Effect of Ultra-High-Pressure Treatment on Gastrodia elata Blume: Drying Characteristics, Components, and Neuroprotective Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ultra-High-Pressure (UHP) Treatment
2.3. Drying Experiments
2.3.1. Hot-Air Drying Treatment
2.3.2. Low-Field Nuclear Magnetic Resonance (LF-NMR) Measurements
2.4. Determination of Active Substances Content of GE
2.5. Measurement of Activity of Enzymes
2.6. Neuroprotective Activity In Vivo
2.7. Statistical Analysis
3. Results and Discussion
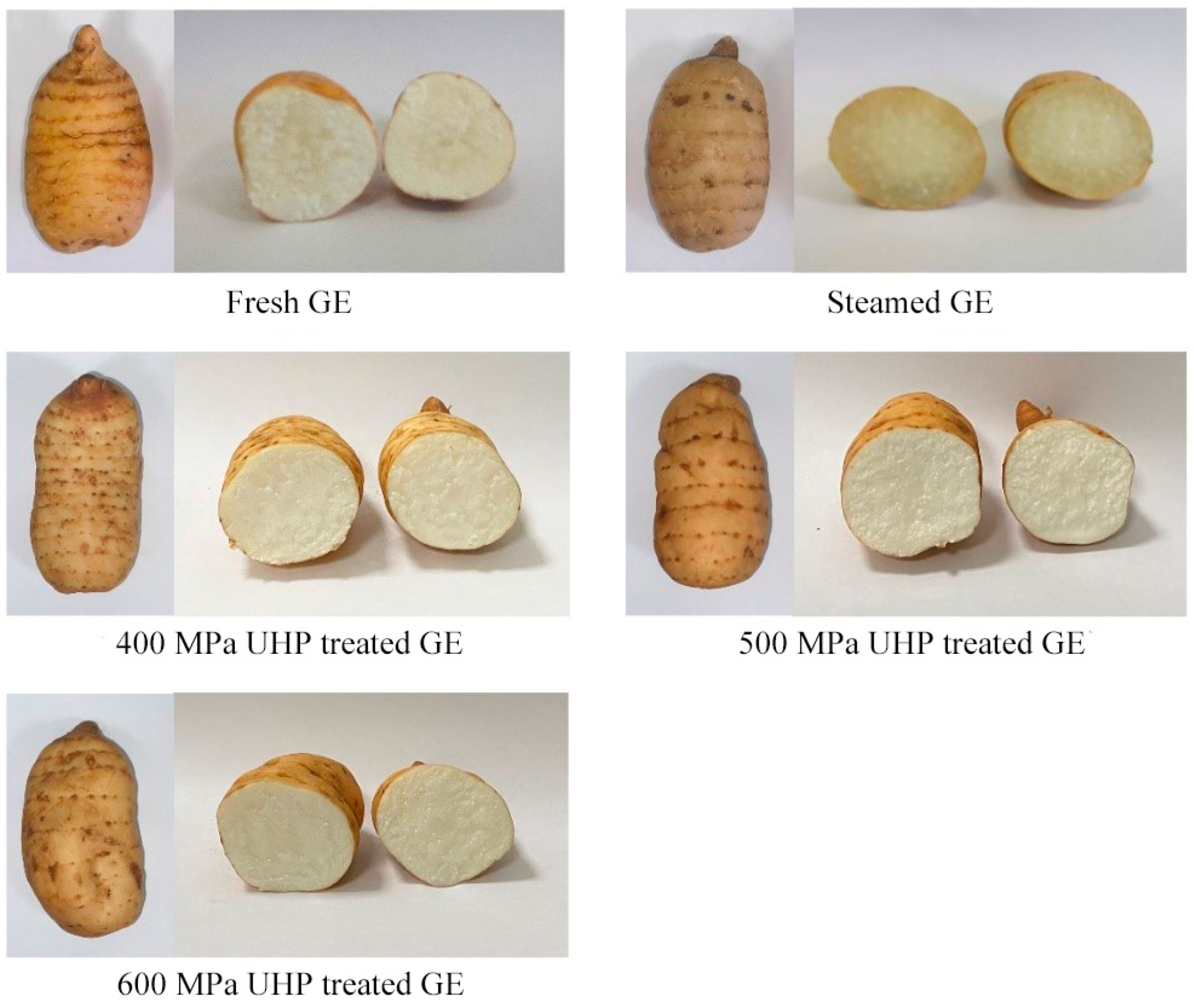
3.1. Color Analysis of UHP-Treated GE
3.2. Water Detection with LF-NMR
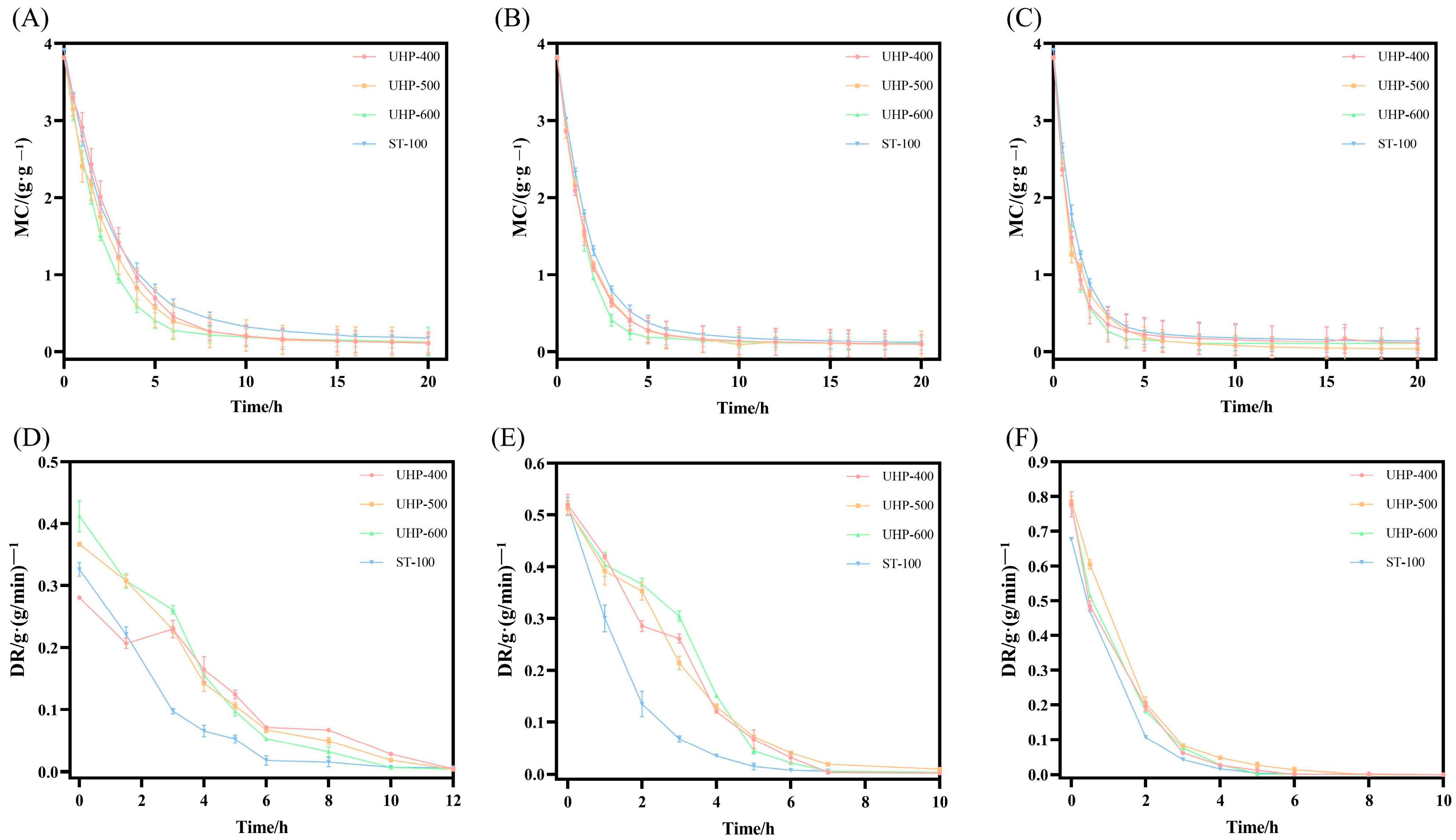
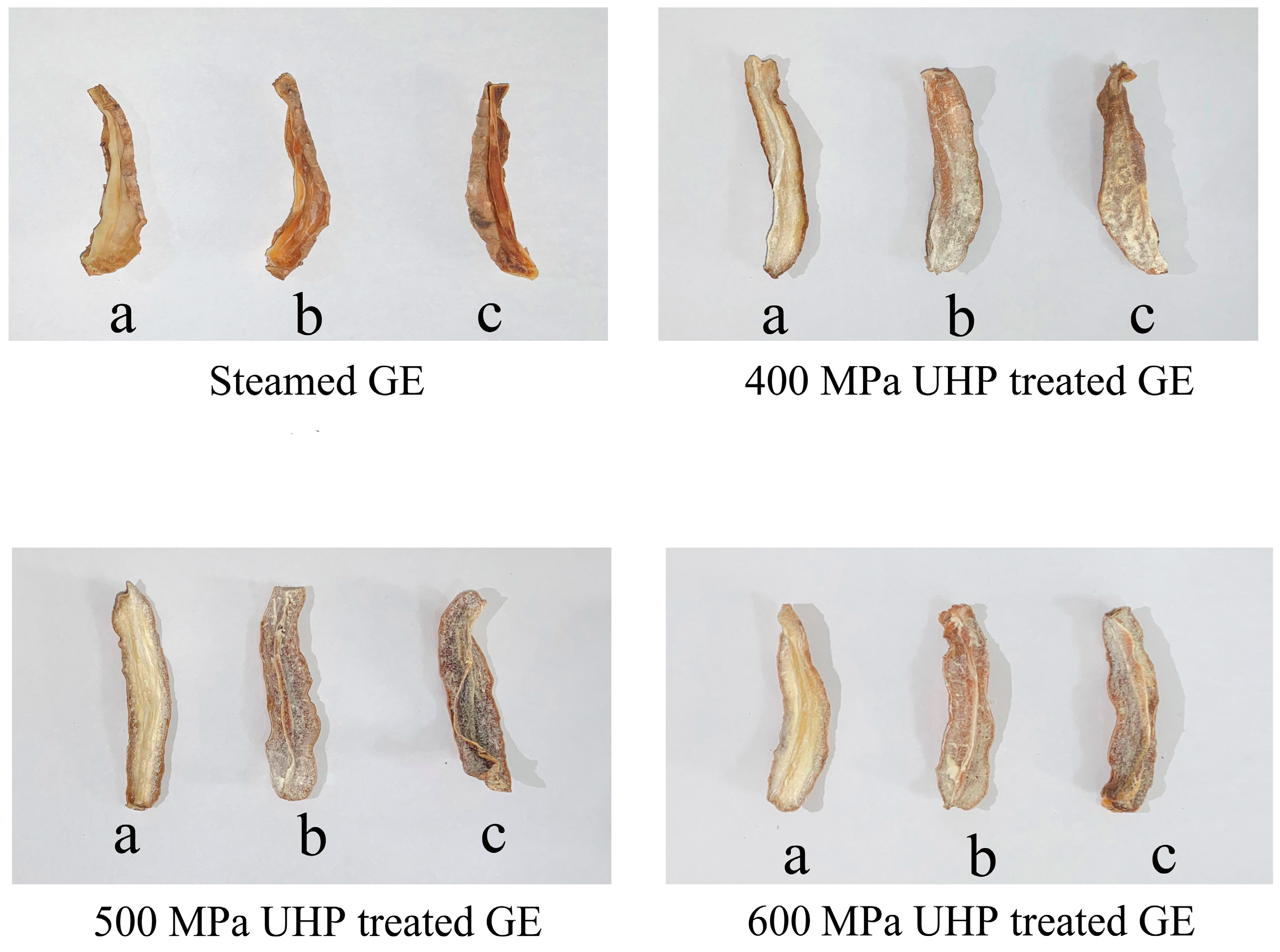
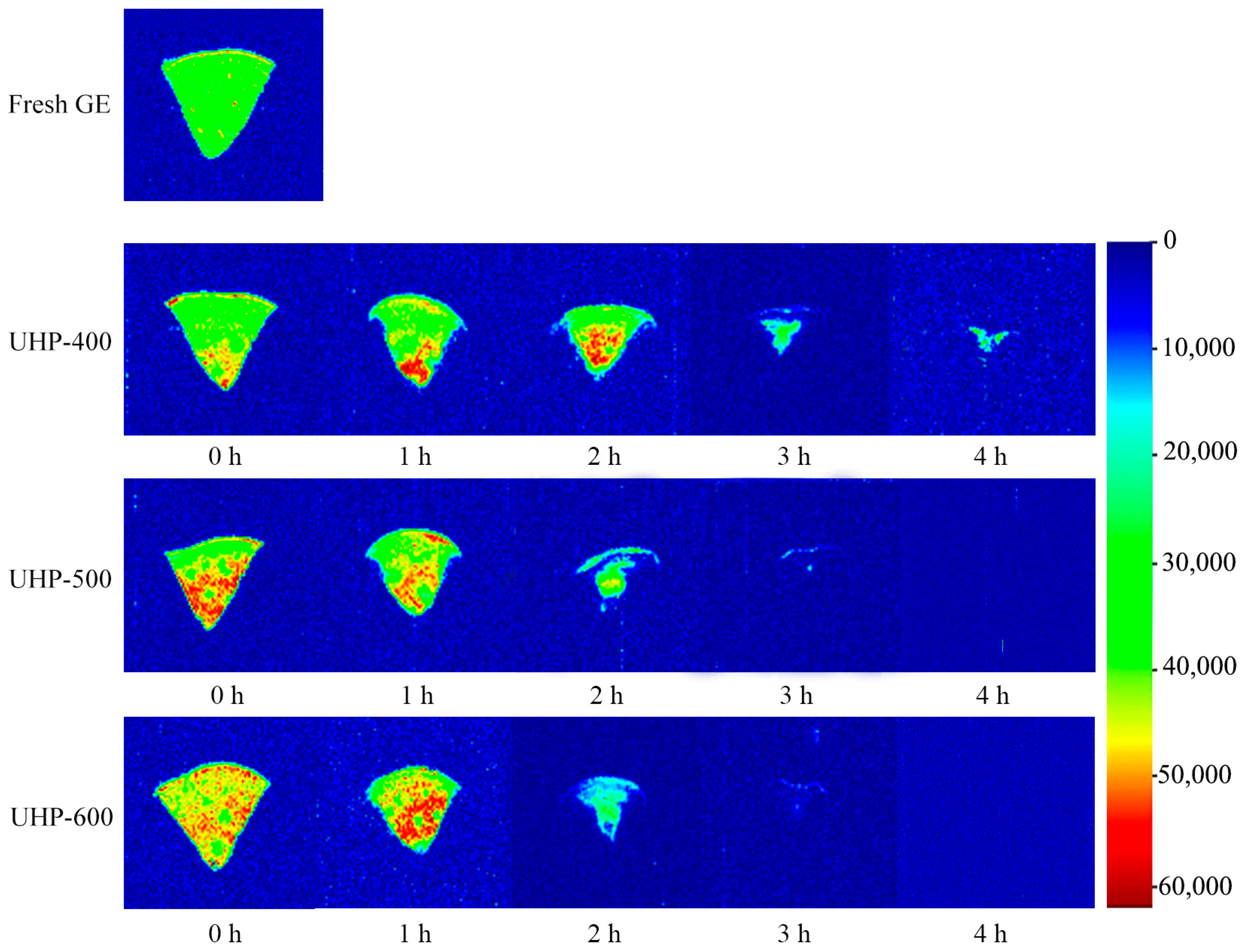
3.2.1. Drying Characteristics Analysis
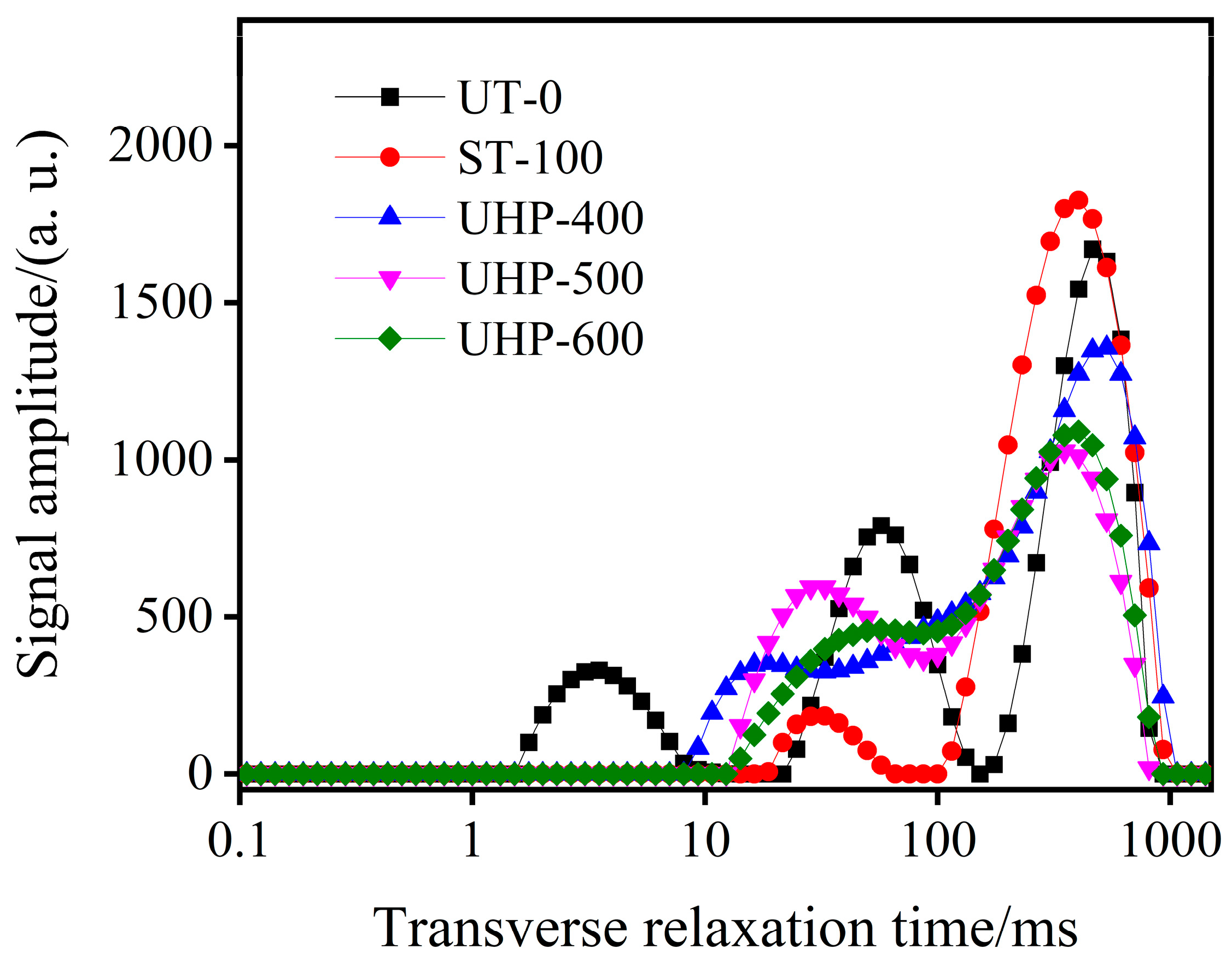
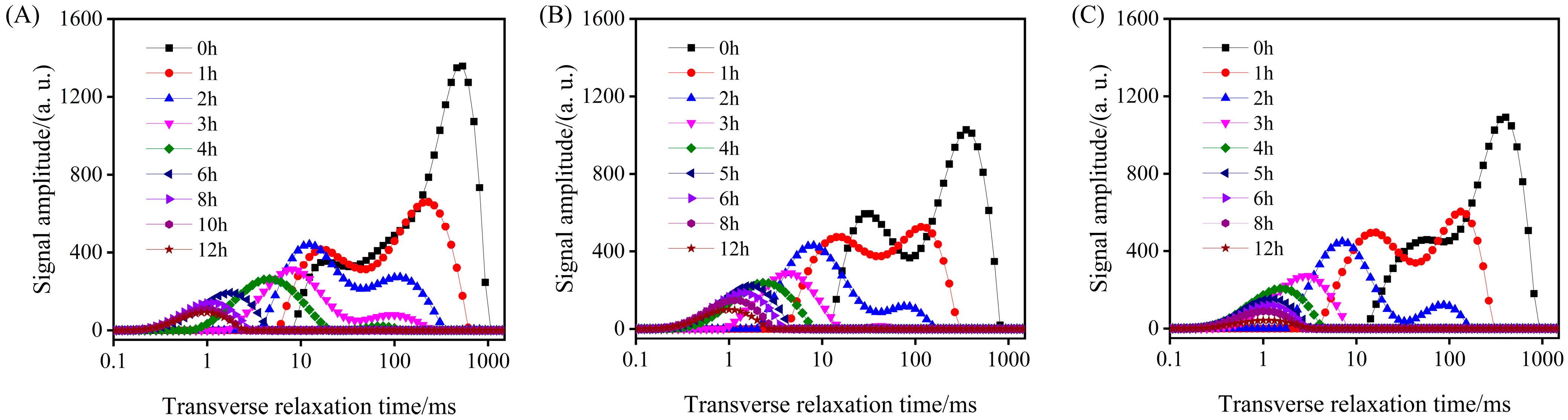
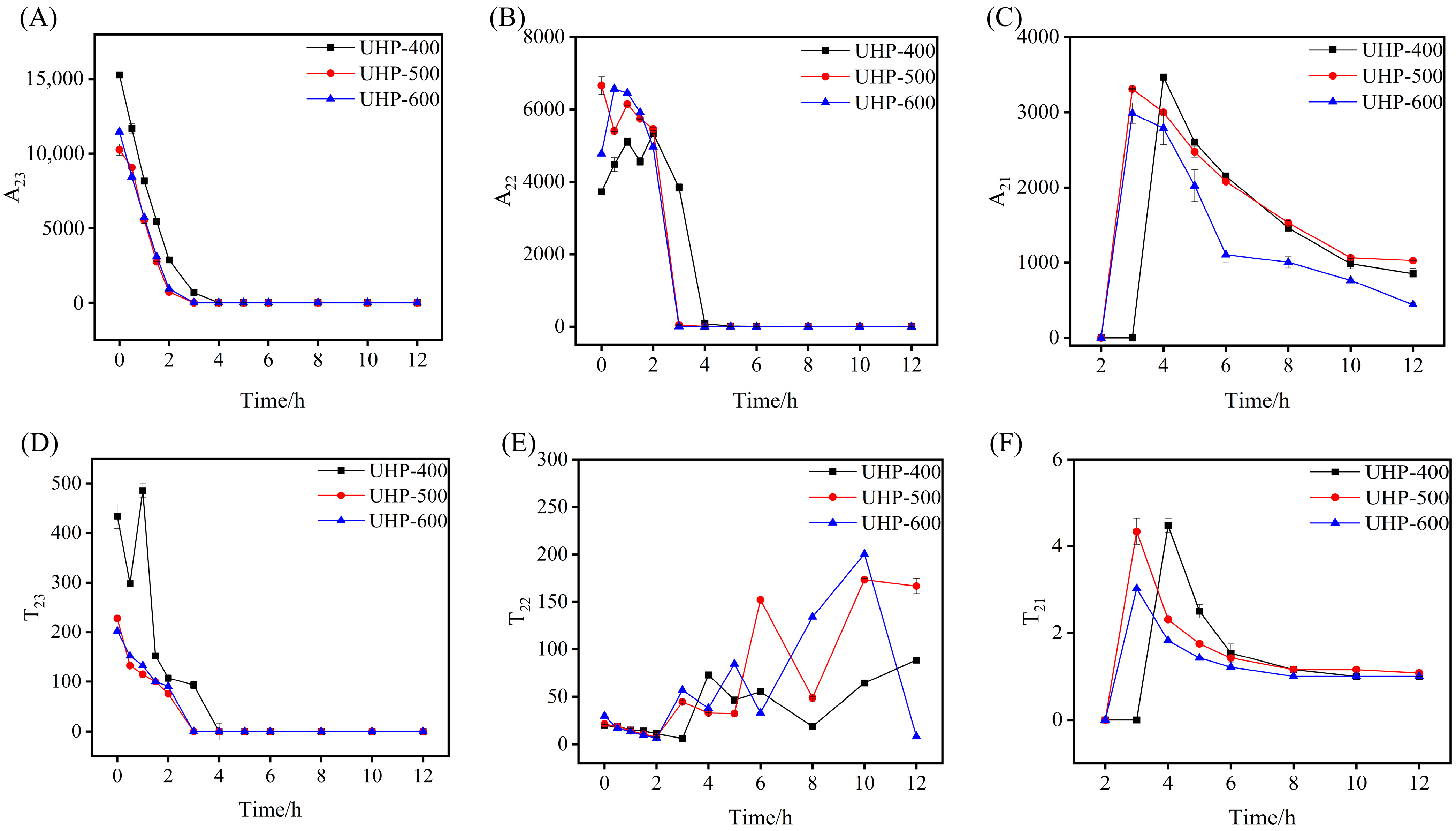
3.2.2. LF-NMR Analysis
3.3. UHP Promotes the Retention of Active Ingredients in GE
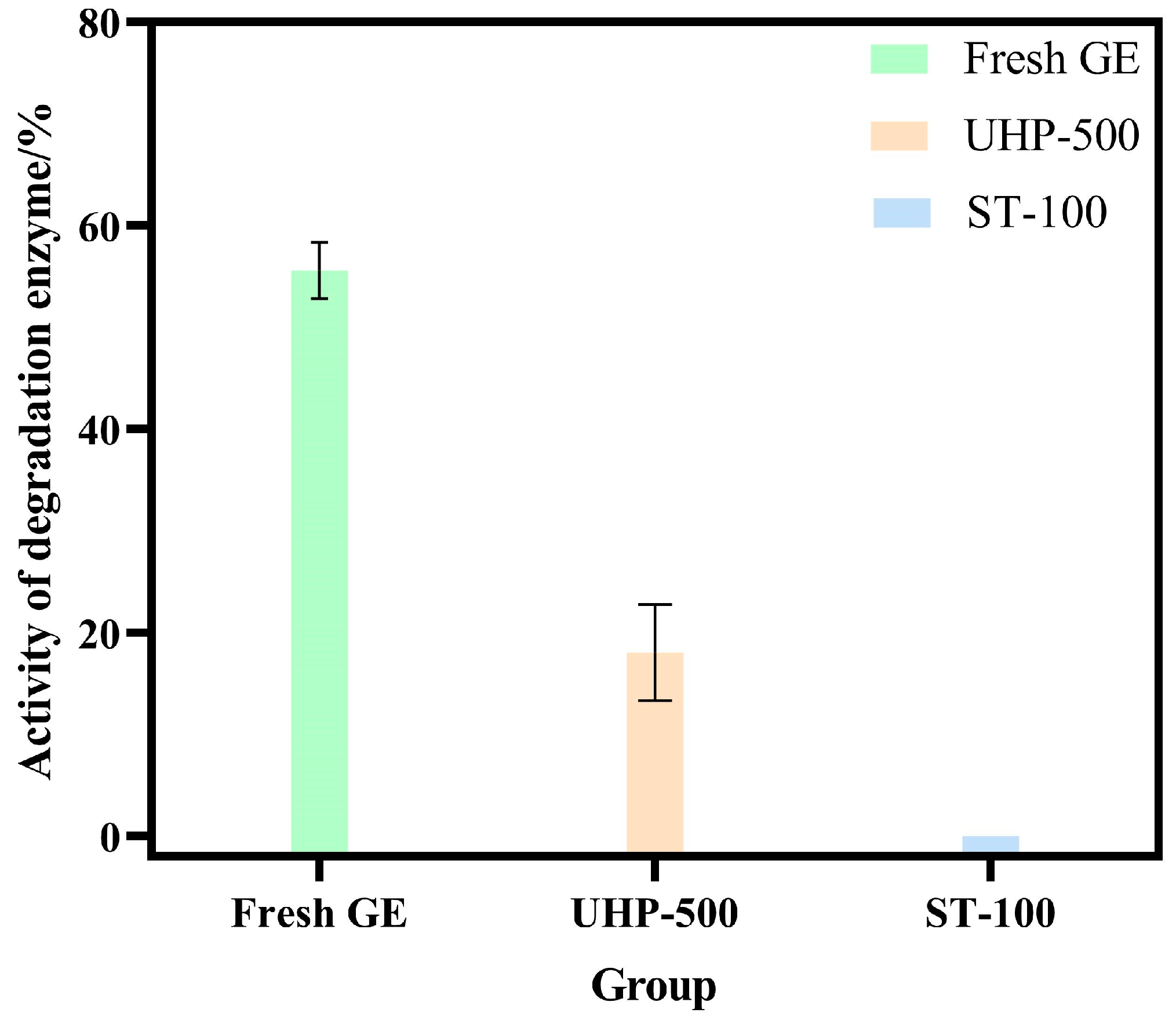
3.4. UHP Reduces the Activity of Degrading Enzymes
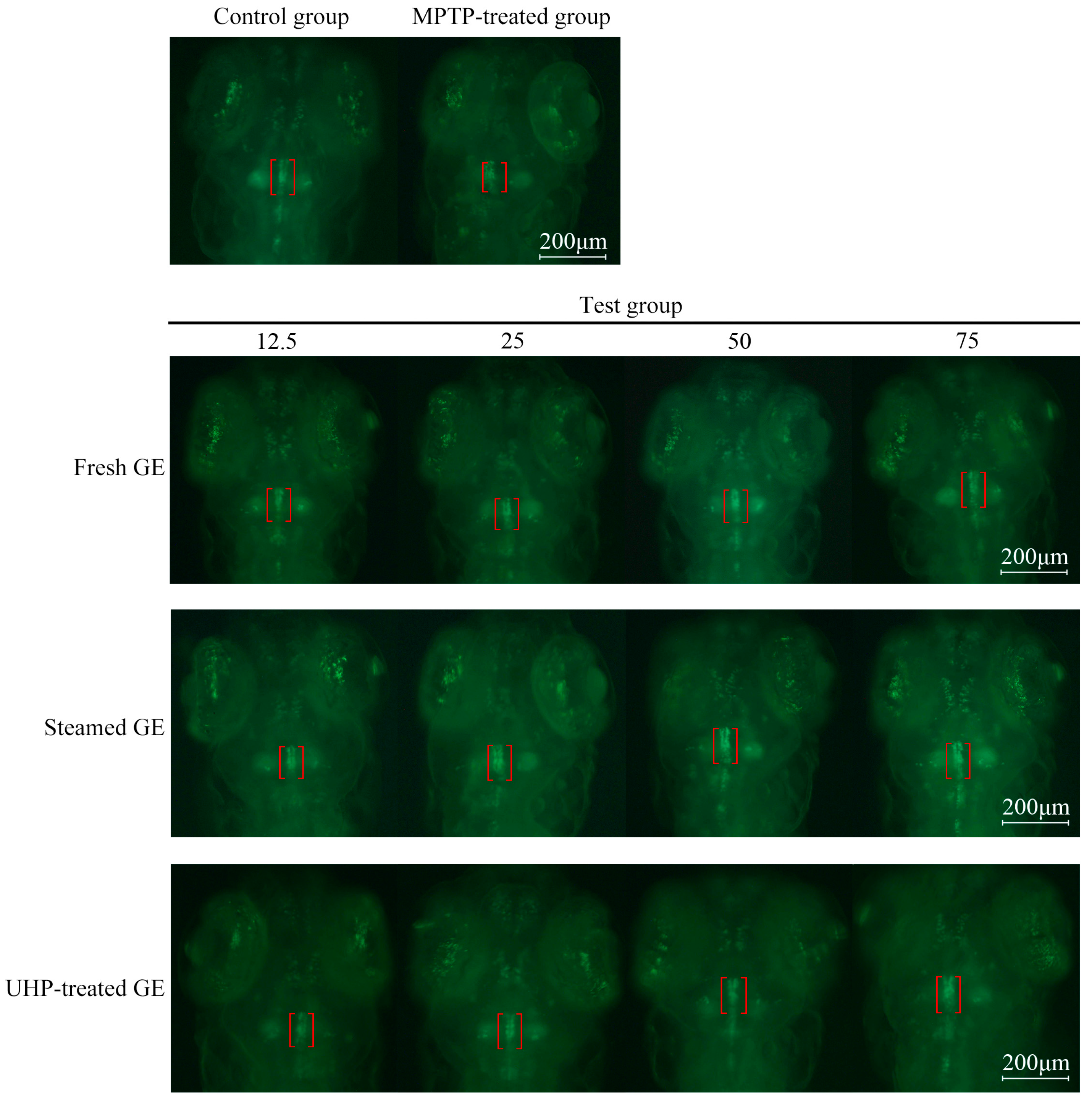
