Optimized Liquid Medium Formulation for Sanghuangporus vaninii and Biological Activity of the Exopolysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Strains of Sanghuangporus vaninii
2.2. Preparation of Culture Medium
2.3. Strain Activation and Shake Flask Culture
2.4. Estimation of Mycelial Biomass
2.5. Single Factor Experiments
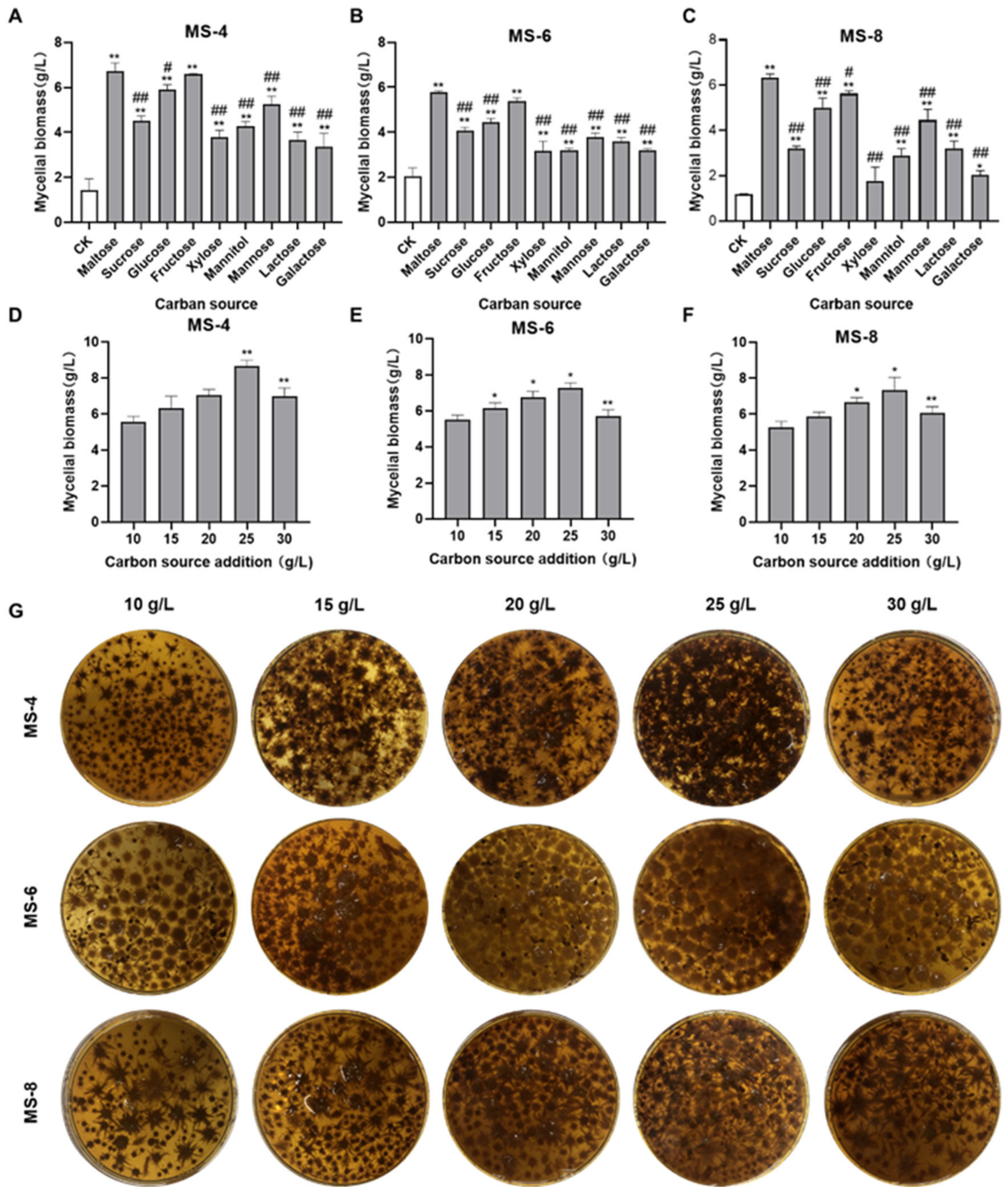
2.6. Single Factor Experiment for Carbon Sources
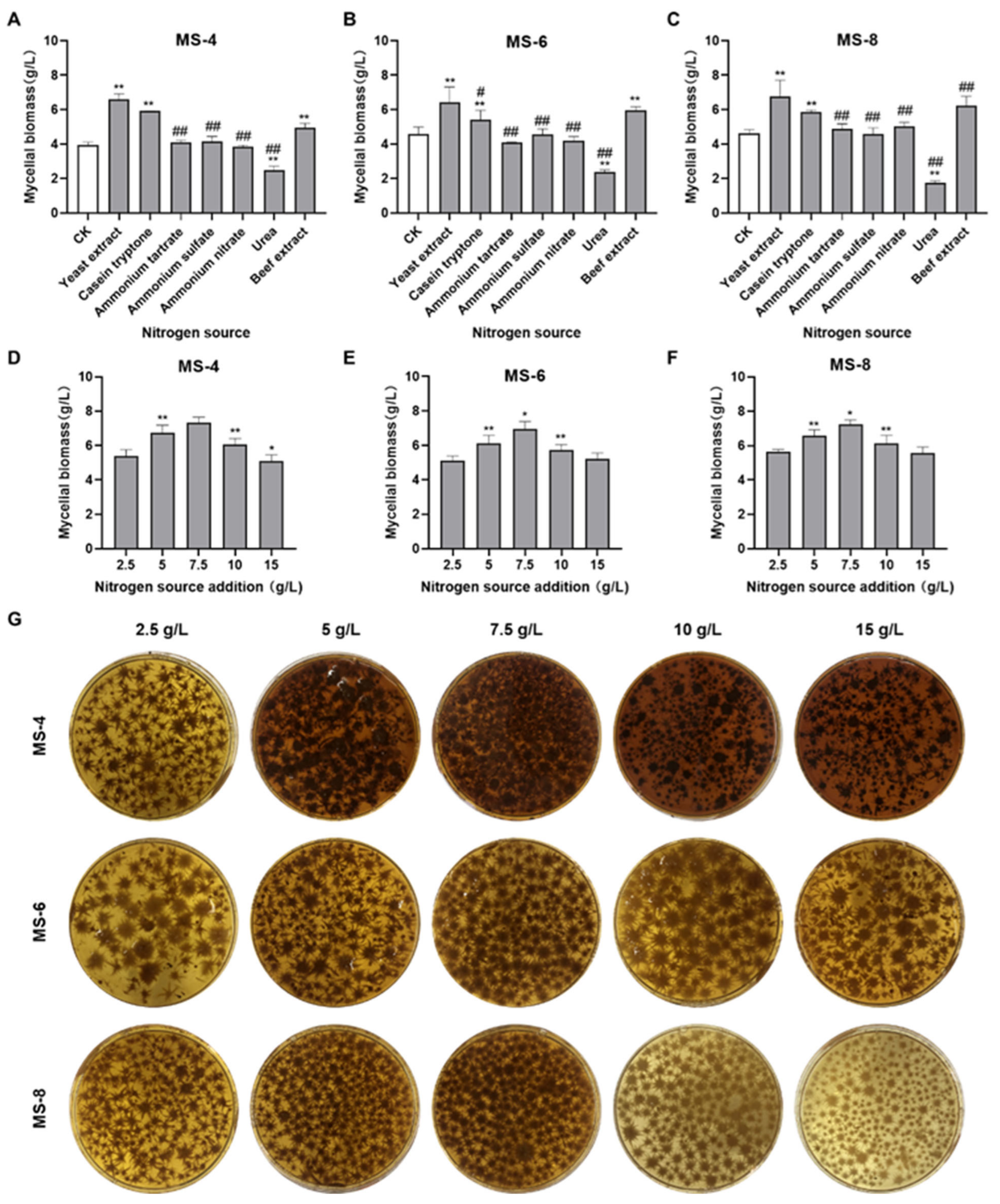
2.7. Single Factor Experiment for Nitrogen Sources
2.8. Screening the Optimal Dosage for the Carbon Source
2.9. Screening the Optimal Dosage of the Nitrogen Source
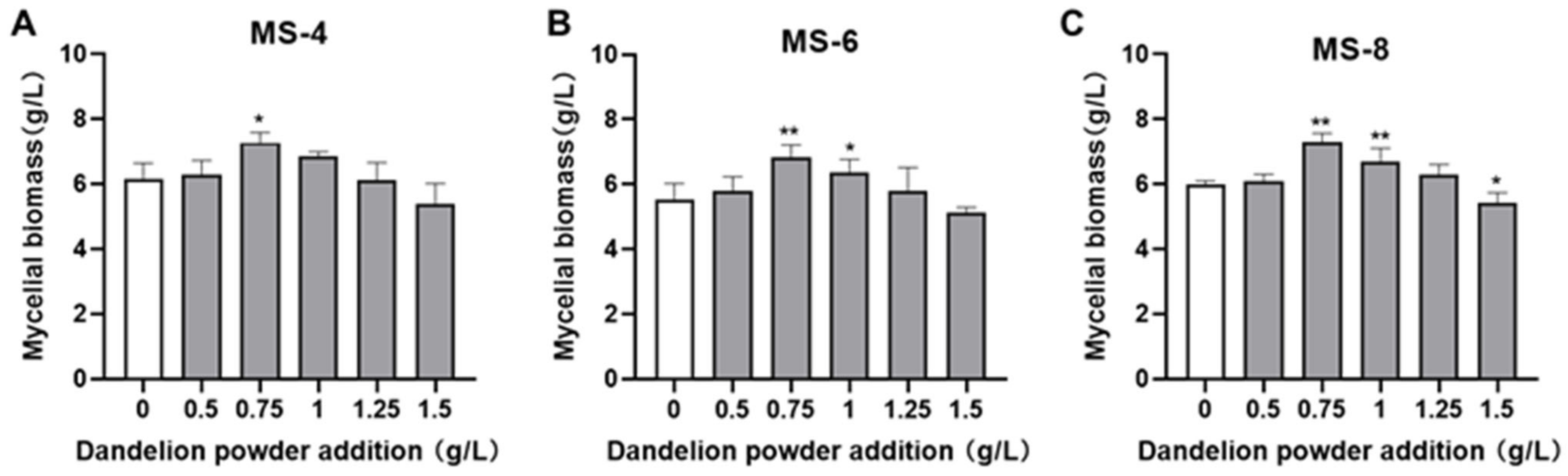
2.10. Screening the Optimal Dosage of the Exogenous Growth Factor
2.11. Response Surface Optimization Experiment Using the Box-Behnken Design
2.12. Validation Test of the Response Surface Method
2.13. Preparation and Purification of Extracellular Polysaccharides
2.14. Determination of Antioxidant Capacity of the Extracellular Polysaccharides
2.15. Determination of Anti-Cancer Activity of the Extracellular Polysaccharides
2.16. Statistical Analysis
3. Results
3.1. Effects of Different Carbon Sources on the Mycelial Biomass of Sanghuangporus vaninii
3.2. Effects of Different Nitrogen Sources on the Mycelial Biomass of Sanghuangporus vaninii
3.3. Optimization of Exogenous Growth Factor Concentration in the Liquid Fermentation Medium
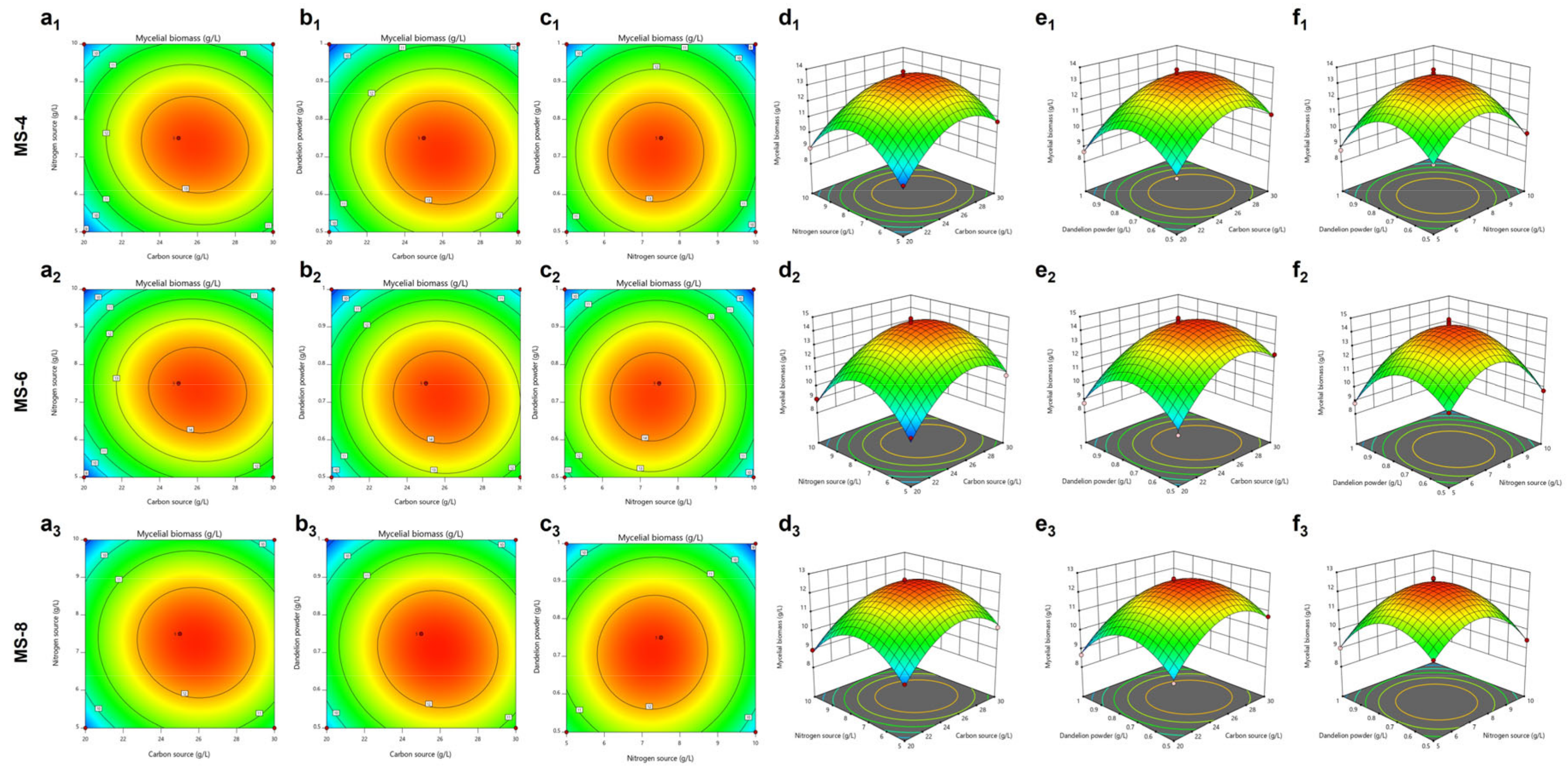
3.4. Response Surface Experimental Design and Results for S. vaninii Strains
3.4.1. Response Surface Experimental Design and Results for the MS-4 Strain
3.4.2. Response Surface Experiment Design and Results for the MS-6 Strain
3.4.3. Response Surface Experiment Design and Results for the MS-8 Strain
