. Food Chem. 2022, 381, 132238. [Google Scholar] [CrossRef]
- Huang, Y.; Guan, Q.; Zhang, Z.; Wang, P.; Li, C. Oleacein: A Comprehensive Review of Its Extraction, Purification, Absorption, Metabolism, and Health Effects. Food Chem. 2024, 433, 137334. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, M.M.; Rauf, A.; Islam, M.R.; Manna, S.J.; Khan, A.A.; Ullah, S.; Akhtar, M.N.; Aljohani, A.S.M.; Abdulmonem, W.A.; et al. Oleuropein: Chemistry, Extraction Techniques and Nutraceutical Perspectives-An Update. Crit. Rev. Food Sci. Nutr. 2024, 64, 9933–9954. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Costa, J.; Sarmento, B.; Araújo, F. 3.3—Cell-Based in Vitro Models for Intestinal Permeability Studies. In Concepts and Models for Drug Permeability Studies; Sarmento, B., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 57–81. ISBN 978-0-08-100094-6. [Google Scholar]
- Pinto, D.; Silva, A.M.; Dall’Acqua, S.; Sut, S.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Simulated Gastrointestinal Digestion of Chestnut (Castanea sativa Mill.) Shell Extract Prepared by Subcritical Water Extraction: Bioaccessibility, Bioactivity, and Intestinal Permeability by In Vitro Assays. Antioxidants 2023, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.D.; Lopes, E.; Afonso, M.; Peixe, A.; Rodrigues, F.M.; Duarte, M.F. Phenolic Profile Characterization of ‘Galega Vulgar’ and ‘Cobrançosa’ Portuguese Olive Cultivars along the Ripening Stages. Appl. Sci. 2020, 10, 3930. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B Coculture Models To Predict Intestinal and Colonic Permeability Compared to Caco-2 Monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of Health-Promoting Phenolic Compounds in Two Extra Virgin Olive Oils and Diversity of Associated Yeasts. J. Food Compos. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Bertini, S.; Ricci, C.; Cascone, M.G.; Danti, S.; Saba, A.; Macchia, M.; Digiacomo, M. Olive Mill Wastewater as Source of Polyphenols with Nutraceutical Properties. Nutrients 2023, 15, 3746. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor Polar Compounds in Extra Virgin Olive Oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu Spectrophotometric Method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel Strong Scavengers of Reactive Oxygen and Nitrogen Species. Bioorganic Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Rodrigues, F.; Braga, N.; Santos, J.; Pimentel, F.B.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P.P. The Castanea Sativa Bur as a New Potential Ingredient for Nutraceutical and Cosmetic Outcomes: Preliminary Studies. Food Funct. 2017, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- González, F.; García-Martínez, E.; Del Mar Camacho, M.; Martínez-Navarrete, N.; Sarmento, B.; Fernandes, I.; Freitas, V.; Rodrigues, F.; Oliveira, B. Insights into the Development of Grapefruit Nutraceutical Powder by Spray Drying: Physical Characterization, Chemical Composition and 3D Intestinal Permeability. J. Sci. Food Agric. 