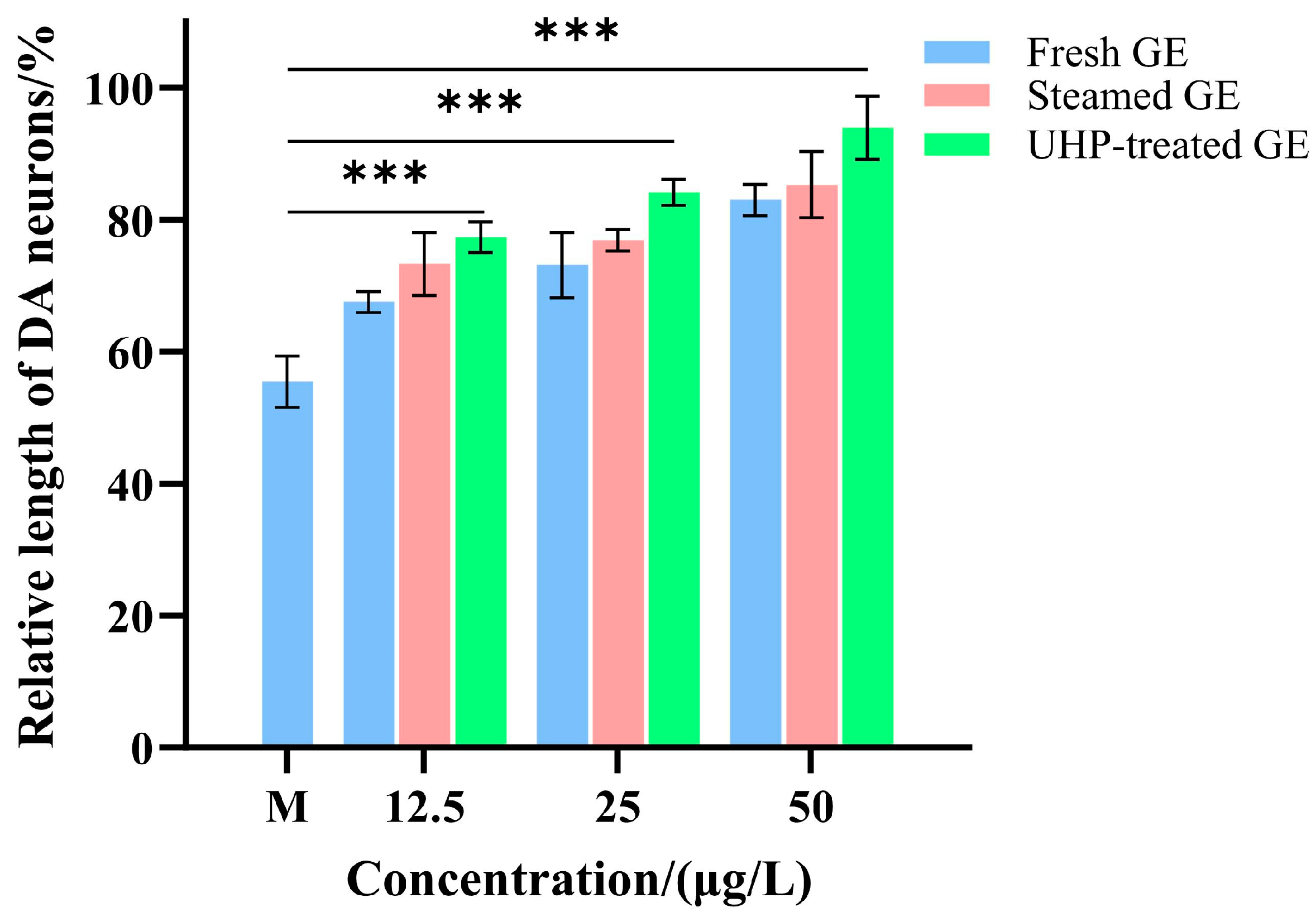
3.5. UHP Enhances the Neuroprotective Activity of GE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.K.; Li, X.Y.; Zhang, Y.; Zheng, Z.A.; Huang, L.Q.; Liu, D.H.; Xiao, H.W.; Liu, Y.H. Effects of high-humidity hot air impingement steaming on Gastrodia elata: Steaming degree, weight loss, texture, drying kinetics, microstructure and active components. Food Bioprod. Process. 2021, 127, 255–265. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Dong, H.J.; Li, J.K.; Guo, L.P.; Wang, X. Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume. Molecules 2019, 24, 236. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, K.; Jenab, E.; Temelli, F. Effects of water on enzyme performance with an emphasis on the reactions in supercritical fluids. Crit. Rev. Biotechnol. 2007, 27, 183–195. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Özbek, B.; Dadali, G. Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. J. Food Eng. 2007, 83, 541–549. [Google Scholar] [CrossRef]
