Research Progress on Tofu Coagulants and Their Coagulation Mechanisms
Abstract
:1. Introduction
2. Research Progress on Different Coagulants
2.1. Salt Coagulants
2.2. Acid Coagulants
2.3. Enzyme Coagulants
2.4. Novel Coagulants
2.4.1. Emulsion Coagulants
2.4.2. Composite Coagulants
2.4.3. Carbohydrate Auxiliary Agents
3. Research Progress on Coagulation Mechanism
3.1. Effect of Phytic Acid on the Coagulation Process
3.2. Coagulation Mechanisms of Different Coagulants
4. Conclusions and Research Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hui, E.; Henning, S.M.; Park, N.; Heber, D.; Liang, V.; Go, W. Genistein and daidzein/glycitein content in tofu. J. Food Compos. Anal. 2001, 14, 199–206. [Google Scholar] [CrossRef]
- Du, L.Q. New Technology of Tofu Production, 1st ed.; Chemical Industry Publishing House: Beijing, China, 2018; pp. 1–64. [Google Scholar]
- Shi, Y.G.; Liu, L.L. Research progress on correlation between soybean protein and tofu quality. J. Food Technol. 2018, 36, 1–8. [Google Scholar]
- Guan, X.F.; Zhong, X.Q.; Lu, Y.H.; Du, X.; Jia, R.; Li, H.S.; Zhang, M.L. Changes of Soybean Protein during Tofu Processing. Foods 2021, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Carter, E.; Chung, R.A. Use of calcium salts for soybean curd preparation. J. Food Sci. 1980, 45, 32–34. [Google Scholar] [CrossRef]
- Liu, Z.S.; Li, L.T.; Eizo, T. Study on properties of tofu salt-coagulant and mechanism of tofu coagulation. Cereals Oils Assoc. 2000, 3, 39–43. [Google Scholar]
- Liu, Z.S.; Chang, S.K.C.; Li, L.T.; Tatsumi, E. Effect of selective thermal denaturation of soybean proteins on soymilk viscosity and tofu’s physical properties. Food Res. Int. 2004, 37, 815–822. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Perera, C.O.; Valiyaveettil, S. Effect of different coagulants on the isoflavone levels and physical properties of prepared firm tofu. Food Chem. 2006, 99, 492–499. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Qu, W.J.; Zhang, A.; Wahia, H.; Gao, X.L.; Ma, H.L.; Zhou, C.S. Evaluation of dual-frequency multi-angle ultrasound on physicochemical properties of tofu gel and its finished product by TOPSIS-entropy weight method. Ultrason. Sonochem. 2022, 90. [Google Scholar] [CrossRef]
- Nguyen, V.T.A.; Nguyen, V.D.; Quoc, L.P.T. Influence of chicken eggshell powder as an alternative coagulant on the yield and textural characteristics of tofu. J. Teknol.-Sci. Eng. 2023, 85, 159–165. [Google Scholar] [CrossRef]
- Hsieh, J.F.; Yu, C.J.; Tsai, T.Y. Proteomic profiling of the coagulation of soymilk proteins induced by magnesium chloride. Food Hydrocoll. 2012, 29, 219–225. [Google Scholar] [CrossRef]
- Arii, Y.; Takenaka, Y. Magnesium Chloride Concentration-Dependent Formation of Tofu-Like Precipitates with Different Physicochemical Properties. Biosci. Biotechnol. Biochem. 2013, 77, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Chen, Y.H.; Xu, J.T.; Guo, S.T. Effect of different modes of magnesium chloride addition on tofu quality properties. Food Sci. 2017, 38, 102–108. [Google Scholar]
- Li, Y.; Wan, Y.L.; Mamu, Y.; Xu, J.T.; Guo, S.T. Aggregation and gelation of soymilk protein after alkaline heat treatment: Effect of coagulants and their addition sequences. Food Hydrocoll. 2023, 135, 108178. [Google Scholar] [CrossRef]
- Chen, J.; Cai, L.; Huang, X.L.; Fu, H.L.; Sun, L.; Yuan, C.W.; Gong, H.; Lyu, B.; Wang, Z.H.; Yu, H.S. Mathematical modeling of optimal coagulant dosage for tofu preparation using MgCl2. Food Chem.-X 2024, 21, 101137. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological Characteristics and Gelation Mechanism of Tofu (Soybean Curd). J. Agric. Food Chem. 1995, 43, 1808–1812. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, G.; Chen, X.W.; Miao, F.; Hou, J.G. Research on the water-retention properties of lactone tofu. Food Ind. 2003, 1, 49–50. [Google Scholar]
- Yu, B.; Wang, X.B. Treatment technology of soy milk improving texture characteristics of lactone tofu. Trans. Chin. Soc. Agric. Eng. 2014, 30, 287–292. [Google Scholar]
- Xu, J.T.; Li, M.Z.; Li, Y.; Guo, S.T. Effect of soaking conditions on properties of soymilk gel. J. Food Sci. Technol. 2022, 40, 139–147. [Google Scholar]
- Li, Q.R.; Hua, Y.F.; Li, X.F.; Kong, X.Z.; Zhang, C.M.; Chen, Y.M. Acid induced gelation of heated soymilks: Protein structure, molecular interactions and gel properties. Food Hydrocoll. 2024, 148, 9. [Google Scholar] [CrossRef]
- Guan, L.J.; Cheng, Y.Q.; Mu, H.L. Study on the yield of tofu made by soymilk fermented with lactic acid bacteria. Food Sci. Tech. 2009, 34, 36–41. [Google Scholar]
- Zhang, Y.; Liu, Z.M.; Liu, W. Processing Technolgy of Acid Slurry Bean Curd. Acad. Period. Farm Prod. Process. 2014, 4, 21–23+26. [Google Scholar]