3.5. Verification Results of the Optimal Fermentation Medium for Sanghuangporus vaninii
3.6. Estimation of the Antioxidant Activities of Extracellular Polysaccharides from the S. vaninii Strains
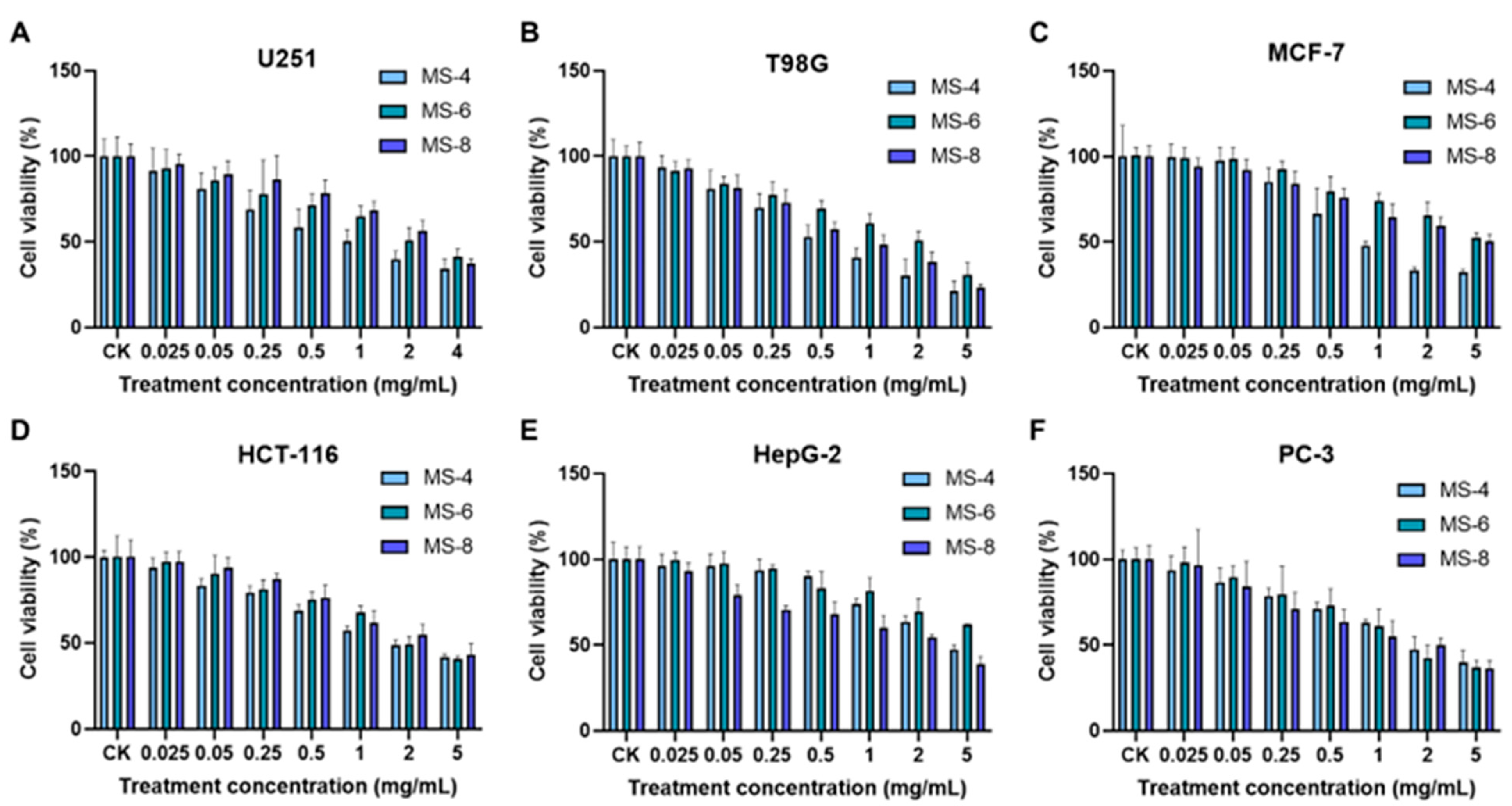
3.7. Estimation of Anti-Cancer Activities of Extracellular Polysaccharides from the S. vaninii Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.C.; Yang, Y.; Zhang, J.S.; Li, Z.P.; Lu, Z.K.; Wang, K. Recent advances in bioactive metabolites from ‘Sanghuang’ mushrooms. Acta Edulis Fungi 2020, 27, 188–201. [Google Scholar] [CrossRef]
- Li, Y.; Bao, H.Y. Chinese Fungal Medicine; Zhongyuan Farmers Publishing House: Zhengzhou, China, 2020. [Google Scholar]
- Li, Q.J. Study on the Active Substances and Quality Standards of Sanghuang Fungus. Ph.D. Thesis, Jilin Agricultural University, Jilin, China, 2017. [Google Scholar]
- Zan, L.F.; Fan, Y.G.; Bao, H.Y.; Tolgor; Ye, J. Characterization of chemical compositions from the wild and cultivated Sanghuangporus vaninii based on UPLC-QTOF-MS. Nat. Prod. Res. Dev. 2021, 33, 1818–1828+1886. [Google Scholar] [CrossRef]
- Xia, Y.J.; Yang, C.Y.; Liu, X.F.; Wang, G.Q.; Xiong, Z.Q.; Song, X.; Yang, Y.J.; Zhang, H.; Ai, L.Z. Enhancement of triterpene production via in situ extractive fermentation of Sanghuangporus vaninii YC-1. Biotechnol. Appl. Biochem. 2022, 69, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Jin, X.; Xie, M.L.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.Y.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Huang, Q.Z.; Chen, K.W.; Huang, Z.F.; Liu, Y.; Jia, R.B.; Liu, B. Sanghuangporus vaninii fruit body polysaccharide alleviates hyperglycemia and hyperlipidemia via modulating intestinal microflora in type 2 diabetic mice. Front. Nutr. 2022, 9, 1013466. [Google Scholar] [CrossRef]
