Comparative Analysis of Nutrients, Phytochemicals, and Minerals in Colored Sweet Potato (Ipomoea batatas L.) Roots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
2.2. Reagents, Solvents, and Standards
2.3. Principal Nutrients, Dietary Fiber, and Resistant Starch
2.4. Soluble Sugars
2.5. β-Carotene Quantification
2.6. Determination of Caffeoylquinic Acids
2.7. Determination of Anthocyanins
2.8. Mineral Content
2.9. Quality Control
2.10. Statistical Analysis
3. Results
3.1. Nutrient Analysis of Sweet Potato Roots
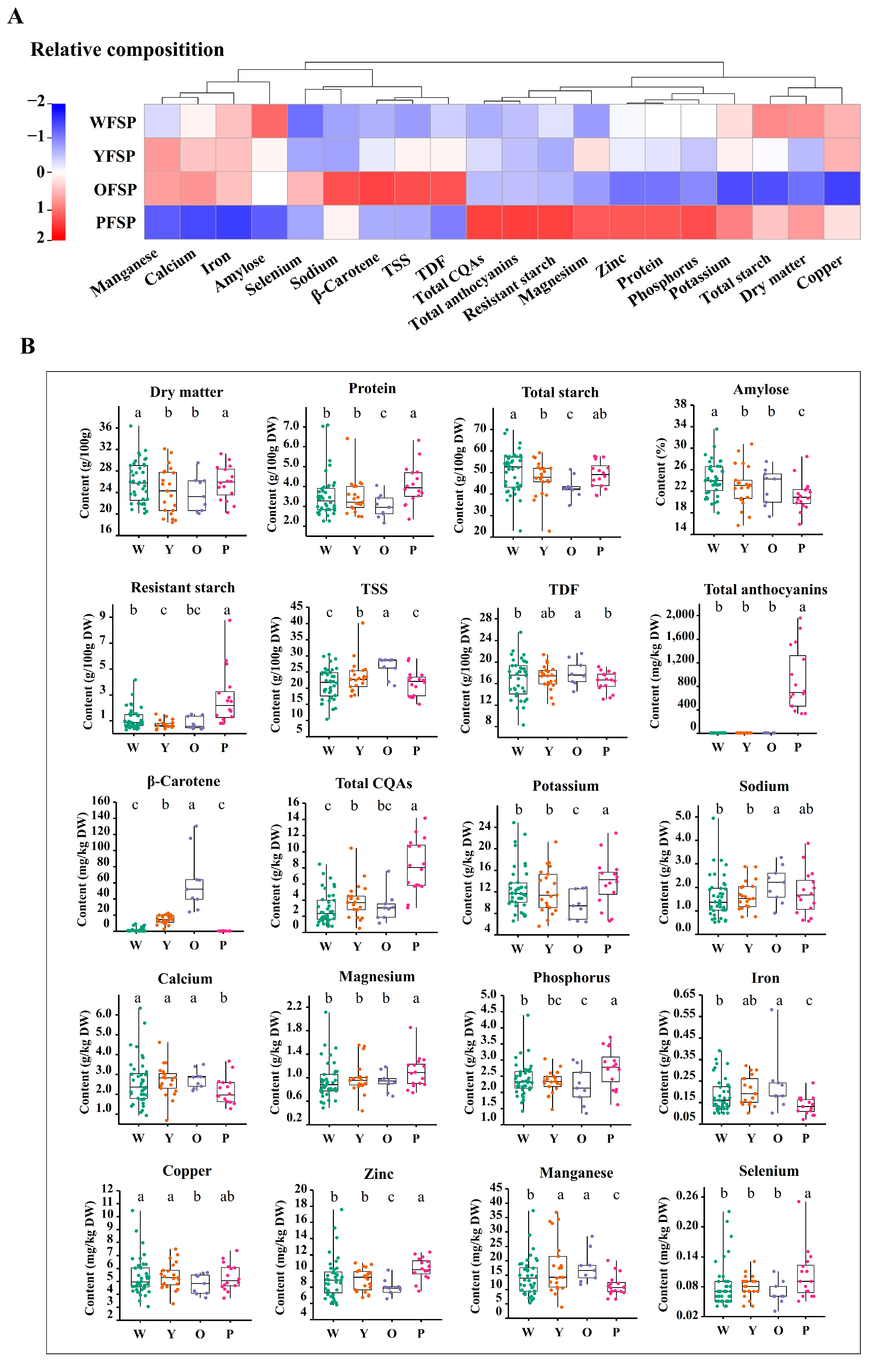
3.2. Comparative Analysis of Nutrients in Colored Roots
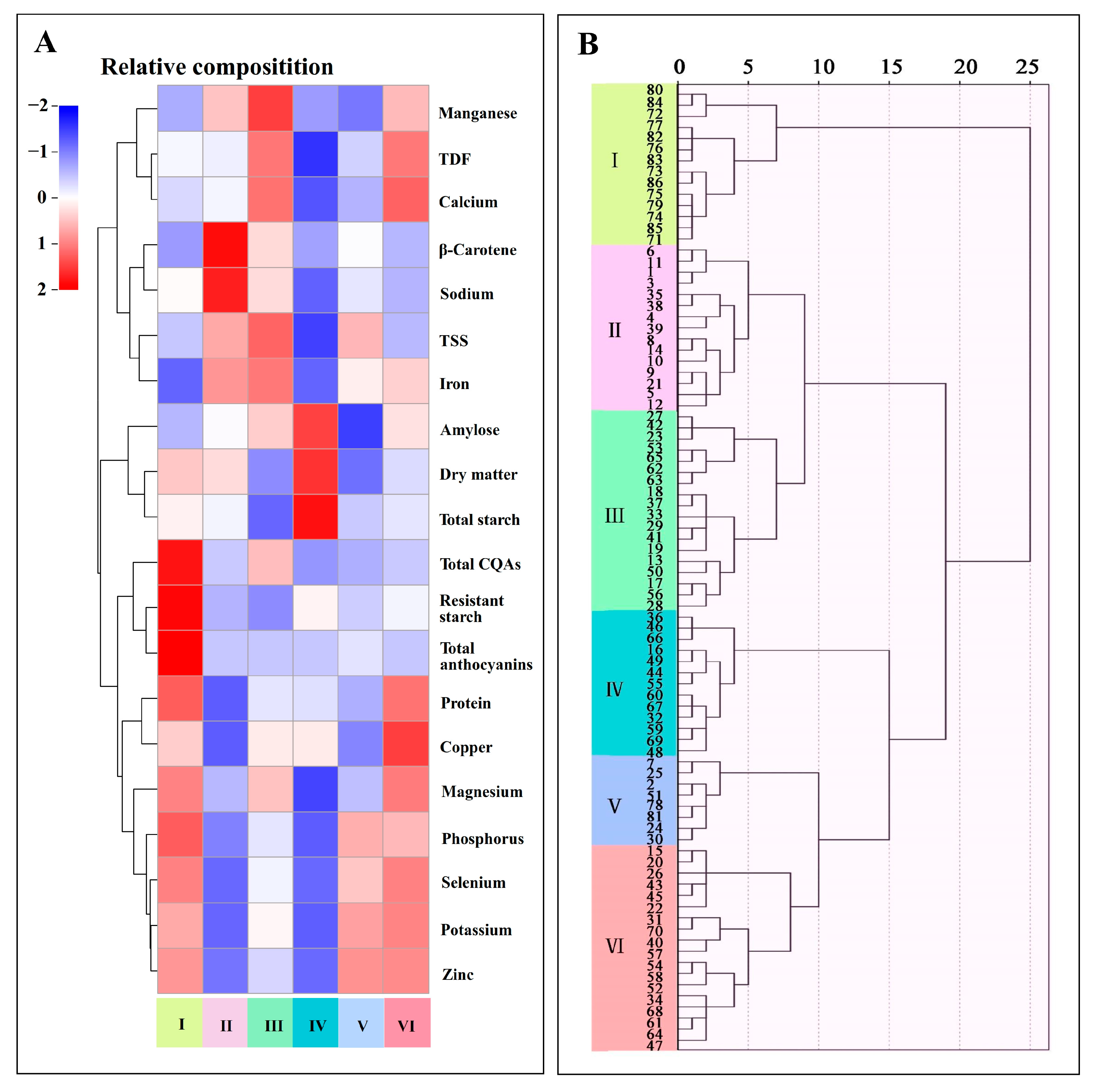
3.3. Cluster Analysis
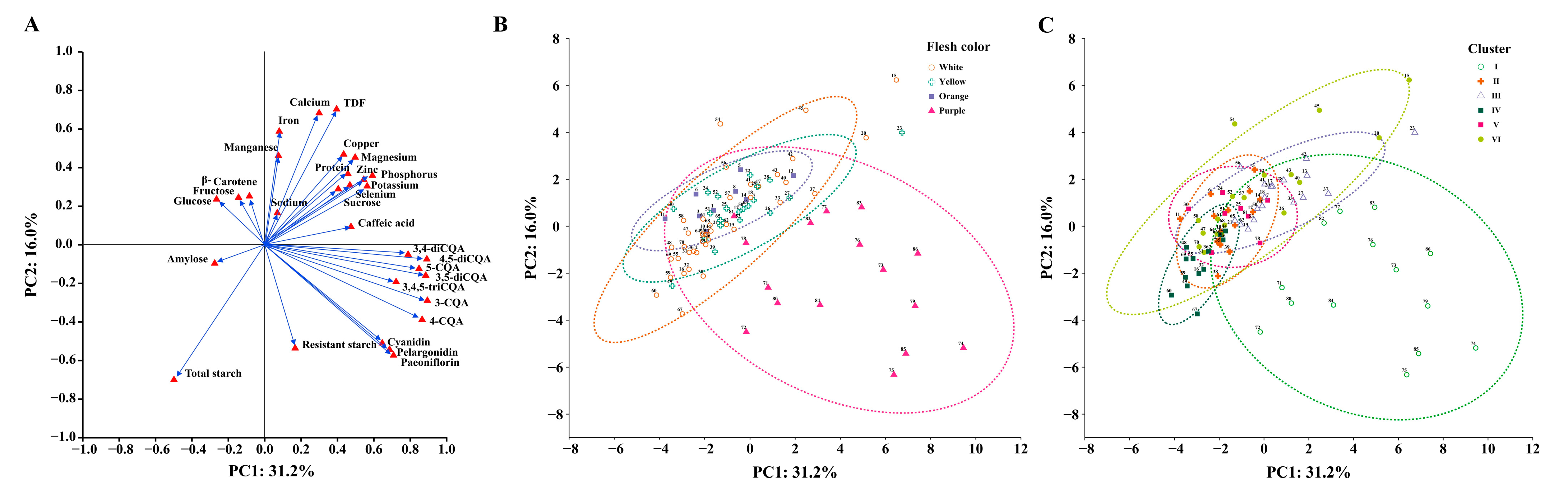
3.4. Principal Component Analysis
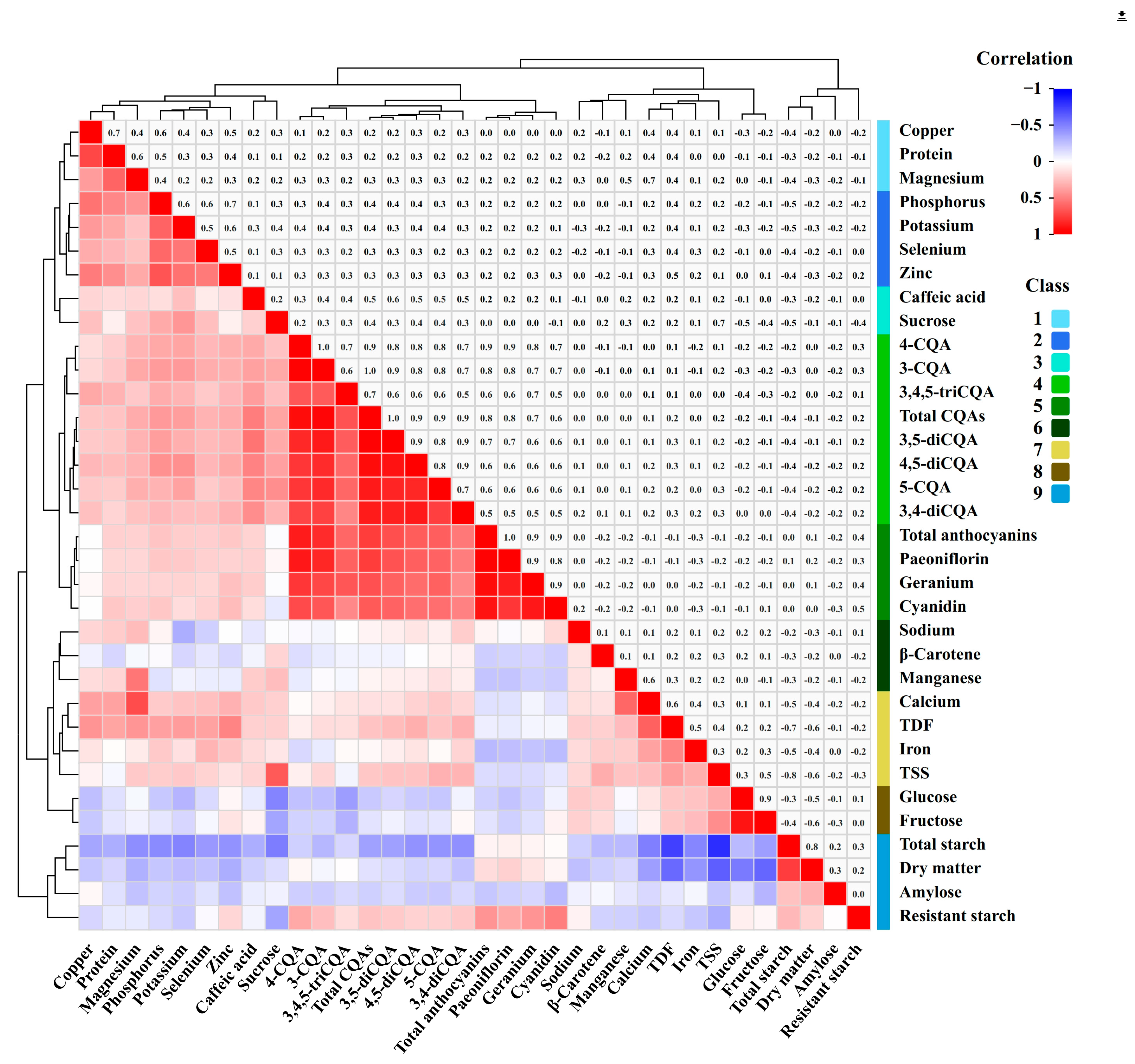
3.5. Correlation Analysisamongnutrients, Phytochemicals, and Minerals
4. Discussion
4.1. Nutrient Composition of Sweet Potato Roots
4.2. Comparative Analysis of Nutrients in Different Flesh-Colored Roots
4.3. Comprehensive Evaluation and Utilization of Sweetpotato Accessions
4.4. Correlation Study Among Nutrients, Phytochemicals, and Minerals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2022; Available online: http://faostat.fao.org (accessed on 8 November 2024).
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Yoshimoto, M. Physiological functions and utilization of sweet potato. In Sweet Potato: Post Harvest Aspects in Food, Feed and Industry; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 59–89. [Google Scholar]
- Katayama, K.; Kitahara, K.; Sakai, T.; Kai, Y.; Yoshinaga, M. Resistant and digestible starch contents in sweet potato cultivars and lines. J. Appl. Glycosci. 2011, 58, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Cartabiano Leite, C.; Porcu, O.; de Francisco, A. Sweet potato (Ipomoea batatas L. Lam) nutritional potential and social relevance: A review. Int. J. Eng. Res. Appl. 2020, 10, 23–40. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2016, 15, 2751–2761. [Google Scholar] [CrossRef]