- Ning, Z.W.; Mao, C.Q.; Lu, T.L.; Ji, D.; Liu, J.; Ji, L.; Yang, H.; Wang, F.Q. Effects of different processing methods on effective components and sulfur dioxide residue in Gastrodiae Rhizoma. China J. Chin. Mater. Med. 2014, 39, 2814–2818. [Google Scholar]
- Wu, Z.; Gao, R.P.; Li, H.; Liao, X.; Tang, X.; Wang, X.G.; Su, Z.M. How steaming and drying processes affect the active compounds and antioxidant types of Gastrodia elata Bl. f. glauca S. chow. Food Res. Int. 2022, 157, 111277. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.Q.; Liu, S.S.; Liu, D.H.; Wang, X.; Wang, Z.M. Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume. Molecules 2019, 24, 3159. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.N.; Geng, Y.L.; Liu, F.; Guo, L.P.; Wang, X. Convenient use of low field nuclear magnetic resonance to determine the drying kinetics and predict the quality properties of mulberries dried in hot-blast air. LWT 2021, 137, 110402. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Drying Technologies: Vehicle to High-Quality Herbs. Food Eng. Rev. 2016, 8, 164–180. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2020, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, B.Y.; Baik, M.Y. Application of ultra high pressure (UHP) in starch chemistry. Crit. Rev. Food Sci. Nutr. 2012, 52, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.P.; Rao, P.S. Kinetic modeling of polyphenoloxidase inactivation during thermal-assisted high pressure processing in black tiger shrimp (Penaeus monodon). Food Control 2017, 80, 388–394. [Google Scholar] [CrossRef]
- Sreedevi, P.; Jayachandran, L.E.; Rao, P.S. Application of high-pressure processing for extending the shelf life of sugarcane juice under refrigerated conditions. J. Food Process. Pres. 2020, 44, e14957. [Google Scholar] [CrossRef]
- Al-Khusaibi, M.; Sablani, S.S.; Perera, C.O. Comparison of Water Blanching and High Hydrostatic Pressure Effects on Drying Kinetics and Quality of Potato. Drying Technol. 2005, 23, 2449–2461. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High hydrostatic pressure increased stability and activity of immobilized lipase in hexane. Enzyme Microb. Technol. 2009, 45, 118–125. [Google Scholar] [CrossRef]