- Liu, L.L.; Zhu, X.Q.; Sun, B.Y.; Zeng, J.H.; Yang, Y.; Shi, Y.G. Change of protein in the processing of acid wheytofu. In Proceedings of the Abstracts of Food Summit in China and 16th Annual Meeting of CIFST, Wuhan, China, 13–14 November 2019. [Google Scholar]
- Zhang, Z.; Chen, Z.; Wang, X.; Li, Y.; Zhang, X. Mechanism of Tofu Protein Precipitation Based on Acid-Pulp Content Change. Soybean Sci. 2022, 41, 345–351. [Google Scholar]
- Song, L.J.; Gao, X.Y.; Hu, L.N.; Wang, D.D.; Zhou, Y.F. Selection of Acid Coagulant for Tofu. Soybean Sci. 2012, 31, 1002–1006. [Google Scholar]
- Li, J.; Wang, L.; Liu, N.; Li, C.C. Effect of Hawthorn-acid Tofu Coagulant on Quality of Tofu; Chinese Institute of Food Science and Technology: Harbin, China, 2012. [Google Scholar]
- Fasoyiro, S.B. Physical, Chemical and Sensory Qualities of Roselle Water Extract-coagulated Tofu Compared with Tofu from Two Natural Coagulants. Niger. Food J. 2014, 32, 97–102. [Google Scholar] [CrossRef]
- Cao, F.H.; Li, X.J.; Luo, S.Z.; Mu, D.D.; Zhong, X.Y.; Jiang, S.T.; Zheng, Z.; Zhao, Y.Y. Effects of organic acid coagulants on the physical properties of and chemical interactions in tofu. LWT-Food Sci. Technol. 2017, 85, 58–65. [Google Scholar] [CrossRef]
- Zeppa, G.; Tedesco, M.; Bertolino, M.; Tatar, B. Grape Pomace as a New Coagulant for Tofu Production: Physicochemical and Sensory Effects. Foods 2021, 10, 17. [Google Scholar] [CrossRef]
- Wu, J.L.; Hu, W.K.; Yang, Z.B.; Zeng, X.F.; Dai, Z.R. Effect of flours addition on the physicochemical and metabolome in Suanjiang, a Chinese traditional fermentation coagulant. Food Biosci. 2023, 53, 102716. [Google Scholar] [CrossRef]
- Fuke, Y.; Sekiguchi, M.; Matsuoka, H. Nature of stem bromelain treatment on the aggregation and gelation of soybean proteins. J. Food Sic. 1985, 50, 1283–1288. [Google Scholar] [CrossRef]
- Murata, K.; Kusakabe, I.; Kobayashi, H.; Kiuchi, H.; Murakami, K. Selection of commercial enzymes suitable for making soymilk-curd. Agric. Biol. Chem. 1987, 51, 2929–2933. [Google Scholar]
- Murata, K.; Kobayashi, H.; Kusakabe, I.; Teramoto, H.; Murakami, K. Preparation of fermented soymilk curd with commercial proteinases. J. Jpn. Soc. Food Sci. 1989, 36, 417–423. [Google Scholar] [CrossRef]
- Yasuda, M.; Aoyama, M.; Sakaguchi, M.; Nakachi, K.; Kobamoto, N. Purification and characterization of a soybean-milk-coagulating enzyme from Bacillus pumilus TYO-67. Appl. Microbiol. Biotechnol. 1999, 51, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Luan, G.Z.; Li, L.T. Study on coagulation of soybean milk by protease. Food Ind. Sci. Technol. 2006, 1, 71–74. [Google Scholar]
- Lo, D.; Steviany; Harris, G.T.; Febrianta, H.; Hutabarat, D.J.C.; Wijaya, C. The effects of various isoelectric points in tofu processing on physicochemical properties and antioxidant capacities. IOP Conf. Ser. Earth Environ. Sci. 2022, 012095. [Google Scholar] [CrossRef]
- Ikura, K.; Kometani, T.; Sasaki, R.; Chiba, H. Crosslinking of soybean 7S and 11S proteins by transglutaminase. Agric. Biol. Chem. 1980, 44, 2979–2984. [Google Scholar]
- Ando, H.; Adachi, M.; Umeda, K.; Matsuura, A.; Nonaka, M.; Uchio, R.; Tanaka, H.; Motoki, M. Purification and characteristics of a novel transglutaminase derived from microorganisms. Agric. Biol. Chem. 1989, 53, 2613–2617. [Google Scholar]
- Nonaka, M.; Sakamoto, H.; Toiguchi, S.; Yamagiwa, K.; Soeda, T.; Motoki, M. Retort-resistant tofu prepared by incubation with microbial transglutaminase. Food Hydrocoll. 1996, 10, 41–44. [Google Scholar] [CrossRef]
- Wang, J.L.; Tang, C.H.; Zhou, Z.H.; Yang, X.Q. The study on the texture of microbial transglutaminase tofu. Grain Process. 2006, 3, 77–80. [Google Scholar]
- Wang, J.L.; Tang, C.H.; Zhou, Z.H.; Yang, X.Q. The effects of different processing methods on the texture of microbial transglutaminase tofu. Mod. Food Sci. Technol. 2006, 2, 1–3+8. [Google Scholar]
- Chang, Y.H.; Shiau, S.Y.; Chen, F.B.; Lin, F.R. Effect of microbial transglutaminase on the rheological and textural characteristics of black soybean packed tofu coagulating with Agar. LWT-Food Sci. Technol. 2010, 44, 1107–1112. [Google Scholar] [CrossRef]
- Rui, X.; Fu, Y.T.; Zhang, Q.Q.; Li, W.; Zare, F.; Chen, X.H.; Jiang, M.; Dong, M.S. A comparison study of bioaccessibility of soy protein gel induced by magnesiumchloride, glucono-δ-lactone and microbial transglutaminase. LWT-Food Sci. Technol. 2016, 71, 234–242. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jiang, S.Q.; Li, L. The gel properties and gastric digestion kinetics of a novel lactic acid bacteria fermented tofu: Focusing on the effects of transglutaminase. LWT-Food Sci. Technol. 2021, 143, 8. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Li, J.L.; Liu, Y.; Yin, L.Y. Effect of new W/O halogen coagulant on moisture change in soybean protein gel. J. Chin. Cereals Oils Assoc. 2014, 29, 100–105. [Google Scholar]
