In Vitro Inhibitory Mechanism of Polyphenol Extracts from Multi-Frequency Power Ultrasound-Pretreated Rose Flower Against α-Glucosidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Polyphenol-Rich Rose Extracts
2.3. Analytical Methods
2.3.1. Determination of Total Phenol Content
2.3.2. Determination of α-Glucosidase Activity
2.3.3. Determination of the Type of α-Glucosidase Inhibition
2.3.4. Fluorescence Spectrometry
2.3.5. Circular Dichroic Scanning
2.3.6. Molecular Docking
2.4. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Inhibitory Activity of Polyphenol-Rich Rose Extract on α-Glucosidase
3.2. Determination of the Type of α-Glucosidase Inhibition by Rose Extracts
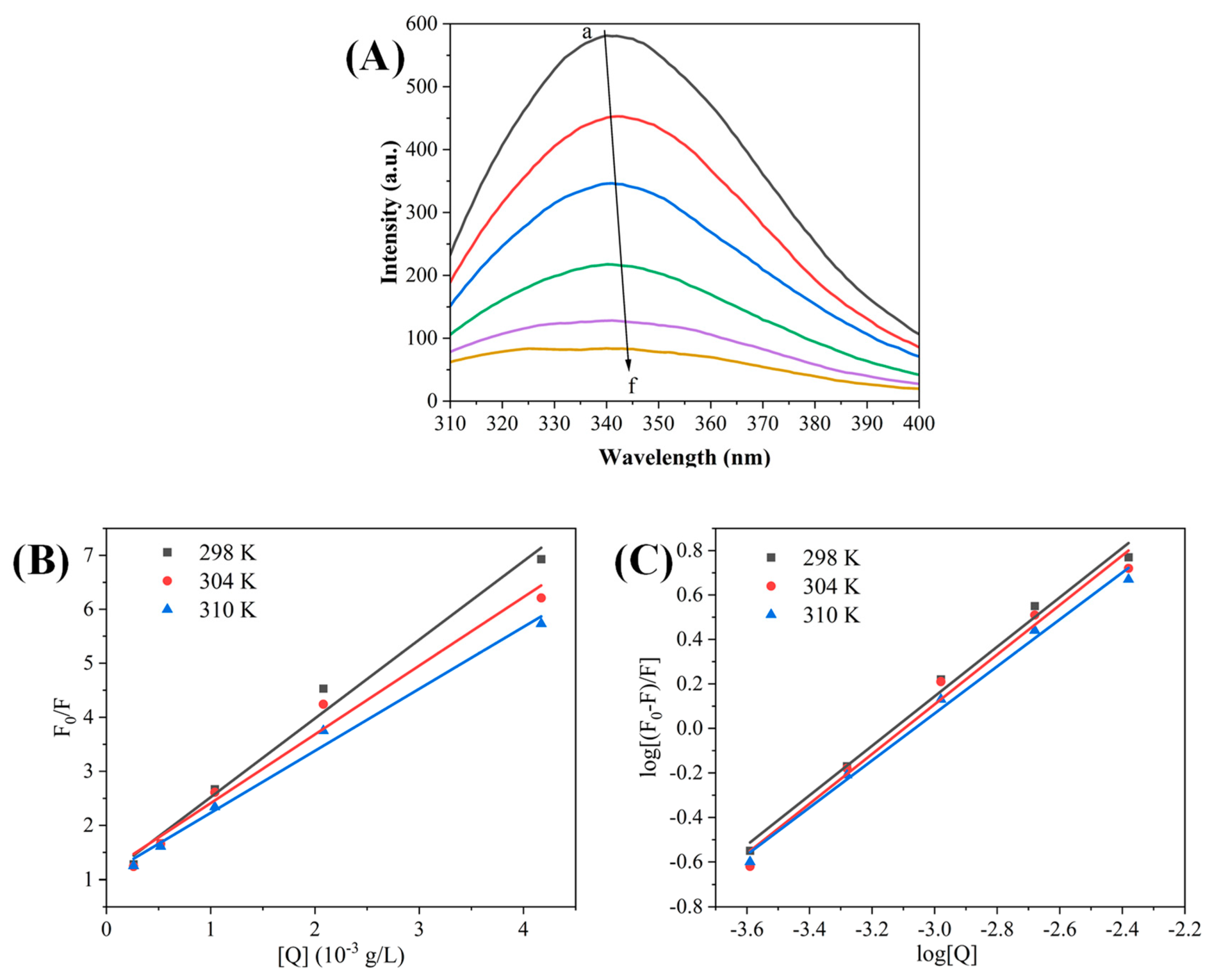
3.3. Effect of Polyphenol-Rich Rose Extract on the Fluorescence Spectra of α-Glucosidase
3.3.1. Endogenous Fluorescence Spectroscopy
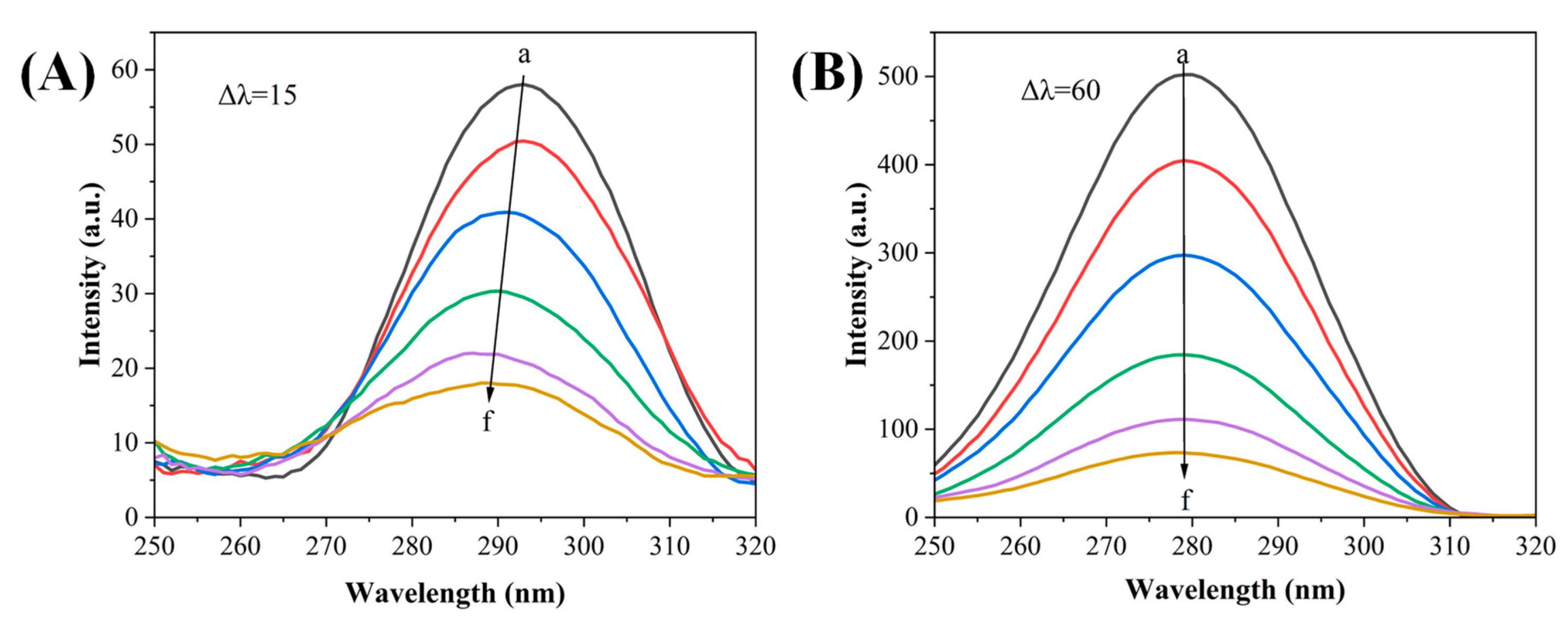
3.3.2. Synchronized Fluorescence Spectroscopy
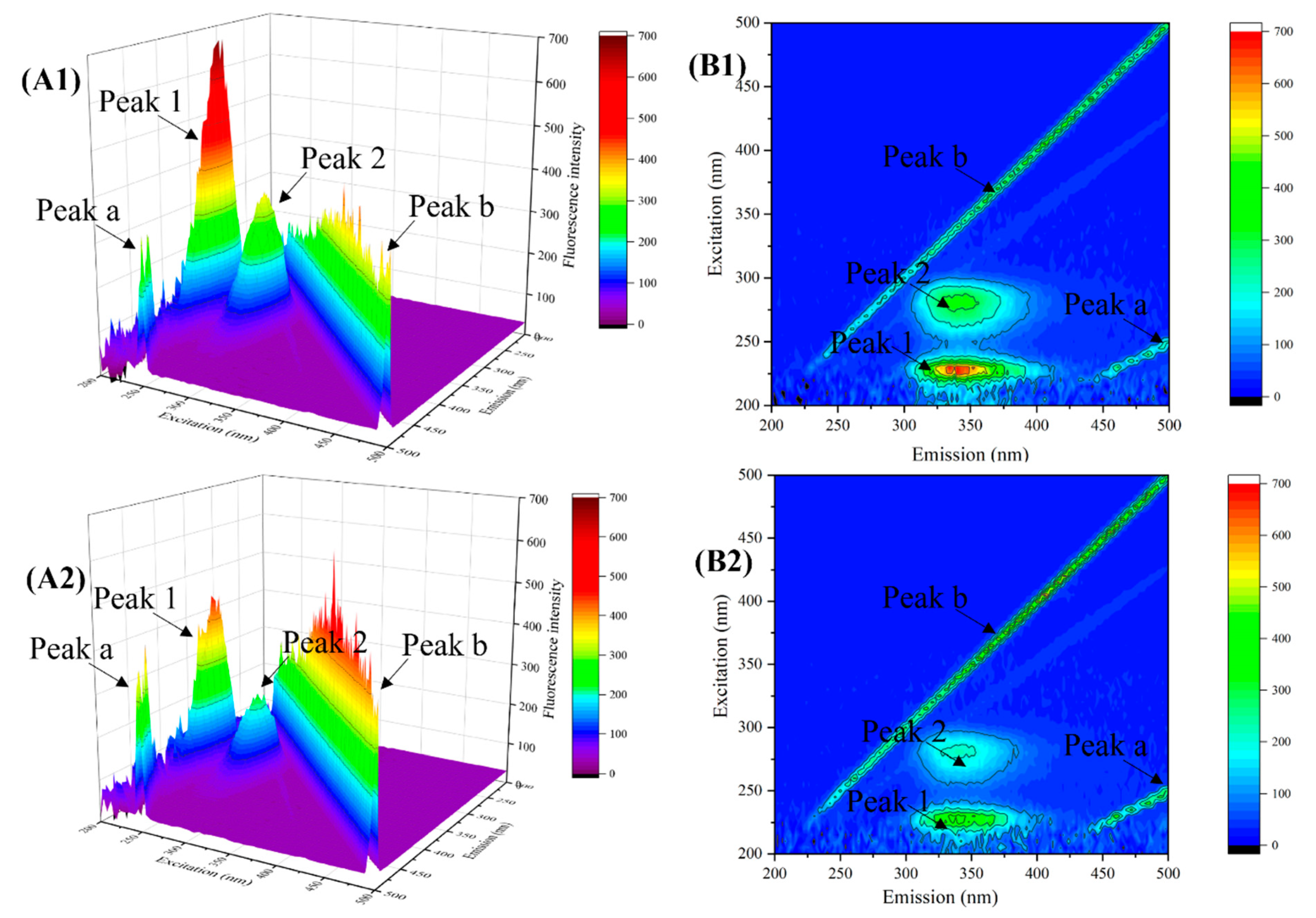
3.3.3. Three-Dimensional Fluorescence Spectroscopy
3.3.4. Effect of Polyphenol-Rich Rose Extract on the Circular Dichroism of α-Glucosidase
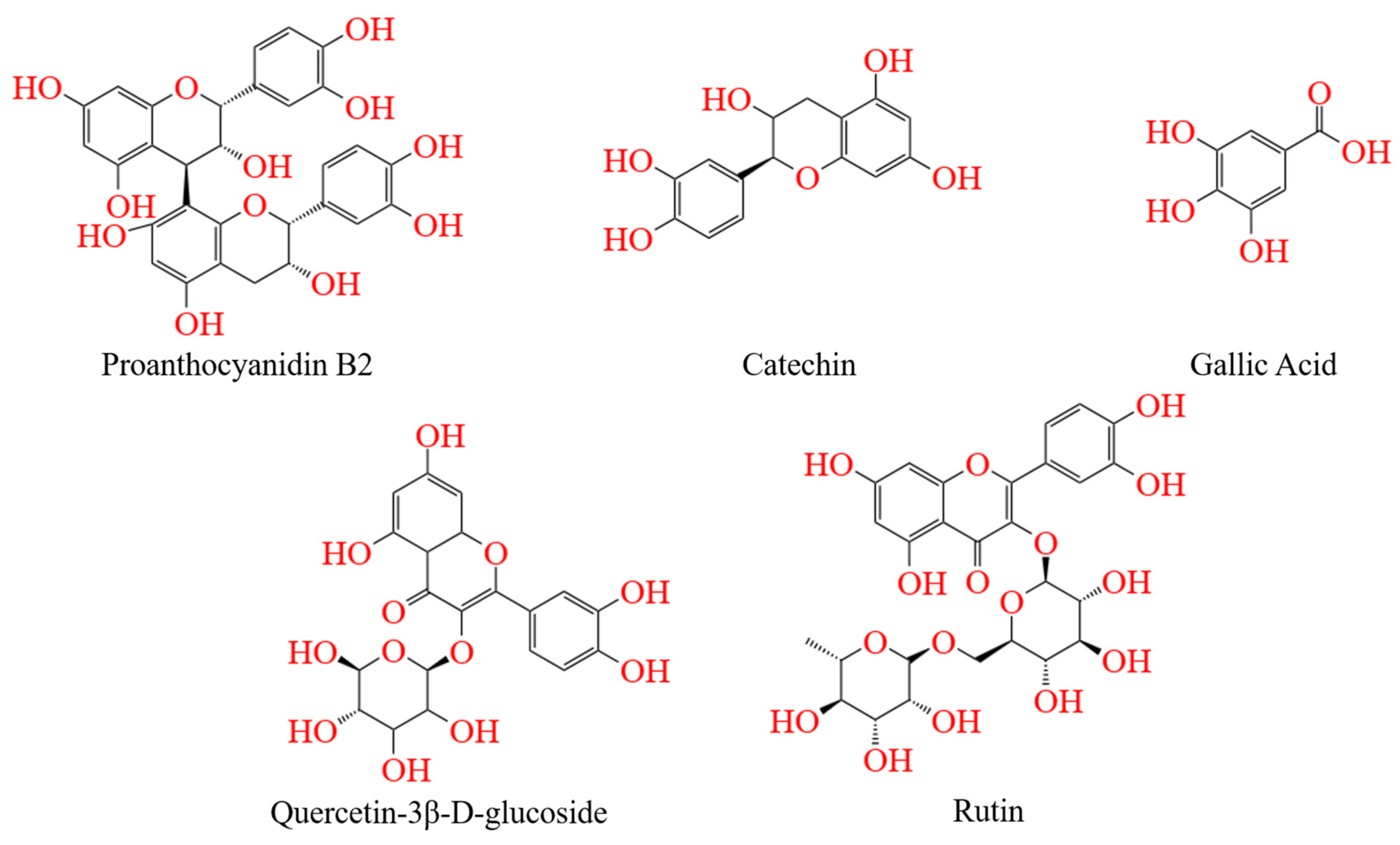
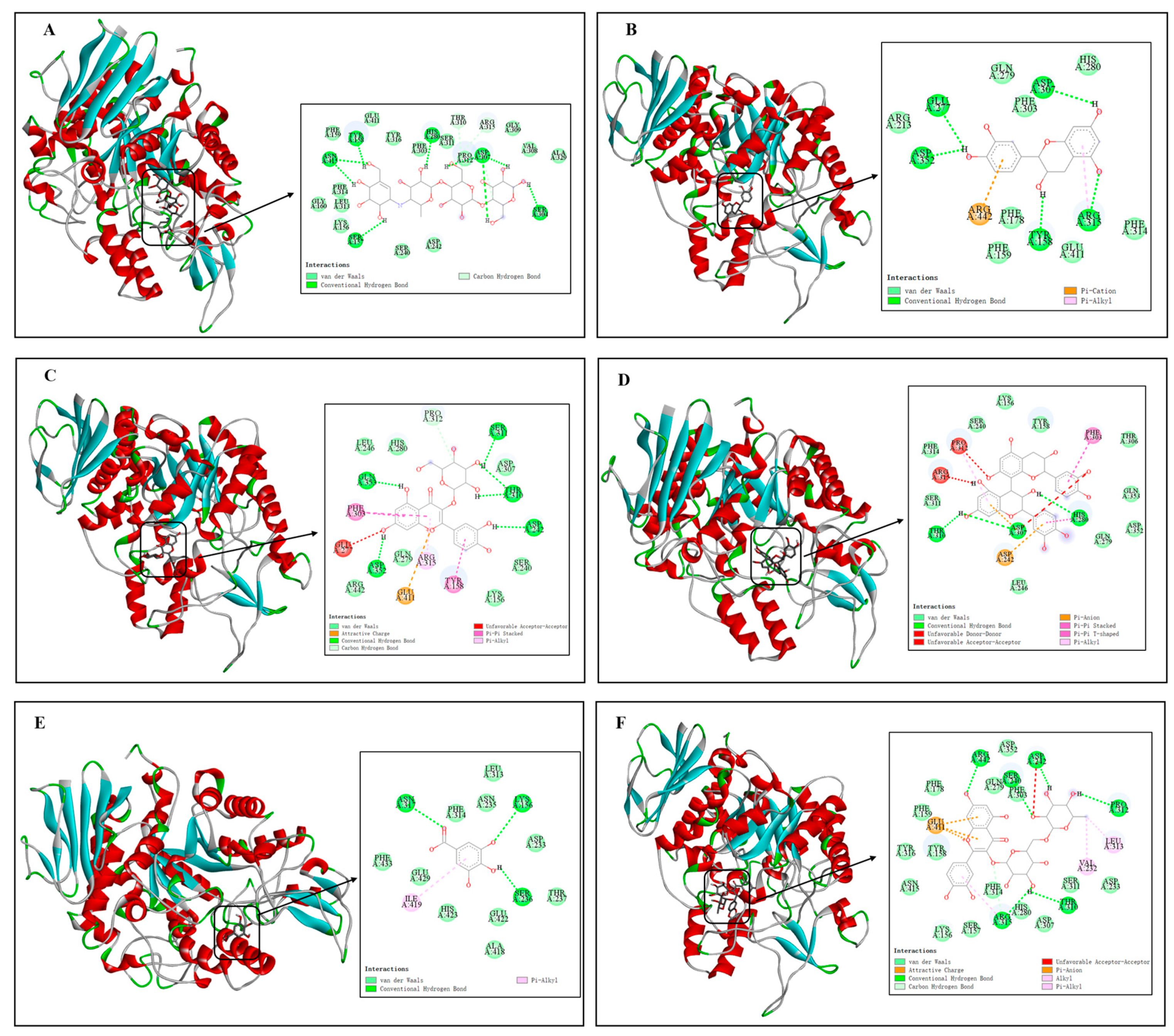
3.3.5. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Shi, J.; Wang, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Inhibitory kinetics and mechanism of flavonoids from lotus (Nelumbo nucifera Gaertn.) leaf against pancreatic alpha-amylase. Int. J. Biol. Macromol. 2018, 120, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Wan, G.-Z.; Xu, F.-C.; Guo, Z.-H.; Chen, J. Screening and identification of a-glucosidase inhibitors from Cyclocarya paliurus leaves by ultrafiltration coupled with liquid chromatography-mass spectrometry and molecular docking. J. Chromatogr. A 2022, 1675, 463160. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.; Du, X.; Li, Z.; Yang, Y.; Jiang, Z.; Ni, H.; Li, Q. Effect of Porphyra haitanensis polyphenols from different harvest periods on hypoglycaemic activity based on in vitro digestion and widely targeted metabolomic analysis. Food Chem. 2024, 437, 137793. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of alpha-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Luo, S.; Yang, W.; Huang, Y.; Peng, Z.; Wang, G. Design, synthesis, biological evaluation, and docking study of new triazole-phenylacetamide derivatives as α-glucosidase inhibitors. Bioorganic Chem. 2023, 141, 106844. [Google Scholar] [CrossRef]
- Cao, P.; Xiang, S.Y.; Liu, S.X.; Feng, Y.; Zhang, X.Y.; Wu, Q.; Hou, J.J.; Liu, H.; Cheng, D.; Liu, X.X. Isolation of an α-glucosidase Inhibitor from Houttuynia cordata Thunb. and Its In vitro and In vivo Hypoglycemic Bioactivity. Plant Foods Hum. Nutr. 2024. [Google Scholar] [CrossRef]
- Ruan, Q.; Chen, Y.; Wen, J.; Qiu, Y.; Huang, Y.; Zhang, Y.; Farag, M.A.; Zhao, C. Regulatory mechanisms of the edible alga Ulva lactuca polysaccharide via modulation of gut microbiota in diabetic mice. Food Chem. 2023, 409, 135287. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Gutierrez, E.; Ramos Solano, B.; Seymour, E.M.; Bolling, S.F. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem. 2018, 252, 142–146. [Google Scholar] [CrossRef]
- Jianzhong, Z.; Chun, C.; Bin, Z.; Qiang, H. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2019, 60, 695–708. [Google Scholar]
- Zhou, Z.; Wang, D.; Xu, X.; Dai, J.; Lao, G.; Zhang, S.; Xu, X.; Dinnyes, A.; Xiong, Y.; Sun, Q. Myofibrillar protein-chlorogenic acid complexes ameliorate glucose metabolism via modulating gut microbiota in a type 2 diabetic rat model. Food Chem. 2023, 409, 135195. [Google Scholar] [CrossRef]
