Effects of Different Packaging Types and Storage Periods on Physicochemical and Antioxidant Properties of Honeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Collection and Experimental Groups
2.2. Determination Physicochemical Properties of Honey
2.2.1. Moisture and pH Analysis
2.2.2. Electrical Conductivity, Free Acidity, Proline Content, and Total Sugar Analysis
2.2.3. Diastase Analysis
2.2.4. Hydroxymethylfurfural (HMF) Analysis
2.3. Determination of Antioxidant–Oxidant Status
2.4. Determination of Catalase and Total Phenolic Content (TPC) Activity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, E. A short history of knowledge about honey bees (Apis) up to 1800. Bee World 2004, 85, 6–11. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Revised Codex Standards for Honey CXS 12-1981. Rev 1(1987), Rev 2, FAO. 2001. Available online: https://www.fao.org/4/w0076e/w0076e30.htm (accessed on 4 September 2024).
- Bergamo, G.; Seraglio, S.K.T.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Physicochemical characteristics of bracatinga honeydew honey and blossom honey produced in the state of Santa Catarina: An approach to honey differentiation. Food Res. Int. 2019, 116, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Ünal, S.; Ayan, S.; Karadeniz, M.; Yer, E. Some forest trees for honeydew honey production in Turkey. Sib. Lesn. Zurnal (Sib. J. For. Sci.) 2017, 101–110. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Kunz, T.; Lee, E.; Schiwek, V.; Seewald, T.; Methner, F. Glucose—A reducing sugar? Reducing properties of sugars in beverages and food. Brew. Sci. 2011, 64, 61–67. [Google Scholar]
- Derebaşı, E.; Bulut, G.; Col, M.; Güney, F.; Yaşar, N.; Ertürk, Ö. Physicochemical and residue analysis of honey from Black Sea region of Turkey. Fresenius Environ. Bull. 2014, 23, 10–17. [Google Scholar]
- Manickavasagam, G.; Saaid, M.; Lim, V.; Saad, M.; Azmi, N.A.S.; Osman, R. Quality assessment and chemometrics application on physicochemical characteristics, antioxidant properties, and 5-HMF content of Malaysian stingless bee honey from different topographical origins. J. Food Sci. 2023, 88, 1466–1481. [Google Scholar] [CrossRef]
- Manickavasagam, G.; Saaid, M.; Lim, V. Impact of prolonged storage on quality assessment properties and constituents of honey: A systematic review. J. Food Sci. 2024, 89, 811–833. [Google Scholar] [CrossRef]
- Fallico, B.; Arena, E.; Zappala, M. Prediction of honey shelf life. J. Food Qual. 2009, 32, 352–368. [Google Scholar] [CrossRef]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Ind. Crop. Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- Zivkov Balos, M.; Popov, N.; Jaksic, S.; Mihaljev, Z.; Pelic, M.; Ratajac, R.; Ljubojevic Pelic, D. Sunflower honey-evaluation of quality and stability during storage. Foods 2023, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.G.M.; Toledo, B.S.; Guimarães, J.T.; Ramos, G.; da Cunha, D.T.; Pimentel, T.C.; Cruz, A.G.; Freitas, M.Q.; Esmerino, E.A.; Mársico, E.T. The impact of packaging design on the perceived quality of honey by Brazilian consumers. Food Res. Int. 2022, 151, 110887. [Google Scholar] [CrossRef] [PubMed]
- Turkish Food Codex Honey Communiqué (Communiqué No: 2020/7), Republic of Turkey Ministry of Food, Agriculture and Livestock. Official Gazette No: 31107. 2020. Available online: https://www.resmigazete.gov.tr/eskiler/2020/04/20200422-13.htm (accessed on 4 September 2024).