- Li, J.L. Preparation of W/O and W/O/W Emulsion Coagulant and Their Improvement to the Quality of Traditional Bittern-Solidified Tofu. Ph.D. Thesis, China Agricultural University, Beijing, China, 2014. [Google Scholar]
- Zhu, Q.M.; Li, J.L.; Liu, H.J.; Saito, M.; Tatsumi, E.; Yin, L.J. Development of stable water-in-oil emulsions using polyglycerol polyricinoleate and whey protein isolate and the impact on the quality of bittern-tofu. J. Dispers. Sci. Technol. 2015, 36, 1548–1555. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Zhao, L.; Zhang, H.; Saito, M.; Yin, L.J. Impact of the release rate of magnesium ions in multiple emulsions (water-in-oil-in-water) containing BSA on the resulting physical properties and microstructure of soy protein gel. Food Chem. 2017, 220, 452–459. [Google Scholar] [CrossRef]
- Zheng, L.H. Studies on new compound coagulant of beau curd. China Food Addit. 2000, 4, 23–26. [Google Scholar]
- Zhang, H. Study on the quality of bean burd affected by composition of the compound coagulant. Food Ferment. Ind. 2002, 11, 21–24. [Google Scholar]
- Wang, R.R.; Wang, J.D.; Liu, E.Q. Studies on compound coagulant of tofu. Chin. Condiment 2006, 6, 25–27. [Google Scholar]
- Shi, Y.G.; Liu, H.B.; Li, G.; Lin, Y.H.; Hu, C.L. Study on the compound coagulant used for Tofu. Sci. Technol. Food Ind. 2007, 6, 171–173. [Google Scholar]
- Wang, Y.D.; Xu, X.X.; Ye, S.P. Study on the quality of tofu affected by the compound coagulant. Cereals Oils Process. 2010, 11, 133–135. [Google Scholar]
- Li, Y.E.; Wang, Y.; Chen, Z.J. Effect of composite coagulant on quality of tofu gel. Food Sci. Tech. 2018, 43, 277–284. [Google Scholar]
- Shi, N.; Xu, H.W.; Chen, H.Q.; Zhang, Y.Y.; Guo, K.Y.; Liu, S.; Dong, B.; Ma, Y.L.; Tan, J.X. Optimization of color soft tofu preparation with combined coagulants using response surface methodology. J. Food Sci. Technol. 2019, 37, 93–99. [Google Scholar]
- Yue, W.T.; Lu, L.X.; Yang, W.Y.; Zhang, Q. Analysis of different compound coagulants on the nutritional quality of whole soybean tofu. Soybean Sci. 2020, 39, 138–145. [Google Scholar]
- Yu, X.L.; Meng, X.; Jin, P.; Ding, N.; Zhang, D.Y. Study on new natural curd coagulant. Food Sci. Tech. 2020, 45, 259–263. [Google Scholar]
- Gao, Y.; Nie, P.; Yang, X.F.; Ma, Z.G.; Du, S.Z.; Huang, Z.P.; Jiang, S.T.; Zheng, Z. Conjugation of soymilk protein and arabinoxylan induced by peroxidase to improve the gel properties of tofu. Food Chem. 2024, 430, 8. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P. Preparation of tofu using chitosan as a coagulant for improved shelf-life. Int. J. Food Sci. Technol. 2010, 39, 133–141. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zheng, W.; Piao, C.H.; Liu, J.M.; Wang, Y.H.; Li, Y.B.; Yu, H.S. Study on the process of making tofu with chitosan mixed coagulant. Farm Mach. 2012, 18, 97–100. [Google Scholar]
- Zhao, X.R.; Wang, S.H.; Deng, C.K. Applied research of chitosan in processing of pressure lactone bean curd. Sci. Technol. Food Ind. 2012, 4, 177–180+186. [Google Scholar]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Kim, G.W.; Sim, J.M.; Nam, S.Y.; Kim, B.M. Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd). Food Sci. Nutr. 2019, 7, 547–553. [Google Scholar] [CrossRef]
- Li, M.; Chen, F.S.; Yang, H.S.; Wang, M.L.; Lai, S.J. Effect of magnesium chloride guar gum mixture on the coagulation process of tofu. Grain Fats 2014, 27, 30–33. [Google Scholar]
- Li, M.; Chen, F.S.; Yang, B.; Lai, S.J.; Yang, H.S.; Liu, K.L.; Bu, G.H.; Fu, C.L.; Deng, Y. Preparation of organic tofu using organic compatible magnesium chloride incorporated with polysaccharide coagulants. Food Chem. 2015, 167, 168–174. [Google Scholar] [CrossRef]
- Cao, F.H. Study on Formation and Gel Mechanism of Bean Curd Induced by Organic Acids. Master’s Thesis, Hefei University of Technology, Hefei, China, 2018. [Google Scholar]
- Zhao, H.B.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate-induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Lan, Q.Y.; Dong, H.M.; Wu, D.T.; Chen, H.; Lin, D.R.; Qin, W. Glycinin-carbohydrate conjugates: Preparation, characterization, and application in processing of whole soybean curd. Food Hydrocoll. 2021, 111, 11. [Google Scholar] [CrossRef]
- Arii, Y.; Takenaka, Y. Initiation of protein association in tofu formation by metal ions. Biosci. Biotechnol. Biochem. 2014, 78, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Murekatete, N.; Zhang, C.M.; Eric, K.; Hua, Y.F. Salt and acid-induced soft tofu-type gels: Rheology, structure and fractal analysis of viscoelastic properties as a function of coagulant concentration. Int. J. Food Eng. 2014, 10, 595–611. [Google Scholar]
- Leiva, J.; Rodriguez, V.; Munoz, E. Influence of calcium chloride concentration on the physicochemical and sensory characteristics of tofu. Cienc. Investig. Agrar. 2011, 38, 435–440. [Google Scholar] [CrossRef]