- Rose, I.M.; Vasanthakaalam, H. Comparison of the nutrient composition of four sweet potato varieties cultivated in Rwanda. Am. J. Food Nutr. 2011, 1, 34–38. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.-D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.-Z.; Yang, X.; Ke, J.; Corke, H. Anthocyanins, hydroxycinnamic acid derivatives, and antioxidant activity in roots of different Chinese purple-fleshed sweetpotato genotypes. J. Agric. Food Chem. 2010, 58, 7588–7596. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, H.; Li, H.; Li, Y. Analysis on the nutrition composition and antioxidant activity of different types of sweet potato cultivars. Food Nutr. Sci. 2015, 6, 161–167. [Google Scholar] [CrossRef]
- Tumwegamire, S.; Kapinga, R.; Rubaihayo, P.R.; LaBonte, D.R.; Grüneberg, W.J.; Burgos, G.; Zum Felde, T.; Carpio, R.; Pawelzik, E.; Mwanga, R.O. Evaluation of dry matter, protein, starch, sucrose, β-carotene, iron, zinc, calcium, and magnesium in East African sweetpotato [Ipomoea batatas (L.) Lam] germplasm. HortScience 2011, 46, 348–357. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Deng, B.; Ru, W.; Tong, C.; Bao, J. Physicochemical, Nutritional, and Antioxidant Properties in Seven Sweet Potato Flours. Front. Nutr. 2022, 9, 923257. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.C.; Yada, B.; Tumuhimbise, G.A.; Amagloh, F.K.; Kaaya, A.N. The Potential of Sweetpotato as a Functional Food in Sub-Saharan Africa and Its Implications for Health: A Review. Molecules 2021, 26, 2971. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Analytical Chemists, 19th ed.; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Hoover, R.; Ratnayake, W. Determination of total amylose content of starch. Curr. Protoc. Food Anal. Chem. 2001, E2.3.1–E2.3.5. [Google Scholar] [CrossRef]
- Trancoso-Reyes, N.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Effect of pre-treatment on physicochemical and structural properties, and the bioaccessibility of β-carotene in sweet potato flour. Food Chem. 2016, 200, 199–205. [Google Scholar] [CrossRef]
- Galvao, A.; Nicoletto, C.; Zanin, G.; Vargas, P.F.; Sambo, P. Nutraceutical Content and Daily Value Contribution of Sweet Potato Accessions for the European Market. Horticulturae 2021, 7, 23. [Google Scholar] [CrossRef]