- Venir, E.; Spaziani, M.; Maltini, E. Crystallization in “Tarassaco” Italian honey studied by DSC. Food Chem. 2010, 122, 410–415. [Google Scholar] [CrossRef]
- Scripca, L.; Amariei, S. Research on honey crystalization. Rev. Chim. 2018, 69, 2953–2957. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Bhat, N.; Neethu, T.; Nayimabanu, T.; Nithin, H. Periodical changes in quality parameters of honey during storage and processing. Int. J. Chem. Stud. 2021, 9, 19–24. [Google Scholar] [CrossRef]
- Solayman, M.; Shapla, U.M.; Khalil, I. Furfural and Hydroxymethylfurfural. In Honey: Composition and Health Benefits; Khalil, M.I., Gan, S.H., Goh, B.H., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 152–166. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Tosi, E.; Ciappini, M.; Re, E.; Lucero, H. Honey thermal treatment effects on hydroxymethylfurfural content. Food Chem. 2002, 77, 71–74. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities L 10/47 2002, 10, 47–52. [Google Scholar]
- Modi, B.; Timilsina, H.; Bhandari, S.; Achhami, A.; Pakka, S.; Shrestha, P.; Kandel, D.; Gc, D.B.; Khatri, S.; Chhetri, P.M.; et al. Current trends of food analysis, safety, and packaging. Int. J. Food Sci. 2021, 2021, 9924667. [Google Scholar] [CrossRef]
- Pasias, I.N.; Raptopoulou, K.G.; Makrigennis, G.; Ntakoulas, D.D.; Lembessis, D.; Dimakis, V.; Katsinas, R.; Proestos, C. Finding the optimum treatment procedure to delay honey crystallization without reducing its quality. Food Chem. 2022, 381, 132301. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Bergamo, G.; Molognoni, L.; Daguer, H.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality changes during long-term storage of a peculiar Brazilian honeydew honey: “Bracatinga”. J. Food Compos. Anal. 2021, 97, 103769. [Google Scholar] [CrossRef]
- Martínez, R.A.; Schvezov, N.; Brumovsky, L.A.; Puccoarello Roman, A.B. Influence of temperature and packaging type on quality parameters and antimicrobial properties during Yateí honey storage. Food Sci. Technol. 2018, 38 (Suppl. 1), 196–202. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association Official Analytical Chemists (AOAC): Washington, DC, USA, 1990. [Google Scholar]
- International Honey Commission. Harmonised methods of the International Honey Commission. 2009, 1–63. Available online: http://www.bee-hexagon.net/files/file/fileE/IHCPapers/IHC-methods_2009.pdf (accessed on 20 March 2024).
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Ramay, M.S.; Yalcin, S. Effects of supplemental pine needles powder (Pinus brutia) on growth performance, breast meat composition, and antioxidant status in broilers fed linseed oil-based diets. Poult. Sci. 2020, 99, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Singleton, V.L. Lamuela-Raventos: Analysis of total phenoles and other oxidation substartes and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152. [Google Scholar]
- Juan-Borrás, M.; Periche, A.; Domenech, E.; Escriche, I. Routine quality control in honey packaging companies as a key to guarantee consumer safety. The case of the presence of sulfonamides analyzed with LC-MS-MS. Food Control 2015, 50, 243–249. [Google Scholar] [CrossRef]
- Minhas, S. Nutritional Storage and Value Addition Studies on Raw and Heat Processed Honey; Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya: Palampur, India, 2010. [Google Scholar]
- da Silva, P.M.; Gonzaga, L.V.; Biluca, F.C.; Schulz, M.; Vitali, L.; Micke, G.A.; Costa, A.C.O.; Fett, R. Stability of Brazilian Apis mellifera L. honey during prolonged storage: Physicochemical parameters and bioactive compounds. LWT 2020, 129, 109521. [Google Scholar] [CrossRef]
- Prica, N.; Baloš, M.Ž.; Jakšić, S.; Mihaljev, Ž.; Kartalović, B.; Babić, J.; Savić, S. Moisture and acidity as indicators of the quality of honey originating from Vojvodina region. Arch. Vet. Med. 2014, 7, 99–109. [Google Scholar] [CrossRef]
- Monggudal, M.; Radzi, M.; Ismail, M.; Ismail, W.W. Effect of six month storage on physicochemical analysis and antioxidant activity of several types of honey. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Terengganu, Malaysia, 25–26 June 2018; p. 012047. [Google Scholar]
- Terrab, A.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- European Commission. Opinion of the Scientific Committee on Veterinary Measures Relating to Public Health on Honey and Microbiological Hazards. Available online: https://food.ec.europa.eu/document/download/7488a863-4179-444a-8eff-72b137ae0240_en?filename=sci-com_scv_out53_en.pdf (accessed on 4 September 2024).