- Yucel, U.; Alpas, H.; Bayindirli, A. Evaluation of high pressure pretreatment for enhancing the drying rates of carrot, apple, and green bean. J. Food Eng. 2010, 98, 266–272. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Cellular permeabilization of cultured plant tissues by high electric field pulses or ultra high pressure for the recovery of secondary metabolites. Food Biotechnology 1993, 7, 35–48. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.N.; Wang, X.; Cheng, S.P.; Liu, F.; Huang, L.Q. Determination of drying kinetics and quality changes of Panax quinquefolium L. dried in hot-blast air. LWT 2019, 116, 108563. [Google Scholar] [CrossRef]
- Su, Z.H.; Yang, Y.G.; Chen, S.Z.; Tang, Z.S.; Xu, H.B. The processing methods, phytochemistry and pharmacology of Gastrodia elata Bl.: A comprehensive review. J. Ethnopharmacol. 2023, 324, 116467. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.P.; Xiao, H.; Feng, S.H.; Liu, D.W.; Cheng, J.T.; Chen, S. Analysis of Degradation Mechanism of Parishin in Gastrodiae Rhizoma. Chin. J. Exp. Tradit. Med. Form. 2017, 23, 18–21. [Google Scholar]
- Mou, L.; Gao, D.L.; Wang, L.Z.; Liu, K.C.; Jin, M. Study on the activity and mechanism of berberine derivative 9-OH berberine against Parkinson’ s disease. Chin. J. Hosp. Pharm. 2022, 42, 884–888. [Google Scholar]