2019, 99, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban Brown Propolis Interferes in the Crosstalk between Colorectal Cancer Cells and M2 Macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; Ledesma-Escobar, C.A.; Parrado-Martínez, M.J.; Marchal-López, R.M.; Olmo-Peinado, J.M.; Espejo-Calvo, J.A.; Priego-Capote, F. Monitoring the Partition of Bioactive Compounds in the Extraction of Extra Virgin Olive Oil. LWT 2022, 162, 113433. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Polyphenolic Content in Olive Oil Waste Waters and Related Olive Samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic Composition and Antimicrobial Activity of Algerian Olive Products and By-Products. LWT 2018, 93, 323–328. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic Profile and Antioxidant Activities of Olive Mill Wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Jarboui, R.; Saber Azab, M.; Bilel, H.; Moustafa, S.M.N. Antifungal Effect of Fresh and Stored Olive Mill Wastewater and Its Ethyl Acetate Extract against Plant Pathogenic Fungi. Plant Prot. Sci. 2024, 60, 65–79. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic Compounds of Olive Fruit: One- and Two-Dimensional Nuclear Magnetic Resonance Characterization of Nüzhenide and Its Distribution in the Constitutive Parts of Fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef]
- Kiritsakis, A.K.; Kiritsakis, K.A.; Tsitsipas, C.K. A Review of the Evolution in the Research of Antioxidants in Olives and Olive Oil during the Last Four Decades. J. Food Bioact. 2020, 31–56, 31–56. [Google Scholar] [CrossRef]
- López-Huertas, E.; Lozano-Sánchez, J.; Segura-Carretero, A. Olive Oil Varieties and Ripening Stages Containing the Antioxidants Hydroxytyrosol and Derivatives in Compliance with EFSA Health Claim. Food Chem. 2021, 342, 128291. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Munier, S.; Paradis, C.; Belmiloudi, S.; Margout-Jantac, D. Validation of Simple UPLC-MS-UV and HPLC-Fluorescence Methods for the Determination of Oleacein in Olive Mill Wastewater. Application in the Analysis of Oleacein in French Cultivars. ACS Food Sci. Technol. 2024, 4, 578–585. [Google Scholar] [CrossRef]
- Silvan, J.M.; Pinto-Bustillos, M.A.; Vásquez-Ponce, P.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive Mill Wastewater as a Potential Source of Antibacterial and Anti-Inflammatory Compounds against the Food-Borne Pathogen Campylobacter. Innov. Food Sci. Emerg. Technol. 2019, 51, 177–185. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Sivakumar, G.; Briccoli Bati, C.; Uccella, N. HPLC-MS Screening of the Antioxidant Profile of Italian Olive Cultivars. Chem. Nat. Compd. 2005, 41, 588–591. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Lavee, S. Changes in Phenolic Content of Olive during Maturation. Int. J. Food Sci. Technol. 1999, 34, 265–274. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Giusti, M.; Zanoni, B.; Innocenti, M.; Mulinacci, N. Phenolic Profiles, Oil Amount and Sugar Content during Olive Ripening of Three Typical Tuscan Cultivars to Detect the Best Harvesting Time for Oil Production. Food Res. Int. 2013, 54, 1876–1884. [Google Scholar] [CrossRef]
