Abstract
Steam explosion (STEX) of peel from commercially juice-extracted oranges was used to convert peel pectin into pectic oligosaccharides (POSs). Surprisingly uniform populations, based on the polydispersity index (PDI; weight-average molecular weight (Mw)/number-average molecular weight (Mn)) of POSs, were obtained from the Hamlin and Valencia varieties of Citrus sinensis. The POSs from Hamlin and Valencia peel had PDI values of (1.23 ± 0.01, 1.24 ± 0.1), respectively. The Mw values for these samples were 14.9 ± 0.2 kDa for Hamlin, and 14.5 ± 0.1 kDa for Valencia, respectively. The degree of methyl-esterification (DM) was 69.64 ± 3.18 for Hamlin and 65.51 ± 1.61 for Valencia. The composition of the recovered POSs was dominated by galacturonic acid, ranging from 89.1% to 99.6% of the major pectic sugars. Only the Hamlin sample had a meaningful amount of rhamnose present, indicating the presence of an RG I domain. Even so, the Hamlin sample’s degree of branching (DBr) was very low (2.95).
Keywords:
pectin; polysaccharide; pectic oligosaccharide; citrus; steam explosion; valorization; value added 1. Introduction
Pectin is a nearly ubiquitous polysaccharide found in plant primary cell walls and middle lamellae [1,2,3]. It is composed primarily of three major domains: homogalacturonan (HG), rhamnogalacturonan I (RG I), and rhamnogalacturonan II (RG II) [4,5,6,7]. HG is a linear homopolymer of a-1,4 linked D-galacturonic acid (GalA) with a degree of polymerization (number of contiguous GalA subunits) generally ranging between 80 and 120 GalAs [8]. A variable proportion of the GalA subunits may be methyl-esterified at the C6 position. The percentage of methyl-esterified GalAs is defined as the degree of methyl-esterification (DM). RG I has a backbone composed of a repeating dimer of GalA and rhamnose (Rha), with neutral sugar side branches of galactans, arabinans, or arabinogalactans off of Rha subunits. RG II is a relatively minor component, but functionally significant [7]. Other, less common, domains are apiogalacturonan and xylogalacturonan [9].
Pectin has biological functions that mirror many of its technological applications. In plant cells, pectin functions include cell–cell adhesion, structural support, hydration control, the positioning of leaf and floral primordia, and acting as signaling molecules in plant biochemical pathways [4,6,10]. Pérez et al. [11] described three-dimensional models of specific pectin structural domains, but a three-dimensional model of its global in vivo structure has not been obtained.
Commercially, pectin’s dominant use is in the food industry, where it is utilized for its functional properties related to gelation, thickening, stabilizing, and emulsification [12,13,14]. More recently, its applications have been expanding into other markets related to health [14]. In many of these markets, it is fragments of pectin (pectic oligosaccharides, or POSs, and modified citrus pectin) that have been reported to have biological activity or pharmaceutical properties [15,16,17,18,19]. The potential applications that have been investigated include uses as prebiotics [16,18,20,21,22,23,24,25,26], apoptosis induction of colon and prostate cancer cells [15,27,28], galectin-3 antagonist during cancer metastasis, fibrosis reduction and heavy metal detoxification [29], and the controlled release of pharmaceuticals [30,31], among others [32]. POSs have also been shown to act as drug delivery excipients [31], and as elicitors for plant defense responses [4,33,34], among others. Udchumpisai et al. [35] demonstrated that POSs can biostimulate rice seed growth activation and that the larger POSs have a positive effect on growth and metabolism.
A variety of methods have been reported for the production of POSs [16,17,18,19,20,21,22,23,24,25,26,36]. STEX was chosen because it can be operated as a continuous process and the pectin recovery is equivalent to the amount of pectin present in raw peel as determined by enzymatic digestion [37]. In this work, data are provided on the major pectic sugar composition of the recovered POSs produced via STEX and their macromolecular and structural properties, including weight fraction (%), weight-average molecular weight (Mw), the calculated poly-dispersity index (PDI, Mw/Mn), intrinsic viscosity (η), radius of gyration, Mark–Houwink–Sakurada exponent values (a), degree of polymerization (DP), degree of methyl-esterification (DM), and degree of branching in RG I (DBr).