- Faleye, F.; Soyinka, J.; Omoniyi, S.; Popoola, O.; Adegbola, A.; Akinpelu, B.; Adekeye, D. Physicochemical properties, antioxidant and antimicrobial activities of Nigerian Polyfloral honeys. J. Pharmacogn. Phytochem. 2021, 10, 01–09. [Google Scholar]
- Bhalchandra, W.; Joshi, M.A.; Jawalkar, N. Effect of storage on various honey quality parameters of Apis mellifera honey harvested from Kannad region, Aurangabad. J. Pharmacogn. Phytochem. 2022, 11, 239–246. [Google Scholar] [CrossRef]
- DeMera, J.H.; Angert, E.R. Comparison of the antimicrobial activity of honey produced by Tetragonisca angustula (Meliponinae) and Apis mellifera from different phytogeographic regions of Costa Rica. Apidologie 2004, 35, 411–417. [Google Scholar] [CrossRef]
- Sanz, S.; Gradillas, G.; Jimeno, F.; Perez, C.; Juan, T. Fermentation problem in Spanish North-Coast Honey. J. Food Prot. 1995, 58, 515–518. [Google Scholar] [CrossRef]
- Silva, M.S.; Rabadzhiev, Y.; Eller, M.R.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in honey. Honey Anal. 2017, 500, 233–257. [Google Scholar]
- Kamal, A.; Raza, S.; Rashid, N.; Hameed, T.; Gilani, M.; Qureshi, M.A.; Nasim, K. Comparative study of honey collected from different flora of Pakistan. Online JB Sci. 2002, 2, 626–627. [Google Scholar]
- Pirdawd, H.K. Physiochemical Analysis of Honey Produced Around the City of Erbil (Iraq). Harran University Graduate School of Natural and Applied Sciences. MSc Thesis, Şanlıurfa. 2022. Available online: http://hdl.handle.net/11513/3266 (accessed on 4 September 2024).
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Brugnerotto, P.; Fuente-Ballesteros, A.; Martín-Gómez, B.; María Ares, A.; Valdemiro Gonzaga, L.; Fett, R.; Carolina Oliveira Costa, A.; Bernal, J. Free amino acid profile in Mimosa scabrella honeydew honey from Brazil and chemometric analysis for geographical discrimination. Food Res. Int. 2024, 177, 113856. [Google Scholar] [CrossRef]