- Ma, L. Extraction of Polysaccharides from Rose Petals and Preparation of Oral Solution. Doctoral Dissertation, Henan University of Science and Technology, Luoyang, China, 2022. [Google Scholar]
- Xu, B.; Feng, M.; Chitrakar, B.; Wei, B.; Wang, B.; Zhou, C.; Ma, H.; Wang, B.; Chang, L.; Ren, G.; et al. Selection of drying techniques for Pingyin rose on the basis of physicochemical properties and volatile compounds retention. Food Chem. 2022, 385, 132539. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Berger, K.; Hogberg, A.; Landin-Olsson, M.; Holm, C. Effects of rose hip intake on risk markers of type 2 diabetes and cardiovascular disease: A randomized, double-blind, cross-over investigation in obese persons. Eur. J. Clin. Nutr. 2012, 66, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xu, B.; Islam, M.N.; Zhou, C.; Wei, B.; Wang, B.; Ma, H.; Chang, L. Individual and synergistic effect of multi-frequency ultrasound and electro-infrared pretreatments on polyphenol accumulation and drying characteristics of edible roses. Food Res. Int. 2023, 163, 112120. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Hosbas, S.; Orhan, D.D. Antidiabetic Effect and Antioxidant Potential of Rosa canina Fruits. Pharmacogn. Mag. 2009, 5, 309–315. [Google Scholar] [CrossRef]
- Wang, C.; Kim, I.-J.; Seong, H.-R.; Noh, C.H.; Park, S.; Kim, T.M.; Jeong, H.S.; Kim, K.Y.; Kim, S.T.; Yuk, H.-G.; et al. Antioxidative and Anti-inflammatory Activities of Rosebud Extracts of Newly-Crossbred Roses. Nutrients 2023, 15, 2376. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, L.; Lu, Q.; Liu, R. Interaction mechanism between alpha-glucosidase and A-type trimer procyanidin revealed by integrated spectroscopic analysis techniques. Int. J. Biol. Macromol. 2020, 143, 173–180. [Google Scholar] [CrossRef]