- Lu, Y.H.; Cui, Z.B.; Guan, X.F.; Lin, J.Y.; Zhong, X.Q.; Zhang, M.L. Effect of magnesium chloride concentration on soymilk coagulation mechanism. LWT-Food Sci. Technol. 2021, 150, 8. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cao, F.H.; Li, X.J.; Mu, D.D.; Zhong, X.Y.; Jiang, S.T.; Zheng, Z.; Luo, S.Z. Effects of different salts on the gelation behaviour and mechanical properties of citric acid-induced tofu. Int. J. Food Sci. Technol. 2020, 55, 785–794. [Google Scholar] [CrossRef]
- Khoder, R.M.; Yin, T.; Liu, R.; Xiong, S.; Huang, Q. Effects of nano fifish bone on gelling properties of tofu gel coagulated by citric acid. Food Chem. 2020, 332. [Google Scholar] [CrossRef]
- Wu, X.; Wan, Y.Q.; Xu, Y.Y.; Wang, Z.C.; Cheng, J.H. Research progress on reduction and cleaning treatment technology of soybean products processing wastewater. J. Anhui Agric. Sci. 2019, 47, 13–16. [Google Scholar]
- Qiao, Z.H. Research on the Shelf Life and Antibacterial Mechanism of Lactic Acid Bacteria. Ph.D. Thesis, China Agricultural University, Beijing, China, 2008. [Google Scholar]
- Huang, Z.R.; He, W.Y.; Zhao, L.Z.; Liu, H.Y.; Zhou, X.J. Processing technology optimization for tofu curded by fermented yellow whey using response surface methodology. Food Sci. Nutr. 2021, 9, 3701–3711. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, C.H.; Wang, B.; Shi, Y.G.; Zhang, X.M.; Liu, Y.; Zhang, N. Analysis of flavor compounds in the processing of fermented soybean whey tofu based on gas chromatography-mass spectrometry. J. Food Saf. Qual. 2020, 11, 5982–5991. [Google Scholar]
- Chen, B.; Yang, Y.X.; Li, Z.C.; Shen, L.Q.; Li, J.J.; Gu, Z.Y. Fermentation optimization of yellow serofluid produced from manufactory of compressed tofu and its application as coagulate for tofu production. J. Chin. Cereals Oils Assoc. 2021, 36, 157–163. [Google Scholar]
- Murata, K.; Kusakabe, I.; Yoshida, S.; Murakami, K. Application of soymilk-whey to production of beverage containing α-d-galactosyl oligosaccharides. Agric. Biol. Chem. 1989, 53, 1997–1998. [Google Scholar]
- Luan, G.Z.; Cheng, Y.Q.; Lu, Z.H.; Li, L.T. Development of soymilk clotting enzyme researching. Acad. Period. Farm. Prod. Process. 2006, 10, 41–43. [Google Scholar]
- Murata, K.; Kusakabe, I.; Kobayashi, H.; Akaike, M.; Park, Y.W.; Murakami, K. Studies on the coagulation of soymilk-protein by commercial proteinases. Agric. Biol. Chem. 2014, 51, 385–389. [Google Scholar]
- Yin, L.Q.; Zhang, Y.Z.; Wu, H.; Wang, Z.; Dai, Y.Q.; Zhou, J.Z.; Liu, X.L.; Dong, M.S.; Xia, X.D. Improvement of the phenolic content, antioxidant activity, and nutritional quality of tofu fermented with Actinomucor elegans. LWT-Food Sci. Technol. 2020, 133. [Google Scholar] [CrossRef]
- Clarke, D.D.; Mycek, M.J.; Neidle, A. The incorporation of amine into proteins. Arch. Biochem. Biophys. 1959, 79, 338–354. [Google Scholar] [CrossRef]
- Xiong, X.H.; Wang, X.L.; Shu, C.F.; Lu, L.X. Effects of transglutaminase treatment on the quality of the quality of glucono-δ-lactone tofu. Food Res. Dev. 2007, 5, 53–56. [Google Scholar]
- Wang, C.Z.; Li, J.Y.; Zhou, S.Y.; Zhou, J.Q.; Lan, Q.Y.; Qin, W.; Wu, D.T.; Liu, J.; Yang, W.Y.; Zhang, Q. Application of transglutaminase for quality improvement of whole soybean curd. J. Food Sci. Technol.-Mysore 2019, 56, 233–244. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.F.; Mu, D.D.; Zhao, Y.Y.; Luo, S.Z.; Zhong, X.Y.; Jiang, S.T.; Li, X.J.; Zheng, Z. Effect of partial hydrolysis with papain on the characteristics of transglutaminase-crosslinked tofu gel. J. Food Sic. 2018, 83, 3092–3098. [Google Scholar] [CrossRef]
- Yang, H.P.; Hua, Y.F.; Chen, Y.M.; Zhang, C.M.; Kong, X.Z. Effect of transglutaminase on rupture strength of GDL Tofu and its mechanism. Food Ferment. Ind. 2018, 44, 8–12+21. [Google Scholar]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Wang, W.S.; Nie, H.L.; Zeng, R.P.; Yu, H.Z.; Yu, B.; Wu, J.J. Effect of crosslinking with transglutaminase on the quality of tofu. Food Ind. 2019, 40, 120–124. [Google Scholar]
- Cheng, Q.; Ye, J.S.; He, W.M.; Wan, M.H.; Li, C.Y.; Huang, D.; Li, B.J.; He, P.H.; Xie, H.L. Effect of transglutaminase on gel hardness of spray dried egg white powder. Genom. Appl. Biol. 2021, 40, 1878–1887. [Google Scholar]
- Li, J.L.; Cheng, Y.Q.; Jiao, X.; Zhu, Q.M.; Yin, L.J. Effect of W/O and W/O/W controlled-release emulsion coagulants on characteristic of bittern-solidified tofu. Trans. Chin. Soc. Agric. Mach. 2013, 44, 162–168. [Google Scholar]
- Li, J.; Qiao, Z.; Tatsumi, E.; Saito, M.; Cheng, Y.; Yin, L. A novel approach to improving thequality of bittern-solidified tofu by W/O controlled-release coagulant. 1: Preparation of W/O bittern coagulant and its controlled-release property. Food Bioprocess Tech. 2013, 6, 1790–1800. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Tatsumi, E.; Saito, M.; Cheng, Y.; Yin, L. A novel approach to improving thequality of bittern-solidified tofu by W/O controlled-release coagulant. 2: Using the improved coagulant in tofu processing and product evaluation. Food Bioprocess Tech. 2013, 6, 1801–1808. [Google Scholar] [CrossRef]