- Janowicz, M.; Lenart, A. Selected physical properties of convection dried apples after HHP treatment. LWT 2015, 63, 828–836. [Google Scholar] [CrossRef]
- Qi, F.M.; Rao, X.Y.; Han, T.T.; Zhong, L.Y.; Luo, X.J.; He, Y.; Shen, R.L. Water migration and kinetics of Arecae Semen during moistening process. China J. Chin. Mater. Med. 2022, 47, 1871–1880. [Google Scholar]
- Zhang, L.H.; Qiao, Y.; Wang, C.; Liao, L.; Shi, D.F.; An, K.J.; Hu, J.Z. Impact of ultrasound combined with ultrahigh pressure pretreatments on color, moisture characteristic, tissue structure, and sensory quality of freeze-dried strawberry slices. J. Food Process. Pres. 2021, 45, e15200. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Loey, A.M.V. Evaluating microalgal cell disruption upon ultra high pressure homogenization. Algal Res. 2019, 42, 101616. [Google Scholar] [CrossRef]
- Tang, C.L.; Wu, B.C.; Wu, J.Y.; Zhang, Z.; Yu, B.C. Novel Strategies Using Total Gastrodin and Gastrodigenin, or Total Gastrodigenin for Quality Control of Gastrodia elata. Molecules 2018, 23, 270. [Google Scholar] [CrossRef]