- Hosotani, K.; Kitagawa, M. Improved simultaneous determination method of β-carotene and retinol with saponification in human serum and rat liver. J. Chromatogr. B 2003, 791, 305–313. [Google Scholar] [CrossRef]
- Truong, V.D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef]
- Sun, S.; Guo, B.; Wei, Y.; Fan, M. Multi-element analysis for determining the geographical origin of mutton from different regions of China. Food Chem. 2011, 124, 1151–1156. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Oboh, S.; Ologhobo, A.; Tewe, O. Some aspects of the biochemistry and nutritional value of the sweet potato (Ipomea batatas). Food Chem. 1989, 31, 9–18. [Google Scholar] [CrossRef]
- Senanayake, S.A.; Ranaweera, K.K.; Gunaratne, A.; Bamunuarachchi, A. Comparative analysis of nutritional quality of five different cultivars of sweet potatoes (Ipomea batatas (L) Lam) in Sri Lanka. Food Sci. Nutr. 2013, 1, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhu, F. Physicochemical properties of whole sweetpotato flour. J. Sci. Food Agric. 2019, 99, 4624–4634. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef]
- Tang, C.; Han, J.; Chen, D.; Zong, S.; Liu, J.; Kan, J.; Qian, C.; Jin, C. Recent advances on the biological activities of purple sweet potato anthocyanins. Food Biosci. 2023, 53, 102670. [Google Scholar] [CrossRef]
- Montilla, E.C.; Hillebrand, S.; Butschbach, D.; Baldermann, S.; Watanabe, N.; Winterhalter, P. Preparative Isolation of Anthocyanins from Japanese Purple Sweet Potato (Ipomoea batatas L.) Varieties by High-Speed Countercurrent Chromatography. J. Agric. Food Chem. 2010, 58, 9899–9904. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties. Food Chem. 2009, 113, 1133–1138. [Google Scholar] [CrossRef]
- Joy, E.J.; Broadley, M.R.; Young, S.D.; Black, C.R.; Chilimba, A.D.; Ander, E.L.; Barlow, T.S.; Watts, M.J. Soil type influences crop mineral composition in Malawi. Sci. Total Environ. 2015, 505, 587–595. [Google Scholar] [CrossRef]
- Kurata, R.; Sun, H.-N.; Oki, T.; Okuno, S.; Ishiguro, K.; Sugawara, T. Chapter 7—Sweet potato polyphenols. In Sweet Potato; Mu, T.-H., Singh, J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 177–222. [Google Scholar]