- Li, J.L.; Cheng, Y.Q.; Tatsumi, E.; Saito, M.; Yin, L.J. The use of W/O/W controlled-release coagulants to improve the quality of bittern-solidified tofu. Food Hydrocoll. 2014, 35, 627–635. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Wu, F.F.; Saito, M.; Tatsumi, E.; Yin, L.J. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204. [Google Scholar] [CrossRef]
- Yu, X.; Huang, X.D. Traditional Soybean Products Processing Technology, 1st ed.; Chemical Industry Press: Beijing, China, 2011; pp. 11–90. ISBN 978-7-122-10594-3. [Google Scholar]
- Luo, C.X. Soy and Soy Products; Light Industry Press: Beijing, China, 1997. [Google Scholar]
- Yang, M.; Yang, Q.C. Studies to improve the quality of lactone tofu. Food Sci. 1997, 2, 73–74. [Google Scholar]
- Hao, Y.D. Soybean Products Production Technology and Deep Processing Technology; Agriculture Press: Beijing, China, 1993. [Google Scholar]
- Xie, C.P.; Zhao, L.Z.; Zhou, X.J.; Li, M.; Mo, X.; Yu, K.; Che, L.N.; Liu, T. Optimization of whole soybean curd compound coagulant using response surface methodology. J. Food Saf. Qual. 2021, 12, 4034–4041. [Google Scholar]
- Lu, W.J.; Zhang, Y.; Zhang, C.; Chen, D.; Xiao, C.G. Influence of calcium sulfate incorporated with gluconolactone coagulant on the quality of whole soybean flour tofu. Food Chem.-X 2023, 17, 100527. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.Z.; Li, K.X.; Qin, W.; Wu, D.T.; Hu, B.; Yang, W.Y.; Dong, H.M.; Zhang, Q. Influence of soybean protein isolate-dextran conjugates on the characteristics of glucono-δ-lactone-induced tofu. LWT-Food Sci. Technol. 2021, 139, 110588. [Google Scholar] [CrossRef]
- Wang, D.F. Food Chemistry, 1st ed.; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- Schaefer, M.J.; Love, J. Relationships between soybean components and tofu texture. J. Food Qual. 2010, 15, 53–66. [Google Scholar] [CrossRef]
- Tsumura, K.; Saito, T.; Kugimiya, W. Influence of Phytase Treatment on the Gelation Property of Soymilk. Food Sci. Technol. Res. 2007, 10, 442–446. [Google Scholar] [CrossRef]
- Toda, K.; Takahashi, K.; Ono, T.; Kitamura, K.; Nakamura, Y. Variation in the phytic acid content of soybeans and its effect on consistency of tofu made from soybean varieties with high protein content. J. Sci. Food Agric. 2006, 86, 212–219. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ono, T.; Wada, T.; Tsukamoto, C.; Kon, Y. Changes in Soybean Phytate Content as a Result of Field Growing Conditions and Influence on Tofu Texture. Biosci. Biotechnol. Biochem. 2006, 70, 874–880. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ono, T.; Nakasato, K. The localization of phytate in tofu curd formation and effects of phytate on tofu texture. J. Food Sci. 2008, 73, C67–C71. [Google Scholar] [CrossRef]
- Huang, M.W. Influence of Soybean Varieties and Their Components on the Quality of Gypsum Tofu and the Establishment of the Classification Standard of Raw Material Soybean. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2015. [Google Scholar]
- Zhang, Y.J. The Suitability Evaluation of Different Soybean Materials in Tofu Making. Master’s Thesis, Northwest A&F University, Xianyang, China, 2016. [Google Scholar]
- Ibrahim, S.G.; Noh, N.A.M.; Ibadullah, W.Z.W.; Saari, N.; Karim, R. Water soaking temperature of kenaf (Hibiscus cannabinus L.) seed, coagulant types, and their concentrations affected the production of kenaf-based tofu. J. Food Process. Preserv. 2020, 44, e14549. [Google Scholar] [CrossRef]
- Wang, R.C.; Guo, S.T. Effects of endogenous small molecular compounds on the rheological properties, texture and microstructure of soymilk coagulum: Removal of phytate using ultrafiltration. Food Chem. 2016, 211, 521–529. [Google Scholar] [CrossRef]
- Wang, R.C. Interactions Among Phytate, Calcium/Magnesium and Proteins in Soymilk and Their Effects on Protein Aggregation. Ph.D. Thesis, China Agriculture University, Beijing, China, 2018. [Google Scholar]
- Wang, R.C.; Liu, J.Y.; GUO, S.T. Binding of phytate to soybean protein during the heat treatment of soymilk and its effect on protein aggregation. Food Hydrocoll. 2018, 84, 368–378. [Google Scholar] [CrossRef]
- Li, X.S.; Toyoda, K.; Hara, I. Coagulation process of soymilk characterized by electrical impedance spectroscopy. J. Food Eng. 2011, 105, 563–568. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, M.J. Application of Food Additive Calcium Sulfate in Tofu Production. Food Res. Dev. 2014, 35, 166–168. [Google Scholar]