2. Materials and Methods
2.1. Static Steam Explosion
Fresh, juice-extracted orange peel (Citrus sinensis var. Hamlin and Valencia) was obtained from a local juice processor in Ft. Pierce, FL, USA. Juice extraction was accomplished using Brown International Juice Extractors (Winter Haven, FL, USA); consequently, the peel was obtained as halved fruit. Hamlin peel was obtained on 24 January 2022, and Valencia peel was obtained on 10 March 2022 (Valencia 1) and 26 April 2022 (Valencia 2). The initial size reduction was accomplished using two passes through the blades of a Fitz-Mill fitted with no screen (Model D-S6, Fitzpatrick Company, Westwood, MA, USA). Further size reduction was completed using a Robot-Coupe (Model R 23, Robot-Coupe, S.N.C., Vincennes Cedex, France) food processor for 15 s. A total of 500 g of the size-reduced peel was added to a clean bucket containing 5 L of deionized water. This slurry was added to the clean Robot-Coupe for an additional 15 s homogenization. Approximately 600 g of this mixture was weighed into a beaker and brought to a pH of 2 using 6 N HCl. It was then placed into the vertical pipe of a static steam explosion system (Figure S1) and held at 140 °C for 30 min (~50 psi). After pressure release, the sample was collected and stored at 4 °C until pectin recovery took place, no longer than 2 h.
2.2. Pectin Recovery
The sample cooled after the steam explosion was vacuum-filtered (Glass Microfiber Filter (125 mm diameter, Whatman GF/F, GE Healthcare, Life Sciences, Malbrough, MA, USA) and the filtrate pH was adjusted to 2 using 6 N HCl. The solution was then precipitated using 95% ethanol to bring the final concentration to 55%. The sample was precipitated overnight, and covered, at room temperature. The sample was then centrifuged at 12,000 rpm for 20 min (Model Avanti JE, Beckman Coulter, Inc. Brea, CA, USA). The pellet was washed with 57% ethanol and centrifuged again under the same conditions. The pellet was stored at −20 °C before it was lyophilized (FreeZone Freeze Dry System; Labconco, Kansas City, MO, USA), or stored at −20 °C until ready for lyophilization.
2.3. Compositional Analysis of Recovered Pectin
The compositional analysis of sugars (rhamnose, arabinose, galactose, glucose, xylose, fructose, sucrose, cellobiose, and galacturonic acid) was determined by hydrolysis of 2 mg of the freeze-dried recovered pectin sample diluted in 2 mL of DI water with 43.2 μL of two pectinases (DSM, PAC, Batch 16B04V1, pectinase activity, 49.43 U mL−1, and Rapidase PNS, pectinase activity, 58.29 U mL−1), 21.6 μL cellulase (Novozyme, Cellic CTec2, VCPI0003, cellulase activity, 208.21 FPU mL−1), and 21.6 μL β-glucosidase (Novozyme 188, DCN00205, β-glucosidase activity, 270.67 U mL−1) enzymes with rotation for 24 h at 45 °C. To prevent microbial growth, 6 μL of cycloheximide (10 mg ml−1 stock) were added. Samples were then filtered, using a 0.45 μm GD/X Nylon syringe filter (Whatman, Cytiva, Marlborough, MA, USA) to remove insoluble solids prior to analysis. Sugars were quantified and identified by direct high-performance ion exchange chromatography (HPIEC) using a Dionex CarboPac PA-1 pellicular anion-exchange column (4 × 250 mm) and pre-column (4 × 50 mm) as described previously [38] with some modification. Specifically, the percent of each buffer used, time, and flow for the sugar analysis can be seen in Table S1. The waveform method and temperature settings were input manually, using the Antec Decade Elite system digital display. Data collection and analysis were completed using Agilent OpenLab CDS Chemstation Rev C. The samples were analyzed in triplicate.