- Franková, H.; Musilová, J.; Árvay, J.; Šnirc, M.; Jančo, I.; Lidiková, J.; Vollmannová, A. Changes in Antioxidant Properties and Phenolics in Sweet Potatoes (Ipomoea batatas L.) Due to Heat Treatments. Molecules 2022, 27, 1884. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, S.Y.; Yang, J.W.; Lee, J.-S.; Oh, S.-D.; Oh, S.; Lee, S.M.; Lim, M.-H.; Park, S.K.; Jang, J.-S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas L.) tubers. Food Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Mishra, D.; Buragohain, A.K.; Chakraborty, S.; Chakraborty, N. Comparative analysis of phytochemicals and nutrient availability in two contrasting cultivars of sweet potato (Ipomoea batatas L.). Food Chem. 2015, 173, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, M.; Tang, C.; Jiang, B.; Yao, Z.; Mo, X.; Wang, Z. Combining Metabolomics and Transcriptomics to Reveal the Mechanism of Coloration in Purple and Cream Mutant of Sweet Potato (Ipomoea batatas L.). Front. Plant Sci. 2022, 13, 877695. [Google Scholar] [CrossRef] [PubMed]
- Gemenet, D.C.; da Silva Pereira, G.; De Boeck, B.; Wood, J.C.; Mollinari, M.; Olukolu, B.A.; Diaz, F.; Mosquera, V.; Ssali, R.T.; David, M.; et al. Quantitative trait loci and differential gene expression analyses reveal the genetic basis for negatively associated β-carotene and starch content in hexaploid sweetpotato [Ipomoea batatas (L.) Lam.]. TAG. Theor. Appl. Genetics. Theor. Und Angew. Genet. 2020, 133, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, Z.; Tang, D.; Luo, K.; Lu, H.; Liu, Y.; Dong, J.; Wang, X.; Lv, C.; Wang, J.; et al. Comparative Transcriptome Analysis Reveals Critical Function of Sucrose Metabolism Related-Enzymes in Starch Accumulation in the Storage Root of Sweet Potato. Front. Plant Sci. 2017, 8, 914. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Jin, Y.; Li, W.; Zhang, L. Effects of phosphorus application on photosynthetic carbon and nitrogen metabolism, water use efficiency and growth of dwarf bamboo (Fargesia rufa) subjected to water deficit. Plant Physiol. Biochem. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Zaheer, A.; Malik, A.; Sher, A.; Mansoor Qaisrani, M.; Mehmood, A.; Ullah Khan, S.; Ashraf, M.; Mirza, Z.; Karim, S.; Rasool, M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019, 26, 1061–1067. [Google Scholar] [CrossRef]