- Wan, X.; Jin, X.; Wu, X.; Yang, X.; Lin, D.; Li, C.; Fu, Y.; Liu, Y.; Liu, X.; Lv, J.; et al. Structural characterisation and antitumor activity against non-small cell lung cancer of polysaccharides from Sanghuangporus vaninii. Carbohyd Polym. 2022, 276, 118798. [Google Scholar] [CrossRef]
- Yin, C.M.; Li, Y.H.; Li, J.T.; Fan, X.Z.; Yao, F.; Shi, D.F.; Cheng, Y.Q.; Liu, M.F.; Lu, Q.; Gao, H. Gastrointestinal digestion, probiotic fermentation behaviors and immunomodulatory effects of polysaccharides from Sanghuangporus vaninii. Int. J. Biol. Macromol. 2022, 223 Pt A, 606–617. [Google Scholar] [CrossRef]
- Liu, M.F.; Yin, C.M.; Zhang, Q.; Fan, X.Z.; Shi, D.F.; Gao, H. Comparison of in vitro antioxidant, hypoglycemic and antibacterial activities of different extracts from 3 kinds of Sanghuangporus vaninii. J. Food Saf. Qual. 2024, 15, 18–25. [Google Scholar]
- Song, J.L.; Wang, W.K.; Yan, J.; Lu, N.; Zhou, Z.F.; Yuan, W.D. Antioxidant substances and activity of medicinal fungus Sanghuangporus. J. JNWAFU (Nat. Sci. Ed.) 2022, 50, 144–154. [Google Scholar] [CrossRef]
- He, P.X.; Wu, S.S.; Zheng, K.; Xu, C.P. Molecular structure and antioxidant activity of polysaccharides from Phellinus vaninii Ljup. J. Food Sci. Biotechnol. 2018, 37, 939–947. [Google Scholar]
- Zhang, Z.F.; Song, T.T.; Chen, J.F.; Lv, G.Y. Recovery of a hypolipidemic polysaccharide from artificially cultivated Sanghuangporus vaninii with an effective method. Front. Nutr. 2023, 9, 1095556. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Guo, S.S.; Sun, C.G.; Shu, Y.T.; Fang, C.Y.; Li, F.S.; Wang, J. Dynamic analysis of active components in different growth years of Phellinus igniarius. Food Res. Dev. 2022, 43, 150–155. [Google Scholar]