2.4. Macromolecular Characterization of Recovered Pectin
Pectin (Hamlin and Valencia 1) samples were prepared in 0.05 M NaNO3/0.01% NaN3 and stirred overnight at room temperature, and a 0.45 μm filter was used to filter the solutions (Millex-HV, PVDF, Millipore Corp., Billerica, MA, USA). The delivery system consisted of a model 1260 series pump, Infinity II degasser, multicolumn thermostat MCT, and auto-sampler (Agilent Technologies, Waldbronn, Germany). Two biocompatible inline solvent filter assemblies, PEEK/SS, 0.5 μm (Cole-Parmer, Burlington, NJ, USA), were placed before and after three size-exclusion columns (TSK GMPWxl, 7.8 × 300 mm, 13 μm particle size, Tosoh Bioscience, Tokyo, Japan). The injection volume was 100 μL and the flow rate was set at 0.7 mL/min. The column set was heated at 35 °C. The Dawn Ambient-D3 multi-angle laser light-scattering photometer (MALLS, Wyatt Technology, Santa Barbara, CA, USA), ViscoStar-V4 differential pressure viscometer (DPV), 1728-TREX differential refractive index (RI, Wyatt Technology, Santa Barbara, CA, USA), and a UV-1260 Infinity spectrophotometer (UV, Agilent Technologies, Waldbronn, Germany) were connected in series. The detectors were aligned with bovine serum albumen (Sigma-Aldrich, St Louis, MO, USA). A narrow-monodispersed Pullulan 50 standard was used to normalize the MALLS 18-angle detector (P-82, JM Science, Grand Island, NY, USA). Astra software (Ver. 8.1.1.12) was used for collecting and analyzing the chromatograms (Wyatt Technology, Santa Barbara, CA, USA). The refractive index increment (dn/dc) value of 0.132 was used. The samples were analyzed in triplicate.
The DM was determined as described by Cameron et al. [39] using a modified titration method found in the United States Pharmacopeia [40]. Four replicates from each sample were performed.
Pectin architecture was explored by using the major pectic sugar composition and DM values. GalA/Rha ratios and the degree of branching (DBr) [41] were calculated. The ratio of GalA to Rha was calculated as a hypothetical representation of the HG/RG I ratio within the pectin samples [42,43]. DBr was calculated as the ratio of Gal + Ara/Rha. DM was used to calculate the average MW of a GalA within the HG domain using the formulas below, where 18.00 Daltons are lost with the water loss in condensation and 32.04 Daltons are added for the addition of a methyl-ester. Estimating the average MW of a GalA allowed us to estimate the DP of the HG domains in the POS from each sample based on the estimated Mw.
2.5. Data Analysis
Statistical analyses were performed using the one-way ANOVA (p < 0.05) function of Microsoft Excel (version 2304) and Tukey’s HSD (honestly significant difference) test at a confidence level of p < 0.05 was performed for multiple comparisons using JMP Pro Statistical Software Version 16 (SAS Institute Inc., Cary, NC, USA).
3. Results and Discussion
3.1. Pectin Yield and Sugar Composition
The highest pectin recovery was seen with the Hamlin sample, and the lowest was in the Valencia 2 sample, while Valencia 1 pectin recovery was similar to Hamlin (Table 1). Average temperatures and pressures within the STEX vessel were very similar in each run. The pectin recovery was similar to the injected steam extraction of Valencia orange pectin for 6 min in pH 2 HCl at 120 °C, 15 psi [44].

Table 1.
Pectin yield obtained from STEX of Hamlin and Valencia juice-extracted orange peel.