- Saqib, M.N.; Tanver Rahman, M.R. Chapter 17—Phenolic acids. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 303–316. [Google Scholar]
- Desjardins, Y. 9—Physiological and ecological functions and biosynthesis of health-promoting compounds in fruit and vegetables. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Tomás-Barberán, F.A., Gil, M.I., Eds.; Woodhead Publishing: Sawston, UK, 2008; pp. 201–247. [Google Scholar]
- Jung, J.K.; Lee, S.U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
| No. | Accession | Flesh Color | Origin | Type |
|---|---|---|---|---|
| 1 | Zheshu851 | Orange | China | Modern cultivar |
| 2 | Huang16 | White | China | Local cultivar |
| 3 | Jihongrouhong | Orange | China | Local cultivar |
| 4 | Xiangshu15 | White | China | Modern cultivar |
| 5 | CA8 | Orange | China | Commercially available |
| 6 | Longshu9 | Orange | China | Modern cultivar |
| 7 | Quanshu830 | Yellow | China | Modern cultivar |
| 8 | Pushu32 | Orange | China | Modern cultivar |
| 9 | Fushu604 | Yellow | China | Modern cultivar |
| 10 | Funingshu12 | Orange | China | Modern cultivar |
| 11 | Chuancaishu211 | Orange | China | Modern cultivar |
| 12 | Beijing553 | Yellow | China | Modern cultivar |
| 13 | Chuanshu20 | Orange | China | Modern cultivar |
| 14 | Quanshu19 | Orange | China | Modern cultivar |
| 15 | ChuanH5 | White | China | Local cultivar |
| 16 | Jishu6-8 | White | China | Modern cultivar |
| 17 | Chao1li | White | China | Modern cultivar |
| 18 | Fushu23 | White | China | Modern cultivar |
| 19 | Jishu25 | White | China | Modern cultivar |
| 20 | Yongchunwuchi | White | China | Local cultivar |
| 21 | Shengnan | Yellow | China | Modern cultivar |
| 22 | Fushu76 | Yellow | China | Modern cultivar |
| 23 | CA2 | Yellow | China | Commercially available |
| 24 | Chaoshu1 | Yellow | China | Modern cultivar |
| 25 | Guangcaishu5 | Yellow | China | Modern cultivar |