- Hermosín, I.; Chicon, R.M.; Cabezudo, M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Tian, J.; Zhang, Y.Z.; Li, S.S.; Zheng, H.Q.; Hu, F.L. Investigation of the maturity evaluation indicator of honey in natural ripening process: The case of rape honey. Foods 2021, 10, 2882. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Adnan, N.A. Biochemical and nutritional components of selected honey samples. Acta. Sci. Pol. Technol. Aliment 2014, 13, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant activity and phenolic compound identification and quantification in Western Australian Honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Fadzil, M.A.M.; Mustar, S.; Rashed, A.A. The potential use of honey as a neuroprotective agent for the management of neurodegenerative diseases. Nutrients 2023, 15, 1558. [Google Scholar] [CrossRef]
- Alaerjani, W.M.A.; Abu-Melha, S.; Alshareef, R.M.H.; Al-Farhan, B.S.; Ghramh, H.A.; Al-Shehri, B.M.A.; Bajaber, M.A.; Khan, K.A.; Alrooqi, M.M.; Modawe, G.A.; et al. Biochemical reactions and their biological contributions in honey. Molecules 2022, 27, 4719. [Google Scholar] [CrossRef]
- Namiki, M. Chemistry of Maillard reactions: Recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. Adv. Food Res. 1988, 32, 115–184. [Google Scholar]
- Wang, X.; Gheldof, N.; Engeseth, N. Effect of processing and storage on antioxidant capacity of honey. J. Food Sci. 2004, 69, fct96–fct101. [Google Scholar] [CrossRef]
- Kedzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skalecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Koç, A.U.; Atakan, Y.; Küçüker, H.; Küçüker, H. Some physicochemical characteristics of Denizli thyme (Origanum onites) honey. Adnan Menderes Üniv. Zir. Fak. Derg. 2023, 20, 127–133. [Google Scholar]
- Kıvrak, Ş.; Kivrak, I.; Karababa, E. Characterization of Turkish honeys regarding of physicochemical properties, and their adulteration analysis. Food Sci. Technol. 2016, 37, 80–89. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
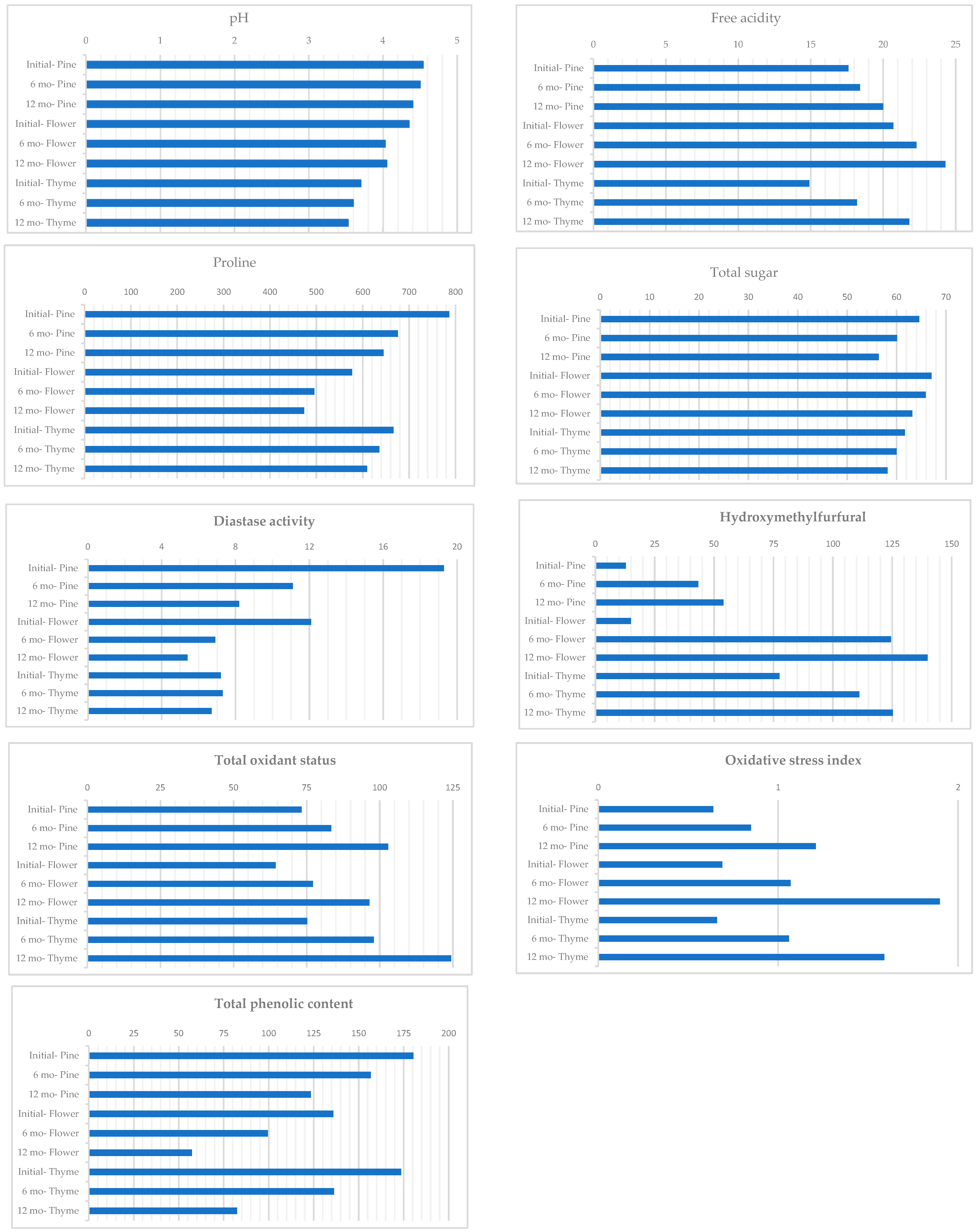
| Groups | Moisture | pH | Electrical Conductivity | Free Acidity | Proline | Total Sugar |
|---|---|---|---|---|---|---|
| Honey type (HT) | ||||||
| Pine | 16.0 | 4.48 a | 937.2 a | 18.6 b | 702.8 | 60.4 |
| Flower | 16.2 | 4.14 b | 397.1 b | 22.5 a | 515.6 | 65.4 |
| Thyme | 16.2 | 3.61 c | 365.6 b | 18.3 b | 637.4 | 60.0 |