- Banerji, S.; Banerjee, S. A formulation of grape seed, Indian gooseberry, turmeric and fenugreek helps controlling type 2 diabetes mellitus in advanced-stage patients. Eur. J. Integr. Med. 2016, 8, 645–653. [Google Scholar] [CrossRef]
- Cvetanovic, A.; Svarc-Gajic, J.; Maskovic, P.; Savic, S.; Nikolic, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Guan, Q.H.; Tang, L.H.; Zhang, L.L.; Huang, L.X.; Xu, M.; Wang, Y.; Zhang, M. Molecular insights into α-glucosidase inhibition and antiglycation properties affected by the galloyl moiety in (-)-epigallocatechin-3-gallate. J. Sci. Food Agric. 2023, 103, 7381–7392. [Google Scholar] [CrossRef]
- Xu, B.; Feng, M.; Tiliwa, E.S.; Yan, W.; Wei, B.; Zhou, C.; Ma, H.; Wang, B.; Chang, L. Multi-frequency power ultrasound green extraction of polyphenols from Pingyin rose: Optimization using the response surface methodology and exploration of the underlying mechanism. LWT-Food Sci. Technol. 2022, 156, 113037. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Huang, Z.; Zhao, Z. Phenolics from Sterculia nobilis Smith pericarp by-products delay carbohydrate digestion by uncompetitively inhibiting alpha-glucosidase and alpha-amylase. LWT-Food Sci. Technol. 2023, 173, 114339. [Google Scholar] [CrossRef]
- Di, Z.; Xuhao, L.; Zhikun, Y.; Jiyong, S.; Lei, Z.; Maurizio, B.; Jianbo, X.; Xinyue, D.; Yanling, W.; Chengtao, W.; et al. Interactions between Phenols and Alkylamides of Sichuan Pepper (Zanthoxylum Genus) in α-Glucosidase Inhibition: A Structural Mechanism Analysis. J. Agric. Food Chem. 2021, 69, 5583–5598. [Google Scholar]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against alpha-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Xu, B.; Chen, J.; Chitrakar, B.; Li, H.; Wang, J.; Wei, B.; Zhou, C.; Ma, H. Effects of flat sweep frequency