| 26 | Jishu19-40 | Yellow | China | Modern cultivar |
| 27 | Fushu22 | Yellow | China | Modern cultivar |
| 28 | Quanshu17 | Yellow | China | Modern cultivar |
| 29 | Fushu18 | Yellow | China | Modern cultivar |
| 30 | Guangcaishu2 | Yellow | China | Modern cultivar |
| 31 | Ningshu10 | White | China | Modern cultivar |
| 32 | Xushu18 | White | China | Modern cultivar |
| 33 | Ganshu9 | White | China | Modern cultivar |
| 34 | Jiangnanshao | White | China | Local cultivar |
| 35 | Qingpizhong | White | China | Local cultivar |
| 36 | Suining16 | White | China | Modern cultivar |
| 37 | Guangcaishu6 | White | China | Modern cultivar |
| 38 | Xichengshu007 | White | China | Modern cultivar |
| 39 | CA4 | Yellow | China | Commercially available |
| 40 | 3-15-53 | White | China | Modern cultivar |
| 41 | 2-16 | White | China | Modern cultivar |
| 42 | Caishu5 | White | China | Modern cultivar |
| 43 | P553 | White | China | Modern cultivar |
| 44 | Ganshu22 | White | China | Modern cultivar |
| 45 | SH5 | White | China | Modern cultivar |
| 46 | CA12 | White | China | Commercially available |
| 47 | CA7 | White | China | Commercially available |
| 48 | Longshu599 | White | China | Modern cultivar |
| 49 | CA6 | Yellow | China | Commercially available |
| 50 | 3-14-52 | Yellow | China | Modern cultivar |
| 51 | Jishu26 | Yellow | China | Modern cultivar |
| 52 | Jinguahuang | Yellow | China | Local cultivar |
| 53 | CA5 | Yellow | China | Commercially available |
| 54 | Sanguishu15 | White | China | Modern cultivar |
| 55 | Liushiri | White | China | Local cultivar |
| 56 | CA13 | White | China | Commercially available |
| 57 | SH3 | White | China | Modern cultivar |
| 58 | Xiangshu1 | White | China | Modern cultivar |
| 59 | Jishu8088 | White | China | Modern cultivar |
| 60 | 2-190 | White | China | Local cultivar |
| 61 | Xushu27 | White | China | Modern cultivar |
| 62 | 3-24 | White | China | Local cultivar |
| 63 | Nanshu88 | Yellow | China | Modern cultivar |
| 64 | Chuanshu383 | White | China | Modern cultivar |
| 65 | CA11 | Yellow | China | Commercially available |
| 66 | Chuanshu217 | White | China | Modern cultivar |