| Packaging type (PT) | ||||||
| Tin cans | 16.1 | 4.11 | 585.3 | 21.1 a | 614.4 | 61.9 |
| Light-colored glass | 16.1 | 4.06 | 550.5 | 17.7 b | 618.5 | 61.8 |
| Dark-colored glass | 16.1 | 4.06 | 564.2 | 20.6 a | 622.8 | 61.9 |
| Storage period (SP) | ||||||
| Initial | 16.5 a | 4.20 a | 498.6 c | 17.7 c | 676.9 a | 64.5 a |
| 6 mo | 16.2 b | 4.04 b | 561.7 b | 19.6 b | 602.6 b | 62.0 b |
| 12 mo | 15.6 c | 3.99 b | 639.7 a | 22.0 a | 576.2 c | 59.3 c |
| SP × HT | ||||||
| Initial—Pine | 16.4 | 4.54 a | 856.1 | 17.6 d | 787.0 a | 64.6 abc |
| 6 mo—Pine | 16.1 | 4.50 ab | 932.2 | 18.4 cd | 676.1 ab | 60.1 cde |
| 12 mo—Pine | 15.4 | 4.40 ab | 1023.3 | 20.0 bcd | 645.2 ab | 56.4 e |
| Initial—Flower | 16.4 | 4.35 b | 331.7 | 20.7 bc | 577.2 bcd | 67.1 a |
| 6 mo—Flower | 16.3 | 4.03 c | 391.4 | 22.3 ab | 495.7 cd | 65.9 ab |
| 12 mo—Flower | 15.8 | 4.05 c | 468.2 | 24.3 a | 473.8 d | 63.2 abc |
| Initial—Thyme | 16.7 | 3.70 d | 308.0 | 14.9 e | 666.6 ab | 61.7 bcd |
| 6 mo—Thyme | 16.2 | 3.60 d | 361.3 | 18.2 cd | 636.1 bc | 60.0 cde |
| 12 mo—Thyme | 15.7 | 3.53 d | 427.4 | 21.8 ab | 609.5 bcd | 58.2 de |
| SP × PT | ||||||
| Initial—Tin cans | 16.5 | 4.25 | 497.4 | 18.2 cd | 676.7 | 64.5 |
| 6 mo—Tin cans | 16.1 | 4.04 | 565.9 | 20.9 bc | 592.3 | 61.7 |
| 12 mo—Tin cans | 15.5 | 4.05 | 692.4 | 24.3 a | 574.2 | 59.7 |
| Initial—Light-colored glass | 16.5 | 4.13 | 502.7 | 16.6 d | 677.2 | 64.5 |
| 6 mo—Light-colored glass | 16.2 | 4.09 | 557.6 | 17.4 d | 605.5 | 61.8 |
| 12 mo—Light-colored glass | 15.7 | 3.97 | 591.2 | 19.0 cd | 572.7 | 59.2 |
| Initial—Dark-colored glass | 16.5 | 4.21 | 495.7 | 18.3 cd | 676.9 | 64.4 |
| 6 mo—Dark-colored glass | 16.2 | 4.00 | 561.6 | 20.6 bc | 610.0 | 62.5 |
| 12 mo—Dark-colored glass | 15.7 | 3.95 | 635.3 | 22.8 ab | 581.5 | 58.9 |
| Overall Estimated mean ± SEM | 16.1 ± 0.1 | 4.08 ± 0.03 | 566.6 ± 10.3 | 19.8 ± 0.3 | 618.6 ± 30.9 | 61.9 ± 1.0 |
| Significance | p | p | p | p | p | p |
| Between subject effects | ||||||
| HT | 0.290 | <0.001 | <0.001 | <0.001 | 0.067 | 0.072 |
| PT | 0.876 | 0.661 | 0.400 | 0.001 | 0.994 | 0.999 |
| HT × PT | 0.323 | 0.821 | 0.285 | 0.140 | 1.000 | 0.999 |