- Dali, I.; Abdelwahab, A.T.; Aydi, A.; Fares, N.; Eladeb, A.; Hamzaoui, M.; Abderrabba, M.; Abdelfattah, M.A.; Guetat, A. Valorization of Lyophilized Olive Mill Wastewater: Chemical and Biochemical Approaches. Sustainability 2023, 15, 3360. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of Mitochondrial Reactive Oxygen Species in Homeostasis Regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, e1245049. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, Isoverbascoside, and Their Derivatives Recovered from Olive Mill Wastewater as Possible Food Antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R.; Francisco-Márquez, M.; Medina, M.E. A Quantum Chemical Study on the Free Radical Scavenging Activity of Tyrosol and Hydroxytyrosol. Theor. Chem. Acc. 2012, 131, 1173. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A Comparison of Antioxidant Activities of Oleuropein and Its Dialdehydic Derivative from Olive Oil, Oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Piscopo, A.; Abdel-Rahman, W.M.; Piga, A.; Pintus, G. Antioxidant Properties of Olive Mill Wastewater Polyphenolic Extracts on Human Endothelial and Vascular Smooth Muscle Cells. Foods 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary Fiber and Phenolic Compounds as Functional Ingredients: Interaction and Possible Effect after Ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of Polyphenols from Lycium Ruthenicum Fruit by UPLC-Q-TOF/MS(E) and Their Antioxidant Activity in Caco-2 Cells. J. Agric. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants 2022, 11, 763. [Google Scholar] [CrossRef]
- López-Yerena, A.; Pérez, M.; Vallverdú-Queralt, A.; Miliarakis, E.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Oleacein Intestinal Permeation and Metabolism in Rats Using an In Situ Perfusion Technique. Pharmaceutics 2021, 13, 719. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Radić, K.; Turčić, P.; Anić, D.; Komar, B.; Šalov, M. Food (Matrix) Effects on Bioaccessibility and Intestinal Permeability of Major Olive Antioxidants. Foods 2020, 9, 1831. [Google Scholar] [CrossRef]
- Nieto, J.A.; Fernández-Jalao, I.; Siles-Sánchez, M.D.L.N.; Santoyo, S.; Jaime, L. Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules 2023, 28, 2461. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Chen, R. An M0 Macrophage-Related Prognostic Model for Hepatocellular Carcinoma. BMC Cancer 2022, 22, 791. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Filipek, A.; Mikołajczyk, T.P.; Guzik, T.J.; Naruszewicz, M. Oleacein and Foam Cell Formation in Human Monocyte-Derived Macrophages: A Potential Strategy Against Early and Advanced Atherosclerotic Lesions. Pharmaceuticals 2020, 13, 64. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein Enhances Anti-Inflammatory Activity of Human Macrophages by Increasing CD163 Receptor Expression. Phytomedicine 2015, 22, 1255–1261. [Google Scholar] [CrossRef]


| Polyphenols mg/kg | OMWW- A1(CF) | EVOO- A1(CF) | OMWW- B1(CL) | EVOO- B1(CL) | OMWW- A2(CF) | EVOO- A2(CF) | OMWW- B2(CL) | EVOO- B2(CL) |
|---|---|---|---|---|---|---|---|---|
| Early stage | Early stage | Early stage | Early stage | Later stage | Later stage | Later stage | Later stage | |
| Hydroxytyrosol | 92.4 ± 7.8 b | 0.99 ± 0.05 c | 172.7 ± 4.2 b | 1.38 ± 0.3 c | 190.7 ± 18 a | 4.2 ± 0.9 c | 339.0 ± 20.5 a | 1.5 ± 0.03 c |
| Tyrosol | 30.0 ± 1.8 e | 1.38 ± 0.2 c | 54.2 ± 1.0 c | 8.2 ± 0.01 c | 61.6 ±10 b | 3.2 ± 0.3 c | 89.6 ± 4.9 c | 10.6 ± 0.2 c |