| Compounds | Regression Equation | Regression Coefficient (R2) | Linear Range (mg/mL) |
|---|---|---|---|
| gastrodin | Y = 35,062X − 92.181 | R2 = 0.9995 | 0.01~0.5 |
| p-hydroxybenzyl alcohol | Y = 56,084X + 114.24 | R2 = 0.9992 | 0.001~0.5 |
| parishin E | Y = 22,813X − 74.322 | R2 = 0.9998 | 0.01~0.25 |
| parishin B | Y = 25,010X − 143.28 | R2 = 0.9995 | 0.01~0.5 |
| parishin C | Y = 36,166X − 115.64 | R2 = 0.9997 | 0.01~0.5 |
| parishin A | Y = 35,846X − 167.72 | R2 = 0.9997 | 0.01~0.5 |
| Treatment Method | The Content of Active Substances (mg/g) | |||||
|---|---|---|---|---|---|---|
| Gastrodin | p-Hydroxybenzyl Alcohol | Parishin E | Parishin B | Parishin C | Parishin A | |
| Fresh GE | 0.31 ± 0.08 | 5.92 ± 0.44 | 1.11 ± 0.24 | 0.93 ± 0.26 | 0.70 ± 0.23 | 1.49 ± 0.44 |
| Steamed GE | 1.00 ± 0.15 | 1.92 ± 0.14 | 8.85 ± 2.67 | 2.99 ± 0.63 | 0.42 ± 0.09 | 5.24 ± 1.49 |