- Liu, X.R.; Xu, J.T.; Li, Y.; Zhao, H.Y.; Guo, S.T. Mechanism of the glucono-δ-lactone induced soymilk gelation: Enthalpy and entropy transformation in the cross-linking of protein molecules. Food Res. Int. 2023, 169, 10. [Google Scholar] [CrossRef]
- Zhong, F. Convenient (Namely Flush Namely Coagulation) Preparation of Bean Curd Flower and Rapid Coagulation Mechanism of Soybean Protein. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2001. [Google Scholar]
- Zhong, F.; Wang, Z.; Xu, S.Y. Instant coagulation of soy protein by proteinases. J. Wuxi Univ. Light Ind. 2002, 21, 47–50. [Google Scholar]
- Zhong, F.; Wang, Z.; Xu, S.Y. Instant-gelation Property of Soy Protein–II Influence of Coagulant. J. Chin. Cereals Oils Assoc. 2002, 17, 50–53. [Google Scholar]
- Zhong, F.; Wang, Z.; Xu, S.Y. Gelation ability of soy 7S and 11S proteins as affected by different coagulants. J. Wuxi Univ. Light. Ind. 2003, 22, 12–17. [Google Scholar]
- Ono, T.; Wada, T.; Imai, A. The structure of tofu for preventing the change of lipid. Soy Protein Res. Jpn. 2004, 7, 42–47. [Google Scholar]
- Lee, C.H.; Rha, C. Microstructure of soybean aggregates and its relation to the physical and textural properties of the curd. J. Food Sci. 1978, 43, 79–84. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, M.M.; Lin, W.F.; Yang, X.Q. Effect of physical force on texture properties of gels of soy protein isolates. Food Sci. 2005, 6, 69–73. [Google Scholar]
- Duan, W.D.; Weng, D.; Pan, S.Y.; Yang, F. Effects of intermolecular forces on gelation of soy protein. Food Sci. 2009, 30, 60–63. [Google Scholar]
- Yang, F.; Pan, S.Y.; Zhang, C.L. Structural change of protein during tofu gelation process. Food Sci. 2009, 30, 120–124. [Google Scholar]
- Zhou, S.H.; Chen, Y.; Zhang, M.; Liu, J.; Wang, J.Y.; Guo, S. Study of molecular forces in formation of brine concentrated tofu gelatin. Food Res. Dev. 2013, 34, 15–19. [Google Scholar]
- Jin, Y.; Liu, L.S.; Zhang, X.F.; Zhang, Q.; Bai, J.; Guo, H.; Peng, Y.J. Effect of coagulation temperature on gelling properties and chemical forces of tofu coagulated with glucono-δ-lactone. Food Sci. 2020, 41, 49–55. [Google Scholar]
- Liu, L.S.; Jin, Y.; Zhang, X.F.; Zhang, Q.; Bai, J.; Guo, H.; Peng, Y.J. Comparative study on structure and chemical interaction between brine tofu and GDL tofu. Food Sci. Technol. 2020, 45, 60–64. [Google Scholar]
- Cavallieri, A.L.F.; Cunha, R.L.D. The effects of acidification rate, ph and ageing time on the acidic cold set gelation of whey proteins. Food Hydrocoll. 2008, 22, 439–448. [Google Scholar] [CrossRef]
- Ju, Q.; Wu, C.; Yuan, Y.Q.; Hu, Y.Y.; Zhou, S.Y.; Luan, G.Z. Insights into the mechanism on Glucono-delta-lactone induced gelation of soybean protein at subunit level. Food Hydrocoll. 2022, 125, 107402. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kumazawa, Y.; Motoki, M. Strength of protein gels prepared with microbial transglutaminase as related to reaction conditions. J. Food Sci. 1994, 59, 866–871. [Google Scholar] [CrossRef]
- Yasir, S.B.; Sutton, K.H.; Newberry, M.P.; Andrews, N.R.; Gerrard, J.A. The impact of transglutaminase on soy proteins and tofu texture. Food Chem. 2007, 104, 1491–1501. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; He, X.; Wu, N.; Wang, J.; Gu, W.; Zhang, Y. Limited aggregation behavior of β-Conglycinin and its terminating effect on glycinin aggregation during heating at pH 7.0. J. Agric. Food Chem. 2012, 60, 3782–3791. [Google Scholar] [CrossRef]
- Tang, G.S.; Ono, T.; Mikami, M. Interaction between protein and lipid in soybean milk at elevated temperature. J. Agric. Food Chem. 1997, 45, 4601–4605. [Google Scholar] [CrossRef]
- Nagano, T.; Tokita, M. Viscoelastic properties and microstructures of 11S globulin and soybean protein isolate gels: Magnesium chloride–induced gels. Food Hydrocoll. 2011, 25, 1647–1654. [Google Scholar] [CrossRef]
- Peng, X.; Ren, C.; Guo, S. Particle formation and gelation of soymilk: Effect of heat. Trends Food Sci. Techol. 2016, 54, 138–147. [Google Scholar] [CrossRef]
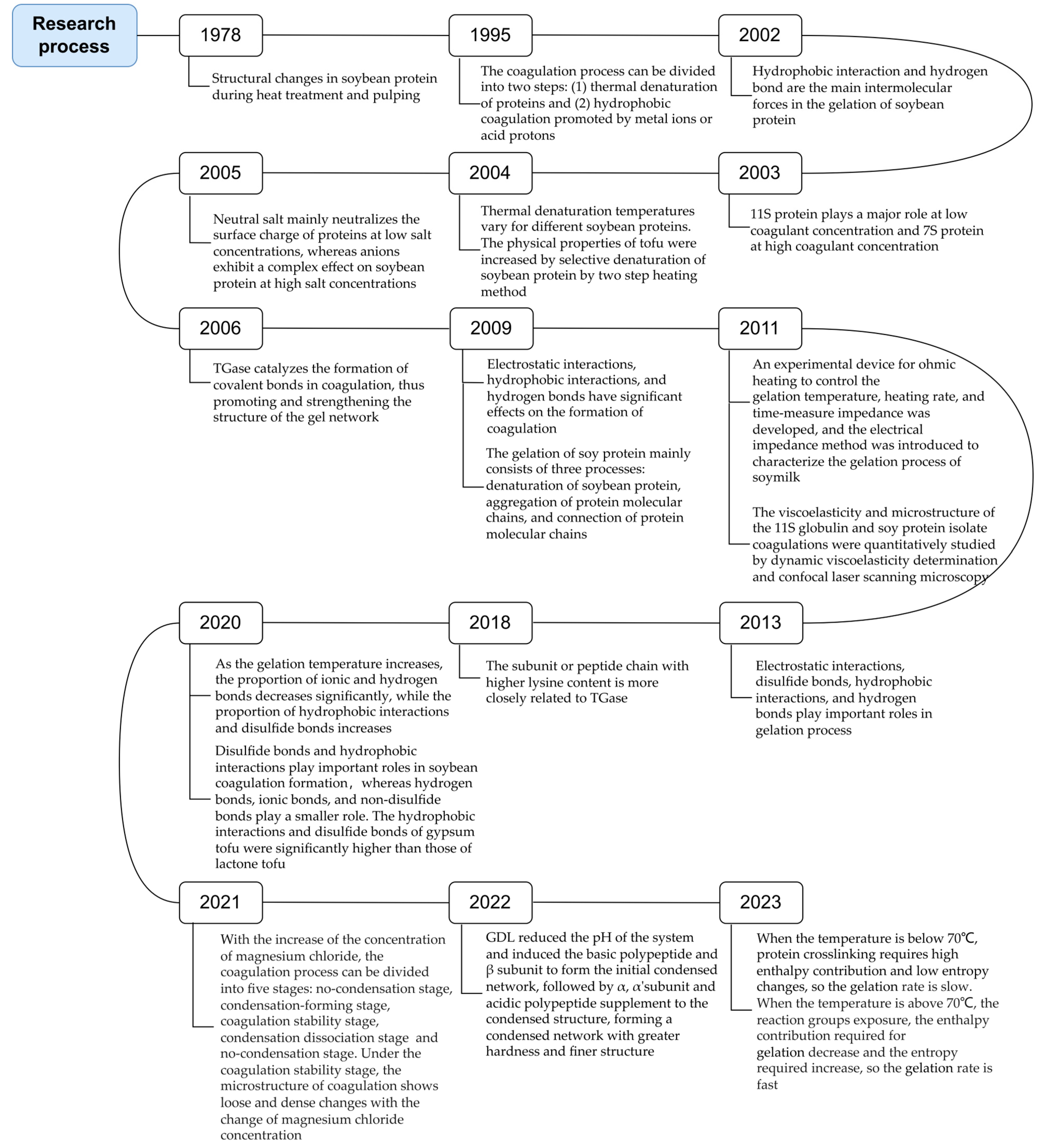
| Type | Type | Year | Research Results |
|---|---|---|---|
| Salt | Calcium salts | 1980 | Calcium salts and two non-calcium compounds induce coagulation production of soybean protein [5] |
| 2000 | Effects of calcium salt coagulants on gel strength and water retention of tofu [6] | ||
| 2004 | Thermal denaturation temperature of soybean protein [7] | ||
| 2006 | Effects of different coagulants on the retention of isoflavones in tofu [8] | ||
| 2022 | Effects of dual-frequency and multi-angle ultrasound on the network structure and texture properties of calcium sulfate tofu [9] | ||
| 2023 | Influence of chicken eggshell powder as an alternative coagulant on the yield and textural characteristics of tofu [10] | ||
| Magnesium salts | 2000 | Effects of magnesium chloride on tofu gel strength and water retention [6] | |
| 2012 | Proteomic analysis of tofu formation induced by magnesium chloride [11] | ||