- Li, X.Q.; Liang, G.Q.; Liu, K.L.; Xiao, X.; Xu, W.W.; Wei, W.; Tang, Y.F.; Lin, Q.X.; Lu, C.X. Extraction methods and application research progress of active ingredients of Phellinus linteus. Guangxi Seric. 2022, 59, 41–50. [Google Scholar]
- Jiang, F.C. Study on Liquid Submerged Fermentation Kinetic and Fermentation Process of High Flavonoid Production by Phellinus baumii. Master's Thesis, Shanghai Ocean University, Shanghai, China, 2017. [Google Scholar] [CrossRef]
- Sun, X.R.; Han, Y.W.; Bian, J.Y. Study on the liquid fermentation conditions for production of mycelium of Stropharia rugoso-annulata. Heilongjiang Agric. Sci. 2016, 114–117. [Google Scholar]
- Zhang, S.Y.; Feng, M.; Li, J.M.; Cai, L.S.; Xu, C.; Guo, L.L.; Guan, Y.L.; Ma, Z.Y. Optimization of the composition of medium for Pleurotus eryngii liquid shake flask fermentation. Edible Fungi 2020, 42, 17–19. [Google Scholar]
- Wang, H.; Lou, L.; Bu, H.S.; Chen, C.B.; Wang, S.M.; Li, Y. Liquid fermentation technology of Marasmius epiphyllus. North. Hortic. 2020, 22, 127–131. [Google Scholar]
- Xu, L.N. Identification, Artificial Cultivation and Fermentation Technology of a Wild Helvella. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2020. [Google Scholar] [CrossRef]
- Teng, C.L.; Liu, D.; Yin, Y.H.; Guo, J.F.; Liu, S.; Zhang, H.; Chang, Y.; Lu, Y.T. Optimization of Sanghuangporus vaninii liquid culture medium based on response surface methodology. Heilongjiang Agric. Sci. 2022, 334, 73–80. [Google Scholar]
- Li, Y.N.; Xu, Z.X.; Shang, D.; Zhang, Y.; Qiu, L.Y.; Gao, Y.Q. Optimization of Agaricus bisporus liquid inoculum cultivation by response surface methodology and preparation of reduced liquid inoculum. North. Hortic. 2021, 14, 134–142. [Google Scholar]