and pulsed ultrasound on the activity, conformation and microstructure of mushroom polyphenol oxidase. Ultrason. Sonochemistry 2022, 82, 105908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, S.; Meng, Y.; Cheng, Y.; Han, Z.; Wang, F. Mechanisms underlying the effect of walnut pellicle extracts and its four representative polyphenols on in vitro digestion of walnut protein isolate. Food Bioprod. Process. 2024, 144, 166–177. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Gan, J.; Zhi-Xiong, X.; Cao, Y. FitDock: Protein-ligand docking by template fitting. Brief. Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, L.; Lin, X.; Hu, X.; Wang, L. Phenolic profiles and screening of potential alpha-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESI-qTOF-MS/MS and molecular docking analysis. Ind. Crops Prod. 2020, 154, 112673. [Google Scholar] [CrossRef]
- Xu, B.-G.; Zhang, M.; Bhandari, B.; Cheng, X.-F.; Islam, M.N. Effect of ultrasound-assisted freezing on the physico-chemical properties and volatile compounds of red radish. Ultrason. Sonochemistry 2015, 27, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochemistry 2018, 40, 619–628. [Google Scholar] [CrossRef]
- Xu, B.; Feng, M.; Chitrakar, B.; Cheng, J.; Wei, B.; Wang, B.; Zhou, C.; Ma, H. Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103295. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Galvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of drying processes on composition, microstructure and health aspects from maqui berries. J. Food Sci. Technol.-Mysore 2020, 57, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.-J.; Kong, J.-B.; Liu, Y.; Zhi, Y.-L.; Cao, Y.-G.; Du, K.; Xue, G.-M.; Li, M.; Zhao, Z.