| 2013 | Effects of magnesium chloride concentration on tofu properties [12] | ||
| 2017 | Effects of mixing speed, mixing time, and magnesium chloride adding batch in the pulping stage on the tofu yield [13] | ||
| 2023 | Effects of alkaline heat treatment on texture properties of magnesium chloride tofu [14] | ||
| 2024 | Mathematical modeling of optimal coagulant dosage for tofu preparation using MgCl2 [15] | ||
| Acid | GDL | 1995 | Dynamic viscoelastic measurements and compression tests infer the gelation of the lactone tofu [16] |
| 2003 | Effects of raw soymilk concentration, heating conditions, and solidification conditions on water retention of lactone tofu [17] | ||
| 2014 | Effects of pretreatment methods of soymilk on texture characteristics of lactone tofu [18] | ||
| 2022 | Effects of soybean soaking conditions on the yield, protein utilization rate, hardness, and water retention of lactone tofu [19] | ||
| 2024 | Effects of soybean milk heating conditions on the formation of coagulation [20] | ||
| Acid slurry | 2009 | Production of acid slurry bean tofu using pure lactic acid bacteria fermentation [21] | |
| 2014 | Optimum conditions for preparing tofu using sour pulp [22] | ||
| 2019 | Mechanism of acid slurry bean tofu and response of soybean protein to heating and pulping [23] | ||
| 2022 | Effects of the added acid slurry amount on protein subunit aggregation [24] | ||
| Organic acid | 2012 | Coagulating properties of various organic acids [25] | |
| 2012 | Tofu preparation using natural hawthorn extract as coagulant [26] | ||
| 2014 | Tofu preparation using roselle water extract as coagulant [27] | ||
| 2017 | Tofu preparation using citric acid, malic acid, and tartaric acid [28] | ||
| 2021 | Tofu preparation using grape pomace as coagulant [29] | ||
| 2023 | Effect of flour addition on the physicochemical and metabolome in acid slurry [30] | ||
| Enzyme | Coagulase | 1985 | Role of bromelain in aggregation and gelation of hot soymilk [31] |
| 1987 | Neutral or alkaline coagulase from microorganisms had a better effect on the coagulation of soymilk [32] | ||
| 1989 | Enzyme coagulated soymilk can be fermented to obtain new soybean tofu products [33] | ||
| 1999 | Serine protease from Bacillus pumilus TYO-67 had an effect on the coagulation of soymilk [34] | ||
| 2006 | Pepsin, chymosin, and flavor protease had no soya bean milk-coagulating activity, whereas alcalase, papain, and bromelain had strong coagulating activity [35] | ||
| 2022 | Effects of Lactobacillus casei FNCC-0090 and Lactobacillus plantarum spp. fermentation on the antioxidant capacity of tofu [36] | ||
| TGase | 1980 | Glutamine aminotransferase from guinea pig liver can catalyze the crosslinking polymerization of soybean protein [37] | |
| 1989 | Extraction of Ca2+ non-dependent glutamine aminotransferase from Streptomyces [38] | ||
| 1996 | Addition of microbial TGase to gypsum tofu and lactone tofu [39] | ||
| 2006 | TGase affects tofu hardness and gelation, but not springiness or cohesion [40,41] | ||
| 2010 | Addition of TGase increases the coagulation temperature of soymilk, and improved the firmness and springiness of tofu [42] | ||
| 2016 | TGase forms strong inter/intra molecular bonds between soy proteins, hindering digestive enzymatic hydrolysis [43] | ||
| 2021 | Combining TGase and lactic acid bacteria can improve the digestibility of tofu [44] | ||
| Novel | Emulsion type | 2014 | Changes in moisture content during the oil–water–brine tofu coagulation formation process [45] |
| 2014 | Method of preparation of two emulsion coagulants (W/O, W/O/W) and their effect on tofu coagulation [46] | ||
| 2015 | Addition of whey protein isolate (WPI) to W/O coagulant improves water retention and quality of tofu [47] | ||
| 2017 | Effect of W/O emulsion on tofu texture properties and microstructure [48] | ||
| Reassortment (composition) | 2000 | GDL (main), gypsum, disodium hydrogen phosphate (modifier), monovinegar (emulsifier) [49] | |
| 2002 | GDL, gypsum [50] | ||
| 2006 | GDL, calcium acetate, magnesium chloride [51] | ||
| 2007 | Gypsum, brine [52] | ||
| 2010 | GDL, gypsum, magnesium chloride [53] | ||
| 2018 | GDL, magnesium chloride hexahydrate, sodium chloride, starch [54] | ||
| 2019 | GDL, TGase [55] | ||
| 2020 | Calcium sulfate, magnesium chloride and GDL respectively compounds with TGase [56] | ||
| 2020 | Chitosan, acetic acid [57] | ||
| 2024 | Arabinoxylan, H2O2, peroxidase, and TGase [58] | ||
| Carbohydrate (chitosan) | 2010 | Tofu ash prepared from chitosan has low protein content, good sensory quality, and long shelf life [59] | |
| 2012 | Preparation of tofu using chitosan and acetic acid complex as coagulant [60] | ||
| 2012 | Addition of chitosan to pressurized lactone tofu improves water retention and increases shelf life [61] | ||