| 67 | CA9 | White | China | Commercially available |
| 68 | Chuanshu211 | White | China | Modern cultivar |
| 69 | Baiguqilong | White | China | Local cultivar |
| 70 | Longshu14 | White | China | Modern cultivar |
| 71 | Hainanzishu | Purple | China | Modern cultivar |
| 72 | CA3 | Purple | China | Commercially available |
| 73 | Fushu404 | Purple | China | Modern cultivar |
| 74 | Xuzishu9 | Purple | China | Modern cultivar |
| 75 | Xuzishu8088 | Purple | China | Modern cultivar |
| 76 | Rizi7 | Purple | Japan | Introduced cultivar |
| 77 | Ribenzi | Purple | Japan | Introduced cultivar |
| 78 | Zisehongshao | Purple | China | Modern cultivar |
| 79 | Xuzishu201 | Purple | China | Modern cultivar |
| 80 | Wanzishu56 | Purple | China | Modern cultivar |
| 81 | Xuzishu7 | Purple | China | Modern cultivar |
| 82 | Chuanzishu4 | Purple | China | Modern cultivar |
| 83 | CA10 | Purple | China | Commercially available |
| 84 | Chuanzishu3 | Purple | China | Modern cultivar |
| 85 | Rizishu9 | Purple | Japan | Introduced cultivar |
| 86 | 1-24 | Purple | China | Modern cultivar |
| Nutrients | Range | Mean | SD | Coefficient of Variation (CV) % |
|---|---|---|---|---|
| Dry matter g/100 g | 18.0–36.5 | 25.2 | 3.8 | 15.2 |
| Protein g/100 g DW | 2.15–7.11 | 3.55 | 1.00 | 28.3 |
| Total starch g/100 g DW | 22.6–69.7 | 48.5 | 8.3 | 17.1 |
| Amylose 1 % | 15.3–34.7 | 23.2 | 3.5 | 15.0 |
| Resistant starch g/100 g DW | 0.254–9.12 | 1.29 | 1.30 | 102 |
| Fructose g/100 g DW | 0.512–10.6 | 3.53 | 1.87 | 53.0 |
| Glucose g/100 g DW | 0.585–10.6 | 4.08 | 2.26 | 55.5 |
| Sucrose g/100 g DW | 2.75–36.2 | 14.7 | 4.9 | 33.2 |
| TSS 2 g/100 g DW | 10.3–40.0 | 22.3 | 4.8 | 21.3 |
| TDF 3 g/100 g DW | 7.99–26.0 | 16.9 | 2.9 | 17.0 |
| Cyanidin mg/kg DW | 0–708 | 61.3 | 150.1 | 245 |
| Paeoniflorin mg/kg DW | 0–1466 | 106 | 288 | 272 |
| Pelargonidin mg/kg DW | 0–23.3 | 1.24 | 3.58 | 288 |
| Total anthocyanins mg/kg DW | 0–2003 | 168 | 419 | 249 |
| β-Carotene mg/kg DW | 0–133 | 10.5 | 21.8 | 207 |
| 5-CQA 4 g/kg DW | 0–0.850 | 0.182 | 0.143 | 78.4 |
| 3-CQA g/kg DW | 0.0922–5.69 | 1.17 | 1.06 | 90.6 |
| 4-CQA g/kg DW | 0–0.878 | 0.148 | 0.157 | 106 |