- Hu, R.K.; Chen, L.B.; Wu, L.X.; Huang, Z.R.; Lv, X.C.; Liu, B. Optimizing culture medium and antioxidant activity of extracellular polysaccharides in liquid fermentation of Flammulina velutipes. Fujian J. Agric. Sci. 2017, 32, 975–980. [Google Scholar] [CrossRef]
- Xie, X.C.; Liu, J.S.; Luo, Y.L.; Deng, B.W.; Bai, Q.Y.; Zhang, Y.W. Comparative analysis of extracellular polysaccharide content and bioactivity associated with 4 strians of Agaricus blazei. Food Res. Dev. 2019, 40, 68–75. [Google Scholar]
- Li, W.; Li, X.S.; Zhao, G.J.; Han, H.; Zhao, T.Y.; Han, H.M.; Shen, Z.Y. Study on the antioxidant activities of active ingredients in black rice anthocyanin compound soft capsule. Food Sci. Technol. 2018, 43, 109–115. [Google Scholar]
- Liu, C.Y.; Yuan, Y.; Hao, X.Y.; Liu, K.; Wu, X.P.; Fu, J.S. A comparative study of intracellular and extracellular polysaccharides in mycelia of Cordyceps cicadae against oxidative damage of hepatocytes. Mycosystema 2020, 39, 421–433. [Google Scholar] [CrossRef]
- Yan, M.Q.; Liu, Y.F.; Zhou, S.; Tang, C.D.; Feng, J.; Zhang, J.S. Liquid fermentation technology and functional components of edible and medicinal fungi. Microbiol. China 2023, 50, 3211–3231. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.S.; Chen, Q.H.; Zhou, Y.P.; Tian, C.E. Liquid fermentation of Ganoderma and application of its products. Microbiol. China 2022, 49, 336–351. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, X.; Zhang, J.Q.; Hu, L.; Jiang, L.L.; Wang, X.; Bian, Y.B. Effects of culture conditions and exogenous additives on cadmium content in mycelia and fruiting body of Lentinula edodes. J. Huazhong Agric. Univ. 2024, 1, 5. [Google Scholar] [CrossRef]
- Zhong, S.; Li, Y.G.; Zhu, J.X.; Lin, T.B.; Lv, Z.Q.; Ye, W.Q. Effect of optimizing medium on the growth of Phellinus igniarius mycelium. J. Zhejiang Agric. Sci. 2011, 1, 173–175. [Google Scholar] [CrossRef]