| UHP-treated GE (500 MPa) | 1.38 ± 0.16 | 0.75 ± 0.04 | 8.02 ± 1.71 | 8.07 ± 0.69 | 0.96 ± 0.08 | 7.21 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, S.; Wang, X.; Li, M.; Perumpuli Arachchige, B.N.; Wang, X. Effect of Ultra-High-Pressure Treatment on Gastrodia elata Blume: Drying Characteristics, Components, and Neuroprotective Activity. Foods 2024, 13, 3534. https://doi.org/10.3390/foods13223534
Dong H, Liu S, Wang X, Li M, Perumpuli Arachchige BN, Wang X. Effect of Ultra-High-Pressure Treatment on Gastrodia elata Blume: Drying Characteristics, Components, and Neuroprotective Activity. Foods. 2024; 13(22):3534. https://doi.org/10.3390/foods13223534
Chicago/Turabian StyleDong, Hongjing, Shuang Liu, Xinming Wang, Meng Li, Buddhika Niroshie Perumpuli Arachchige, and Xiao Wang. 2024. "Effect of Ultra-High-Pressure Treatment on Gastrodia elata Blume: Drying Characteristics, Components, and Neuroprotective Activity" Foods 13, no. 22: 3534. https://doi.org/10.3390/foods13223534
APA StyleDong, H., Liu, S., Wang, X., Li, M., Perumpuli Arachchige, B. N., & Wang, X. (2024). Effect of Ultra-High-Pressure Treatment on Gastrodia elata Blume: Drying Characteristics, Components, and Neuroprotective Activity. Foods, 13(22), 3534. https://doi.org/10.3390/foods13223534