The pectin sugar composition (Table 2) indicated that GalA was the dominant sugar in each sample, ranging from 89.15% in the Hamlin sample to 99.61 and 96.59 in Valencia 1 and Valencia 2, respectively. Only the Hamlin sample appeared to contain any RG I component, with a GalA/Rha ratio of 20.36 and a DBr of 2.95. For Valencia 1, the GalA/Rha ratio was 190.79, with a minimal DBr of 0.51, and no Gal was detected. No Rha was detected in the Valencia 2 sample although minimal amounts of Gal and Ara were detected. Since it is possible that the sugar composition analysis enzymes did not hydrolyze the rhamnogalacturonan I, we repeated the monosaccharide analysis using methanolysis [21] and found similar results (Hamlin: 82.57% GalA, 7.59% Xyl, 4.76% Glc, 2.38% Gal, 1.22% GlcA, 1.08% Ara, 0.37% Rha; Valencia 1: 99.1% GalA, 0.23% Xyl, 0.21% Ara, 0.18% Gal, 0.15% GlcA, 0.07% Rha, 0.07% Glc); Valencia 2: 98.08% GalA, 0.74% Ara, 0.62% Gal, 0.17% GlcA, 0.16% Xyl, 0.15% Rha, 0.07% Glc. Therefore, the STEX process produced a homogalacturonan structure by preferentially hydrolyzing the rhamnogalacturonan I domains of pectin that are known to be less acid-resistant [45]. The combination of homogenization and steam explosion may have been similar to high-pressure homogenization that depolymerized citrus pectin [46] and increased the uronic acid content of carrot pectin [47]. STEX POSs have similar sugar composition to POS1 and modified citrus pectin, which are reported to have anti-adhesive activity for Shiga toxin-producing Eschericia coli [25]. In previous reports of orange peel POSs with prebiotic properties, the POSs had high levels of arabinose and relatively lower levels of galacturonic acid compared to the STEX POSs [21,24,25].

Table 2.
The average (Mean) and standard error (SE) of the major pectic sugars given in percent dry weight (% dw) and descriptors of pectin architecture (GalA/Rha, DBr (GalA + Ara/Rha)). Percent GalA (%GalA) was the percentage of the total sample that was GalA. The percentage of the major pectin sugars (%) was calculated using Equation (6). ND = Not Detected.
3.2. Macromolecular Characterization and Architecture
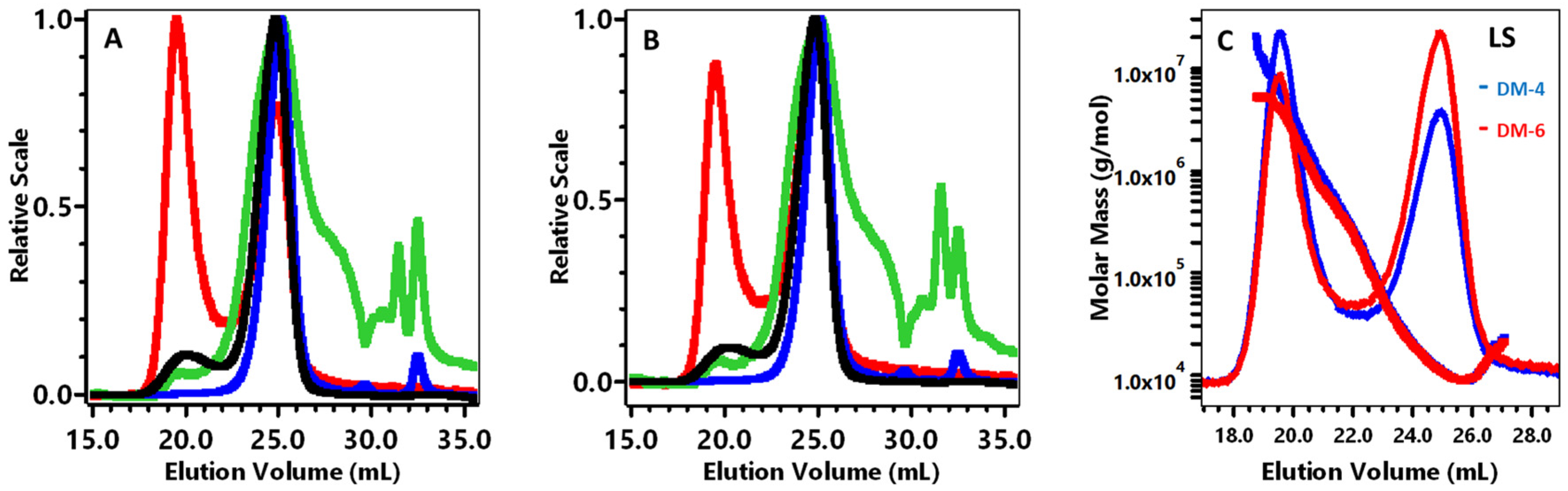
Representative HPSEC-MALLS chromatograms for each sample demonstrated a bimodal POS peak (Figure 1A,B, light scattering), with peak 1 eluting 18.7–22.1 mL, which represented 1% of an integrated peak area of the sample, and the major peak 2 eluting 22.1–26.6 mL, which represented 99% of the sample for both Hamlin and Valencia 1 (Table 3).