| Caffeic acid | 16.2 ± 1.3 e,f | nd | 12.9 ± 0.8 d | nd | 59.4 ± 3.6 b | nd | 55.7 ± 9 d | nd |
| Vanillic acid | 4.1 ± 0.4 f | nd | 4.4 ± 0.02 d | nd | 5.8 ± 1.5 c | nd | 7.0 ± 1 e | nd |
| Verbascoside | 72.0 ± 6.9 c | nd | 190.1 ± 2.5 b | nd | nd | nd | 164.7 ± 27 b | nd |
| Oleuropein | 36.1 ± 3.4 e | nd | 15.1 ± 5.3 d | nd | nd | nd | nd | nd |
| Pinoresinol | 13.1 ± 3.7 f | nd | 36.6 ± 3.6 c,d | nd | nd | nd | 30.1 ± 3.2 d | nd |
| Apigenin-7-glucoside | 50.8 ± 1.5 d | nd | 96.8 ± 7.3 c | nd | nd | nd | 44.6 ± 0.2 d | nd |
| Oleacein | 153.8 ± 13 a | 49.3 ± 2.1 b | 672.6 ± 55.1 a | 157 ± 5.7 b | nd | 29.2 ± 2.1 b | nd | 180.7 ± 4.9 b |
| Oleocanthal | nd | 224.9 ± 14.7 a | nd | 369.4 ± 4.8 a | nd | 216 ± 11.7 a | nd | 379.6 ± 8 a |
| Total phenols | 455.5 ± 17.6 | 276.6 ± 14.8 | 1255.4 ± 56.17 | 536.0 ± 7.45 | 317.5 ± 20.9 | 252.6 ± 11.9 | 730.8 ± 35.6 | 572.4 ± 9.38 |
| TPC FC | 987.8 ± 29.1 | 307.6 ± 73.2 | 1529 ± 24.6 | 616.9 ± 3.00 | 457.8 ± 9.7 | 268 ± 30.8 | 1512.2 ± 23.6 | 609.1 ± 77 |
| Samples |
DPPH IC50 mg/mL |
ABTS IC50 mg/mL |
|---|---|---|
| OMWW A1 | 0.792 ± 0.02 c | 0.325 ± 0.006 f |
| OMWW A2 | 0.769 ± 0.05 c | 0.440 ± 0.02 e |
| OMWW B1 | 0.165 ± 0.02 g | 0.216 ± 0.006 g |
| OMWW B2 | 0.258 ± 0.007 f | 0.144 ± 0.01 h |
| EVOO A1 | 1.16 ± 0.02 b | 1.83 ± 0.01 a |
| EVOO A2 | 1.61± 0.02 a | 1.72 ± 0.05 b |
| EVOO B1 | 0.47 ± 0.004 e | 0.73 ± 0.01 d |
| EVOO B2 | 0.58 ± 0.009 d | 0.84 ± 0.02 c |
| Trolox | 0.007 ± 0.001 h | 0.003 ± 0.0004 i |
| Samples | ROS | |
|---|---|---|
| O2− | HOCl− | |
| IC50 (μg/mL) | ||
| OMWW A1 | 134.07 ± 17.44 a | 120.94 ± 9.99 a |
| OMWW A2 | 48.76 ± 4.53 b,c | 113.88 ± 3.79 a |
| OMWW B1 | 52.48 ± 1.58 b | 78.07 ± 3.04 a |
| OMWW B2 | 40.07 ± 3.93 b,c | 96.34 ± 1.83 a |
| Positive Controls | ||
| Gallic acid | 6.34 ± 0.21 d | 2.60 ± 0.05 b |
| Catechin | 18.01 ± 0.34 c,d | 0.20 ± 0.01 b |
| Analyte | Mean Permeation Rate (±SD) at 240 min (%) |
|---|---|
| Tyrosol | 59 ± 29 |
| Hydroxytyrosol | 34 ± 5 |
| Caffeic acid | 26 ± 3 |
| Vanillic acid | 48 ± 2 |
| Pinoresinol | 44 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuffaro, D.; Bertolini, A.; Silva, A.M.; Rodrigues, F.; Gabbia, D.; De Martin, S.; Saba, A.; Bertini, S.; Digiacomo, M.; Macchia, M. Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies. Foods 2024, 13, 3312. https://doi.org/10.3390/foods13203312
Cuffaro D, Bertolini A, Silva AM, Rodrigues F, Gabbia D, De Martin S, Saba A, Bertini S, Digiacomo M, Macchia M. Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies. Foods. 2024; 13(20):3312. https://doi.org/10.3390/foods13203312
Chicago/Turabian StyleCuffaro, Doretta, Andrea Bertolini, Ana Margarida Silva, Francisca Rodrigues, Daniela Gabbia, Sara De Martin, Alessandro Saba, Simone Bertini, Maria Digiacomo, and Marco Macchia. 2024. "Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies" Foods 13, no. 20: 3312. https://doi.org/10.3390/foods13203312
APA StyleCuffaro, D., Bertolini, A., Silva, A. M., Rodrigues, F., Gabbia, D., De Martin, S., Saba, A., Bertini, S., Digiacomo, M., & Macchia, M. (2024). Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies. Foods, 13(20), 3312. https://doi.org/10.3390/foods13203312