| Within subject contrasts | ||||||
| SP | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| SP × HT | 0.318 | 0.023 | 0.387 | <0.001 | <0.001 | 0.005 |
| SP × PT | 0.744 | 0.232 | 0.208 | 0.003 | 0.824 | 0.874 |
| SP × HT × PT | 0.319 | 0.733 | 0.038 | 0.876 | 0.852 | 0.997 |
| Groups | Diastase Activity | Hydroxymethylfurfural | Total Antioxidant Status | Total Oxidant Status | Oxidative Stress Index | Catalase | Total Phenolic Content |
|---|---|---|---|---|---|---|---|
| Honey type (HT) | |||||||
| Pine | 12.9 a | 36.8 b | 10.0 a | 86.5 | 0.90 | 104.0 a | 153.6 a |
| Flower | 8.2 b | 93.1 a | 7.2 b | 79.3 | 1.22 | 90.8 b | 97.7 c |
| Thyme | 7.0 c | 104.7 a | 9.6 a | 99.2 | 1.10 | 97.2 ab | 130.8 b |
| Packaging type (PT) | |||||||
| Tin cans | 9.3 | 85.9 | 8.9 | 87.2 | 1.05 | 94.9 | 124.6 |
| Light-colored glass | 9.4 | 75.8 | 9.3 | 88.5 | 1.02 | 94.0 | 129.5 |
| Dark-colored glass | 9.4 | 72.9 | 8.6 | 89.4 | 1.16 | 103.1 | 127.9 |
| Storage period (SP) | |||||||
| Initial | 12.9 a | 35.2 c | 10.7 a | 71.0 c | 0.67 c | 108.9 a | 163.4 a |
| 6 mo | 8.4 b | 93.0 b | 8.9 b | 86.2 b | 0.99 b | 97.6 b | 130.9 b |
| 12 mo | 6.8 c | 106.4 a | 7.3 c | 107.9 a | 1.57 a | 85.5 b | 87.7 c |
| SP × HT | |||||||
| Initial—Pine | 19.3 a | 12.9 d | 11.4 | 73.3 e | 0.64 e | 114.3 | 180.5 a |
| 6 mo—Pine | 11.1 b | 43.4 cd | 10.1 | 83.4 bcde | 0.85 de | 104.0 | 156.8 b |
| 12 mo—Pine | 8.2 c | 54.0 cd | 8.6 | 102.9 b | 1.21 c | 93.6 | 123.5 c |
| Initial—Flower | 12.1 b | 15.0 d | 9.3 | 64.4 e | 0.69 e | 101.8 | 136.0 c |
| 6 mo—Flower | 6.9 cd | 124.5 ab | 7.2 | 77.2 cde | 1.07 cd | 93.1 | 99.6 d |
| 12 mo—Flower | 5.4 e | 139.9 a | 5.2 | 96.5 bcd | 1.90 a | 77.6 | 57.3 e |
| Initial—Thyme | 7.2 cd | 77.6 bc | 11.4 | 75.2 de | 0.66 e | 110.6 | 173.7 ab |
| 6 mo—Thyme | 7.3 cd | 111.2 ab | 9.4 | 98.0 bc | 1.06 cd | 95.6 | 136.3 c |
| 12 mo—Thyme | 6.7 de | 125.3 ab | 8.1 | 124.4 a | 1.59 b | 85.3 | 82.4 d |
| SP × PT | |||||||
| Initial—Tin cans | 12.8 | 35.1 | 10.5 | 71.1 | 0.68 | 109.2 | 160.6 |
| 6 mo—Tin cans | 8.3 | 104.5 | 8.9 | 85.9 | 0.97 | 95.9 | 128.1 |
| 12 mo—Tin cans | 6.6 | 118.1 | 7.4 | 104.5 | 1.49 | 79.6 | 85.0 |