Figure 1.
High-performance size-exclusion chromatography analysis of the Hamlin (DM-D4) (A) and Valencia (DM-D6) (B) varieties; superimposed calibration curve of Hamlin and Valencia (DM-D4, DM-D6) (C). In Figure 1A,B, HPSEC detectors were light scattering at 90 °C (-), differential pressure viscometer (-), refractive index (-), and ultraviolet absorption at 280 nm (-). In Figure 1C, HPSEC detectors were light scattering at 90 °C, (DM-D4) and HPSEC detectors were light scattering at 90 °C, DM-D6.

Table 3.
The polydispersity (Mw/Mn), weight-average molecular weight (Mw), intrinsic viscosity (η), radius of gyration (Rgz), and Mark–Houwink–Sakurada exponent (a) of Hamlin and Valencia as studied by HPSEC. The total area values are the average of a triplicate set of RI measurements ± standard deviations.
The polydispersity index is defined as Mw/Mn and used to determine the broadness of molecular weight distribution as demonstrated in Table 3. The total chromatogram (TC) including all peak areas 18.7–26.6 mL, had an Mw/Mn of 2.52 for Hamlin and 2.14 for Valencia 1. Compared to peak 2, the peak 1 polydispersity was much broader, the molar mass (Mw) was much higher, and the Mark–Houwink constant (a) was more compact, which indicated pectin aggregation. In contrast, the peak 2 Mw and intrinsic viscosity (η) for both samples were the same (Table 3, Figure 1C). The peak 2 14 kDa Mw was much lower than the 367–414 kDa Mw for orange pectin that was acid extracted by other methods [22,28], which indicated that depolymerization occurred during STEX. The radius of gyration (Rg) (Table 3) and UV chromatograms (Figure 1A,B) for both samples were similar. A comprehensive study described the conformation of polysaccharides with the Mark–Houwink–Sakurada exponent [48,49]. The M-H exponent (a) for Hamlin and Valencia 1 in this experiment indicated a random coil shape molecule. Pectin with a random coil shape was reported to bind to anthocyanins with anti-oxidant properties [50]. Hellin et al. [51] used commercial lime pectin de-esterified by 0.1 M NaOH at 4 °C and hydrolyzed with 0.1 M HCL at 80 °C for 72 h, that revealed a bimodal light-scattering peak with the first peak reported as aggregates. The main peak 2 was reported to possess values of 18.5 kDa Mw and 0.75 dL/g η, which were higher than the Hamlin and Valencia STEX peak 2 values. However, their α was 0.87, which agreed with the random coil shape that we observed for Hamlin and Valencia STEX peak 2.
Singh and Tingirikari [26] reported a wide range of sizes for POSs, from a degree of polymerization (DP) of 1–10, with Mw up to 4000 kDa, depending on the production method. Cano et al. [24] also reported a wide range of Mw ranging from 1200 to 3600 kDa obtained from citrus fruit peel. Much smaller POSs were reported by Di et al. [25], with Mw ranging between 9.2 and 811 kDa. The arabinose-rich POS was the most bifidogenic, while a lower molecular weight and de-methyl-esterification enhanced anti-adhesive activity against E. coli O157:H7 binding to human HT29 cells [25].