- Wang, R.R. Study on Sulfated Modification of Exopolysaccharides from Fungi Fermentation and Its Biological Activity. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2015. [Google Scholar]


| No. | A: Carbon Source (g/L) | B: Nitrogen Source (g/L) | C: Dandelion Powder (g/L) | Mycelial Biomass (g/L) |
|---|---|---|---|---|
| 1 | 30 | 7.5 | 1 | 9.76 |
| 2 | 30 | 5 | 0.75 | 10.73 |
| 3 | 25 | 10 | 1 | 8.53 |
| 4 | 20 | 5 | 0.75 | 9.06 |
| 5 | 25 | 7.5 | 0.75 | 13.33 |
| 6 | 20 | 7.5 | 1 | 8.66 |
| 7 | 25 | 5 | 1 | 8.76 |
| 8 | 25 | 7.5 | 0.75 | 13.46 |
| 9 | 25 | 10 | 0.5 | 9.86 |
| 10 | 20 | 10 | 0.75 | 9.03 |
| 11 | 25 | 5 | 0.5 | 10.16 |
| 12 | 30 | 7.5 | 0.5 | 11.06 |
| 13 | 25 | 7.5 | 0.75 | 13.65 |
| 14 | 30 | 10 | 0.75 | 9.36 |
| 15 | 25 | 7.5 | 0.75 | 13.86 |
| 16 | 25 | 7.5 | 0.75 | 13.26 |
| 17 | 20 | 7.5 | 0.5 | 9.36 |
| Source | Sum of Squares | Df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 63.01 | 9 | 7.00 | 101.95 | <0.0001 |
| A | 2.88 | 1 | 2.88 | 41.94 | 0.0003 |
| B | 0.4656 | 1 | 0.4656 | 6.78 | 0.0352 |
| C | 2.80 | 1 | 2.80 | 40.72 | 0.0004 |
| AB | 0.4489 | 1 | 0.4489 | 6.54 | 0.0377 |
| AC | 0.0900 | 1 | 0.0900 | 1.31 | 0.2899 |
| BC | 0.0012 | 1 | 0.0012 | 0.0178 | 0.8975 |
| A2 | 13.52 | 1 | 13.52 | 196.95 | <0.0001 |
| B2 | 19.91 | 1 | 19.91 | 289.98 | <0.0001 |
| C2 | 17.01 | 1 | 17.01 | 247.65 | <0.0001 |
| Residual | 0.4807 | 7 | 0.0687 | ||
| Lack of fit | 0.2412 | 3 | 0.0804 | 1.34 | 0.3789 |
| Pure error | 0.2395 | 4 | 0.0599 | ||
| Cor Total | 63.49 | 16 |
| No. | A: Carbon Source (g/L) | B: Nitrogen Source (g/L) | C: Dandelion Powder (g/L) | Mycelial Biomass (g/L) |
|---|---|---|---|---|
| 1 | 25 | 5 | 0.5 | 10.8 |
| 2 | 20 | 7.5 | 1 | 8.7 |