-Z.; et al. Structurally Diverse Phenolic Amides from the Fruits of Lycium barbarum with Potent a-Glucosidase, Dipeptidyl Peptidase-4 Inhibitory, and PPAR-? Agonistic Activities. J. Agric. Food Chem. 2023, 71, 11080–11093. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-L.; Si, X.; Wang, Y.-H.; Gong, E.-S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit alpha-glucosidase and alpha-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Rayan, A.M.; Akhtar, H.M.S.; Zeng, X. Synergistic inhibition of isolated flavonoids from Moringa oleifera leaf on alpha-glucosidase activity. LWT-Food Sci. Technol. 2021, 141, 111081. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, X.; Tang, J.; Zhang, X.; Wang, C.; Li, H. Characterization of the binding mechanism and conformational changes of bovine serum albumin upon interaction with aluminum-maltol: A spectroscopic and molecular docking study. Metallomics 2019, 11, 1625–1634. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between beta-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Karrar, E.; Jin, Q.; Zhang, H.; Wu, G.; Wang, X. Mechanisms of sesamol and sesamin inhibiting alpha-glucosidase activity by spectroscopy and molecular docking. Food Biosci. 2023, 53, 102680. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Wang, M.; Ma, X.; Peng, X. Studies on the inhibition of a-glucosidase by biflavonoids and their interaction mechanisms. Food Chem. 2023, 420, 136113. [Google Scholar] [CrossRef]
- Ma, F.; Huang, H.-Y.; Zhou, L.; Yang, C.; Zhou, J.-H.; Liu, Z.-M. Study on the conformation changes of Lysozyme induced by Hypocrellin A: The mechanism investigation. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2012, 97, 1159–1165. [Google Scholar] [CrossRef]