| 2019 | Crab shell extract treated with acetic acid as coagulant [62] | ||
| Carbohydrate (other polysaccharides) | 2014 | Magnesium chloride and guar gum were compounded; guar gum modified soymilk solidification rate [63] | |
| 2015 | Three polysaccharides: carrageenan, guar gum, and gum Arabic, complexed with MgCl [64] | ||
| 2018 | Citric acid, salt, and polysaccharide compounding significantly improves tofu texture [65] | ||
| 2020 | Konjac, gellan, and kodeland added to calcium sulfate induces soy sepharose system [66] | ||
| 2021 | Glycinin–dextran conjugate and TGase compounding improve the coagulation texture characteristics and microscopic morphology [67] |
| Composition | Evaluation Index | Optimum Ratio and Conditions |
|---|---|---|
| GDL, gypsum, disodium hydrogen phosphate (modifier), monoglyceride (emulsifier) [49] | Gel strength | GDL 0.3%, gypsum 0.069%, disodium hydrogen phosphate 0.047%, monoglyceride 0.019% (calculated by soymilk) |
| GDL, gypsum [50] | Yield, water content, protein content, sensory characteristics | Ratio of GDL and gypsum is 2:1 |
| GDL, calcium acetate, magnesium chloride [51] | Gel strength, water retention, sensory characteristics | Optimal ratio of GDL, calcium acetate, magnesium chloride is 2:1:1 |
| Gypsum, brine [52] | Strength, chewiness, sensory characteristics | Ratio of gypsum and brine is 4:6 |
| GDL, gypsum, magnesium chloride [53] | Yield, water content, water retention, protein content, sensory characteristics | Ratio of GDL, gypsum, magnesium chloride is 2:1:1 |
| GDL, magnesium chloride hexahydrate, sodium chloride, starch [54] | Gel strength, water-leaving rate, sensory characteristics | Ratio of coagulants (mass ratio of GDL and magnesium chloride hexahydrate) is 0.2:0.15, 0.1 mol/L NaCl 0.6 mL, 0.2% starch 3 mL |
| GDL, TGase [55] | springiness, coherence | Concentration of GDL is 1.7 g/L and TGase is 1.4 g/L, the temperature of pulping is 53 °C |
| Calcium sulfate, magnesium chloride and GDL respectively compounds with TGase [56] | Gel strength, the contents of amino acids, fats and so on | 0.4% GDL + 0.1% TGase, 0.5%CaSO4 + 0.3% TGase (prepared tofu with highest quality), 0.4% MgCl2 + 0.3% TGase |
| Chitosan, acetic acid [57] | Gel strength, dehydration rate, sensory characteristics | Concentrations of chitosan is 1.0% and acetic acid is 1.2%, pulping temperature is 90 °C, set time is 30 min |
| Gypsum, soybean fermentation broth, TGase [100] | Water retention, springiness | Addition amount of soybean fermentation broth is 17%, CaSO4 is 0.2%, and TGase is 0.2‰ |
| Arabinoxylan, H2O2, peroxidase and TGase [58] | Water retention, hardness, storage modulus, loss modulus | 1.0% Arabinoxylan + 100 U peroxidase/g Arabinoxylan + 1 mL 3% H2O2/g Arabinoxylan + 25 U TGase/g protein |
| Coagulant | Research Contents | Results |
|---|---|---|
| GDL | Effect of phytic acid on the quality of GDL tofu | Phytase-treated GDL tofu has higher fracture stress than untreated tofu [105] |
| MgCl2 | Relationship between phytic acid, protein content, and tofu fracture stress | Soybean phytic acid content is more influenced than protein content by environmental factors; tofu fracture stress is negatively associated with phytic acid content; the association between fracture stress and phytic acid content decreases with an increase in magnesium chloride concentration [106] |
| CaSO4/GDL | Phytic acid content and its influence on tofu quality | Tofu with higher phytic acid content has a softer texture [107] |
| CaCl2 | Effect of phytic acid concentration on hardness and viscosity of tofu curd | The condensation hardness, viscosity, and fragility of tofu decreases with an increase in phytic acid concentration [108] |
| CaSO4 | Binding form of phytic acid in soybean milk and its effect on the coagulation rate | Phytic acid slows the protein coagulation reaction, affecting gel strength [112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Du, X.; Jia, R.; Zhu, Y.; Lu, Y.; Guan, X.; Hu, Y.; Zhu, X.; Zhang, M. Research Progress on Tofu Coagulants and Their Coagulation Mechanisms. Foods 2024, 13, 3475. https://doi.org/10.3390/foods13213475
Geng Y, Du X, Jia R, Zhu Y, Lu Y, Guan X, Hu Y, Zhu X, Zhang M. Research Progress on Tofu Coagulants and Their Coagulation Mechanisms. Foods. 2024; 13(21):3475. https://doi.org/10.3390/foods13213475
Chicago/Turabian StyleGeng, Yuhan, Xin Du, Rui Jia, Yi Zhu, Yuhao Lu, Xiangfei Guan, Yuehan Hu, Xinyu Zhu, and Minlian Zhang. 2024. "Research Progress on Tofu Coagulants and Their Coagulation Mechanisms" Foods 13, no. 21: 3475. https://doi.org/10.3390/foods13213475
APA StyleGeng, Y., Du, X., Jia, R., Zhu, Y., Lu, Y., Guan, X., Hu, Y., Zhu, X., & Zhang, M. (2024). Research Progress on Tofu Coagulants and Their Coagulation Mechanisms. Foods, 13(21), 3475. https://doi.org/10.3390/foods13213475