| 3 | 30 | 10 | 0.75 | 9.56 |
| 4 | 30 | 5 | 0.75 | 10.8 |
| 5 | 20 | 10 | 0.75 | 9.05 |
| 6 | 30 | 7.5 | 1 | 9.86 |
| 7 | 25 | 7.5 | 0.75 | 14.2 |
| 8 | 25 | 10 | 0.5 | 9.76 |
| 9 | 25 | 7.5 | 0.75 | 14.9 |
| 10 | 25 | 7.5 | 0.75 | 14 |
| 11 | 20 | 5 | 0.75 | 8.03 |
| 12 | 30 | 7.5 | 0.5 | 11.4 |
| 13 | 25 | 5 | 1 | 8.8 |
| 14 | 25 | 10 | 1 | 8.5 |
| 15 | 20 | 7.5 | 0.5 | 9.26 |
| 16 | 25 | 7.5 | 0.75 | 14.6 |
| 17 | 25 | 7.5 | 0.75 | 14.67 |
| Source | Sum of Squares | Df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 97.03 | 9 | 10.78 | 88.52 | <0.0001 |
| A | 5.36 | 1 | 5.36 | 44.03 | 0.0003 |
| B | 0.3160 | 1 | 0.3160 | 2.59 | 0.1513 |
| C | 3.59 | 1 | 3.59 | 29.49 | 0.0010 |
| AB | 1.24 | 1 | 1.24 | 10.21 | 0.0152 |
| AC | 0.2401 | 1 | 0.2401 | 1.97 | 0.2031 |
| BC | 0.1369 | 1 | 0.1369 | 1.12 | 0.3243 |
| A2 | 23.92 | 1 | 23.92 | 196.36 | <0.0001 |
| B2 | 31.23 | 1 | 31.23 | 256.38 | <0.0001 |
| C2 | 22.00 | 1 | 22.00 | 180.62 | <0.0001 |
| Residual | 0.8525 | 7 | 0.1218 | ||
| Lack of fit | 0.3170 | 3 | 0.1057 | 0.7893 | 0.5596 |
| Pure error | 0.5355 | 4 | 0.1339 | ||
| Cor Total | 97.88 | 16 |
| No. | A: Carbon Source (g/L) | B: Nitrogen Source (g/L) | C: Dandelion Powder (g/L) | Mycelial Biomass (g/L) |
|---|---|---|---|---|
| 1 | 25 | 10 | 1 | 8.63 |
| 2 | 25 | 10 | 0.5 | 9.46 |
| 3 | 30 | 5 | 0.75 | 10.2 |
| 4 | 30 | 7.5 | 1 | 8.83 |
| 5 | 30 | 7.5 | 0.5 | 10.73 |
| 6 | 20 | 5 | 0.75 | 9.13 |
| 7 | 25 | 7.5 | 0.75 | 12.67 |
| 8 | 20 | 10 | 0.75 | 8.96 |
| 9 | 20 | 7.5 | 0.5 | 9.16 |
| 10 | 25 | 5 | 0.5 | 10.26 |
| 11 | 30 | 10 | 0.75 | 9.33 |
| 12 | 25 | 7.5 | 0.75 | 12.33 |
| 13 | 20 | 7.5 | 1 | 8.67 |
| 14 | 25 | 7.5 | 0.75 | 12.7 |
| 15 | 25 | 7.5 | 0.75 | 12.46 |
| 16 | 25 | 7.5 | 0.75 | 12.4 |
| 17 | 25 | 5 | 1 | 9.03 |