- Ding, F.; Diao, J.-X.; Sun, Y.; Sun, Y. Bioevaluation of Human Serum Albumin-Hesperidin Bioconjugate: Insight into Protein Vector Function and Conformation. J. Agric. Food Chem. 2012, 60, 7218–7228. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Ye, X.; Liu, D. In vitro inhibitory effects of Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins on pancreatic alpha-amylase and their interaction. Bioorganic Chem. 2020, 101, 104029. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, W.; Feng, Y.; Li, Y.; Liu, K.; Liu, L.; Lin, D.; He, Z.; Wu, X. Acteoside and Acyl-Migrated Acteoside, Compounds in Chinese Kudingcha Tea, Inhibit alpha-Amylase In Vitro. J. Med. Food 2017, 20, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tian, Y.; Yang, N.; Xu, X.; Jin, Z. A study on the inhibition mechanism of beta-cyclodextrin on pullulanase. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 161–165. [Google Scholar] [CrossRef]



| Samples | REs (Control) | REs (UP) | Acarbose |
|---|---|---|---|
| Total phenolic content (mg/g) | 134.91 ± 5.69 a | 167.40 ± 2.67 b | / |
| IC50 α-Glu (μg/mL) | 1.96 ± 0.07 A,B | 1.33 ± 0.10 C | 0.17 ± 0.01 |
| T (K) | Ksv (L/g) | Ka (L/g) | n | ΔH° (kJ/g) | ΔG° (kJ/g) | ΔS° (J/g/K) |
|---|---|---|---|---|---|---|
| 298 K | 1457.11 | 3019.95 | 1.11 | −35.04 | −20.05 | −50.31 |
| 304 K | 1271.63 | 2884.03 | 1.12 | −19.75 | ||
| 310 K | 1147.02 | 1737.80 | 1.06 | −19.44 |
| Chemical Compound | Affinities (kcal/mol) | Active Amino Acid Residues |
|---|---|---|
| Acarbose | −8.70 | Tyr158, Asn415, Asp307, Ser304, Ser157, Thr310, Arg315, Glu411, Phe159, Tyr316, Gly309, Phe303, Ser311, Pro312, Val308, Ala329, Phe314, Gly160, Leu313, Lys156, Ser240, Asp242 |
| Catechin | −8.60 | Glu277, Asp307, Asp352, Tyr158, Arg315, Arg442, Gln279, His280, Phe303, Arg213, Phe178, Phe314, Phe159, Glu411 |
| Quercetin-3β-D-glucoside | −9.60 | Pro312, Lfu246, His280, Ser311, Gin353, Asp307, Thr310, Phe303, Asp242, Glu277, Gln279, Arg315, Ser240, Arg442, Glu411, Tyr158, Lys156 |