| Caffeic acid g/kg DW | 0.0220–0.678 | 0.177 | 0.100 | 56.4 |
| 3,4-diCQA g/kg DW | 0.0536–2.02 | 0.569 | 0.423 | 74.5 |
| 3,5-diCQA g/kg DW | 0.131–4.04 | 1.39 | 0.97 | 69.6 |
| 4,5-diCQA g/kg DW | 0.0516–1.37 | 0.399 | 0.272 | 68.1 |
| 3,4,5-triCQA g/kg DW | 0–0.979 | 0.120 | 0.135 | 112 |
| Total CQAs g/kg DW | 0.478–14.2 | 4.16 | 2.98 | 71.7 |
| Potassium g/kg DW | 5.56–24.8 | 12.4 | 4.2 | 33.6 |
| Sodium g/kg DW | 0.492–4.95 | 1.68 | 0.84 | 49.9 |
| Calcium g/kg DW | 0.638–6.33 | 2.56 | 0.95 | 37.2 |
| Magnesium g/kg DW | 0.373–2.16 | 0.981 | 0.284 | 29.0 |
| Phosphorus g/kg DW | 1.32–4.40 | 2.42 | 0.52 | 21.6 |
| Iron g/kg DW | 0.0731–0.593 | 0.186 | 0.081 | 43.8 |
| Copper mg/kg DW | 3.03–10.4 | 5.29 | 1.21 | 22.8 |
| Zinc mg/kg DW | 5.69–17.6 | 9.11 | 2.05 | 22.5 |
| Manganese mg/kg DW | 3.72–37.3 | 15.0 | 7.1 | 47.4 |
| Selenium mg/kg DW | 0.0294–0.247 | 0.0831 | 0.0399 | 48.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Zhong, L.; Li, X.; Qin, L.; Zhou, Y.; Lei, X.; Zheng, X.; Jin, K.; Pu, Z.; Hou, X.; et al. Comparative Analysis of Nutrients, Phytochemicals, and Minerals in Colored Sweet Potato (Ipomoea batatas L.) Roots. Foods 2024, 13, 3636. https://doi.org/10.3390/foods13223636
Zhao S, Zhong L, Li X, Qin L, Zhou Y, Lei X, Zheng X, Jin K, Pu Z, Hou X, et al. Comparative Analysis of Nutrients, Phytochemicals, and Minerals in Colored Sweet Potato (Ipomoea batatas L.) Roots. Foods. 2024; 13(22):3636. https://doi.org/10.3390/foods13223636
Chicago/Turabian StyleZhao, Shan, Lingli Zhong, Xi Li, Lin Qin, Ya Zhou, Xinyu Lei, Xingguo Zheng, Keting Jin, Zhigang Pu, Xue Hou, and et al. 2024. "Comparative Analysis of Nutrients, Phytochemicals, and Minerals in Colored Sweet Potato (Ipomoea batatas L.) Roots" Foods 13, no. 22: 3636. https://doi.org/10.3390/foods13223636
APA StyleZhao, S., Zhong, L., Li, X., Qin, L., Zhou, Y., Lei, X., Zheng, X., Jin, K., Pu, Z., Hou, X., Song, J., Lang, T., Zhang, C., & Feng, J. (2024). Comparative Analysis of Nutrients, Phytochemicals, and Minerals in Colored Sweet Potato (Ipomoea batatas L.) Roots. Foods, 13(22), 3636. https://doi.org/10.3390/foods13223636