| Source | Sum of Squares | Df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 39.96 | 9 | 4.44 | 228.54 | <0.0001 |
| A | 1.26 | 1 | 1.26 | 64.65 | <0.0001 |
| B | 0.6272 | 1 | 0.6272 | 32.28 | 0.0007 |
| C | 2.48 | 1 | 2.48 | 127.40 | <0.0001 |
| AB | 0.1225 | 1 | 0.1225 | 6.30 | 0.0403 |
| AC | 0.4970 | 1 | 0.4970 | 25.58 | 0.0015 |
| BC | 0.0400 | 1 | 0.0400 | 2.06 | 0.1945 |
| A2 | 10.15 | 1 | 10.15 | 522.16 | <0.0001 |
| B2 | 10.18 | 1 | 10.18 | 523.84 | <0.0001 |
| C2 | 10.94 | 1 | 10.94 | 563.31 | <0.0001 |
| Residual | 0.1360 | 7 | 0.0194 | ||
| Lack of fit | 0.0273 | 3 | 0.0091 | 0.3352 | 0.8020 |
| Pure error | 0.1087 | 4 | 0.0272 | ||
| Cor Total | 40.10 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, X.; Lu, Q.; Xu, H.; Sun, H.; Zhang, J.; Wu, X.; Fu, J. Optimized Liquid Medium Formulation for Sanghuangporus vaninii and Biological Activity of the Exopolysaccharides. Foods 2024, 13, 3574. https://doi.org/10.3390/foods13223574
Huang H, Li X, Lu Q, Xu H, Sun H, Zhang J, Wu X, Fu J. Optimized Liquid Medium Formulation for Sanghuangporus vaninii and Biological Activity of the Exopolysaccharides. Foods. 2024; 13(22):3574. https://doi.org/10.3390/foods13223574
Chicago/Turabian StyleHuang, Haichen, Xiaomin Li, Qi Lu, Hui Xu, Huijuan Sun, Junli Zhang, Xiaoping Wu, and Junsheng Fu. 2024. "Optimized Liquid Medium Formulation for Sanghuangporus vaninii and Biological Activity of the Exopolysaccharides" Foods 13, no. 22: 3574. https://doi.org/10.3390/foods13223574
APA StyleHuang, H., Li, X., Lu, Q., Xu, H., Sun, H., Zhang, J., Wu, X., & Fu, J. (2024). Optimized Liquid Medium Formulation for Sanghuangporus vaninii and Biological Activity of the Exopolysaccharides. Foods, 13(22), 3574. https://doi.org/10.3390/foods13223574