| Initial—Light-colored glass | 12.8 | 35.2 | 11.0 | 70.7 | 0.64 | 103.9 | 169.1 |
| 6 mo—Light-colored glass | 8.7 | 88.7 | 9.3 | 86.8 | 0.96 | 93.7 | 135.0 |
| 12 mo—Light-colored glass | 6.7 | 103.5 | 7.5 | 107.9 | 1.46 | 84.3 | 84.5 |
| Initial—Dark-colored glass | 12.9 | 35.2 | 10.6 | 71.0 | 0.67 | 113.5 | 160.5 |
| 6 mo—Dark-colored glass | 8.3 | 85.9 | 8.5 | 85.9 | 1.05 | 103.2 | 129.6 |
| 12 mo—Dark-colored glass | 7.0 | 97.6 | 6.9 | 111.4 | 1.75 | 92.7 | 93.7 |
| Overall Estimated mean ± SEM | 9.4 ± 0.2 | 78.2 ± 10.7 | 9.0 ± 0.2 | 88.4 ± 4.3 | 1.07 ± 0.05 | 97.3 ± 1.6 | 127.3 ± 3.3 |
| Significance | p | p | p | p | p | p | p |
| Between subject effects | |||||||
| HT | <0.001 | 0.040 | <0.001 | 0.187 | 0.053 | 0.013 | <0.001 |
| PT | 0.901 | 0.873 | 0.407 | 0.977 | 0.521 | 0.061 | 0.822 |
| HT × PT | 0.964 | 0.979 | 0.218 | 0.843 | 0.792 | 0.563 | 0.962 |
| Within subject contrasts | |||||||
| SP | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| SP × HT | <0.001 | 0.004 | 0.207 | 0.043 | 0.010 | 0.581 | 0.035 |
| SP × PT | 0.969 | 0.680 | 0.684 | 0.670 | 0.295 | 0.082 | 0.363 |
| SP × HT × PT | 0.995 | 0.881 | 0.381 | 0.284 | 0.355 | 0.422 | 0.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiğit, Y.; Yalçın, S.; Onbaşılar, E.E. Effects of Different Packaging Types and Storage Periods on Physicochemical and Antioxidant Properties of Honeys. Foods 2024, 13, 3594. https://doi.org/10.3390/foods13223594
Yiğit Y, Yalçın S, Onbaşılar EE. Effects of Different Packaging Types and Storage Periods on Physicochemical and Antioxidant Properties of Honeys. Foods. 2024; 13(22):3594. https://doi.org/10.3390/foods13223594
Chicago/Turabian StyleYiğit, Yusuf, Suzan Yalçın, and Esin Ebru Onbaşılar. 2024. "Effects of Different Packaging Types and Storage Periods on Physicochemical and Antioxidant Properties of Honeys" Foods 13, no. 22: 3594. https://doi.org/10.3390/foods13223594
APA StyleYiğit, Y., Yalçın, S., & Onbaşılar, E. E. (2024). Effects of Different Packaging Types and Storage Periods on Physicochemical and Antioxidant Properties of Honeys. Foods, 13(22), 3594. https://doi.org/10.3390/foods13223594