| Proanthocyanidin B2 | −9.90 | Lys156, Ser240, Tyr158, Phe314, Pro312, Phe303, Thr3306, Arg315, Ser311, Gln353, Thr310, Asp307, His280, Asp242, Gln279, Asp352, Leu246 |
| Gallic acid | −6.50 | Leu313, Asn317, Phe314, Asn235, Lys156, Phe433, Glu429, ILE419, His423, Glu422, Ser236, Thr237, Ala418 |
| Rutin | −10.10 | Asp352, Arg442, Asp242, Phe178, Gln279, Ser40, Phe303, Phe159, Glu411, Pro312, Tyr316, Tyr158, Leu313, Val232, Asn415, Phe314, Ser311, Asp233, Thr310, His280, Arg315, Asp307, Lys156, Ser157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Feng, M.; Chitrakar, B.; Yang, F.; Wei, B.; Wang, B.; Zhou, C.; Ma, H.; Gao, X.; Xu, B. In Vitro Inhibitory Mechanism of Polyphenol Extracts from Multi-Frequency Power Ultrasound-Pretreated Rose Flower Against α-Glucosidase. Foods 2024, 13, 3421. https://doi.org/10.3390/foods13213421
Zhang C, Feng M, Chitrakar B, Yang F, Wei B, Wang B, Zhou C, Ma H, Gao X, Xu B. In Vitro Inhibitory Mechanism of Polyphenol Extracts from Multi-Frequency Power Ultrasound-Pretreated Rose Flower Against α-Glucosidase. Foods. 2024; 13(21):3421. https://doi.org/10.3390/foods13213421
Chicago/Turabian StyleZhang, Chao, Ming Feng, Bimal Chitrakar, Fan Yang, Benxi Wei, Bo Wang, Cunshan Zhou, Haile Ma, Xianli Gao, and Baoguo Xu. 2024. "In Vitro Inhibitory Mechanism of Polyphenol Extracts from Multi-Frequency Power Ultrasound-Pretreated Rose Flower Against α-Glucosidase" Foods 13, no. 21: 3421. https://doi.org/10.3390/foods13213421
APA StyleZhang, C., Feng, M., Chitrakar, B., Yang, F., Wei, B., Wang, B., Zhou, C., Ma, H., Gao, X., & Xu, B. (2024). In Vitro Inhibitory Mechanism of Polyphenol Extracts from Multi-Frequency Power Ultrasound-Pretreated Rose Flower Against α-Glucosidase. Foods, 13(21), 3421. https://doi.org/10.3390/foods13213421