Single-factor ANOVA of Mn, Mw, polydispersity, and (η) (Table 3) demonstrated significant differences between the means (F = 553, p-value = 1.57−7; F = 456.8, p-value = 2.77−7; F = 300.45, p-value = 9.66−7, and F = 12,757.5, p-value = 1.30−11, respectively). All samples could be considered high-DM pectins (>50%; Table 4). No significant difference was found for the DMs of the samples (Table 4, F = 1.05, p-value = 0.3893). Based on the estimated DM, Mw, and the calculations from Formulas (1)–(5) we estimated the degree of polymerization (DP) for each of the POSs. Hamlin was the largest with a DP of 51.8. Valencia 1 had a DP of 33.1, and Valencia 2 had a DP of 27.7. Since the POSs are mainly homogalacturonan, they are oligogalacturonic acids with DPs of up to 50 galacturonic acid residues detected with HPAEC-PAD [52]. The HPAEC-PAD method can also resolve individual malto-oligosaccharides up to 70 glucose residues [53], which expands the traditional oligosaccharide definition beyond DP 10. Therefore, our POS DP calculations appear to be reasonable.

Table 4.
Degree of methyl-esterification (DM) of extracted pectins. SD = Standard deviation, SE = standard error of the mean. Averages with the same superscript are not significantly different.
4. Conclusions
Steam explosion of commercial orange peel from Hamlin and Valencia oranges produced pectic oligosaccharides and polysaccharides with homogalacturonan composition, high degree of esterification, low molar mass, and a random coil shape. Acidic steam explosion extraction provides a rapid, continuous method that preferentially hydrolyzes the pectin rhamnogalacturonan I domain during POS preparation. The application of these pectic oligosaccharides remains to be investigated, but their structure and composition suggests that they may be able to prevent the adhesion of pathogenic Shiga toxin-producing Eschericia coli and bind anthocyanins with anti-oxidant properties. Orange peel POSs represent a potentially valuable co-product of orange juice manufacturing with functional food properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13233738/s1, Figure S1. Static steam explosion system; Table S1. HPLC method time, buffer concentration and flow.
Author Contributions
T.-A.M., formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization; K.L.F., conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—review and editing, funding acquisition; R.G.C., conceptualization, methodology, formal analysis, resources, data curation, writing—original draft preparation, writing—review and editing, visualization; W.Z., formal analysis, resources, data curation, writing—review and editing; A.T.H., formal analysis, resources, data curation, writing—original draft, writing—review and editing; H.K.C., formal analysis, writing—review and editing; C.D., conceptualization, methodology, formal analysis, resources, visualization, supervision, project administration, funding acquisition, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Department of Agriculture, Agricultural Research Service under project number 6034-41000-018-00D and Innovation Fund Round 11.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Sandra Matlack, PeiLing Li, and Elena Branca for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Note
The U.S. Department of Agriculture prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
References
- Jarvis, M.C. Structure and properties of pectin gels in plant cell walls. Plant Cell Environ. 1984, 7, 153–164. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Forsyth, W.; Duncan, H.J. A survey of the pectic content of nonlignified monocot cell walls. Plant Physiol. 1988, 88, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Shi, D.C.; Wang, J.; Hu, R.B.; Zhou, G.K.; O’Neill, M.A.; Kong, Y.Z. Boron-bridged RG-II and calcium are required to maintain the pectin network of the Arabidopsis seed mucilage ultrastructure. Plant Mol. Biol. 2017, 94, 267–280. [Google Scholar] [CrossRef]
- Tanhatan-Nasseri, A.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Isolation and characterization of model homogalacturonans of tailored methylesterification patterns. Carbohydr. Polym. 2011, 86, 1236–1243. [Google Scholar] [CrossRef]
- Utku, A.; Peña, M.J.; O’Neill, M.A. Changes in the abundance of cell wall apiogalacturonan and xylogalacturonan and conservation of rhamnogalacturonan II structure during the diversification of the Lemnoideae. Planta 2018, 247, 953–971. [Google Scholar]
- Peaucelle, A.; Louvet, R.; Johansen, J.N.; Höfte, H.; Laufs, P.; Pelloux, J.; Mouille, G. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 2008, 18, 1943–1948. [Google Scholar] [CrossRef]
- Pérez, S.; Karim Mazeau, K.; Catherine Hervé du Penhoat, C. The three-dimensional structures of the pectic polysaccharides. Plant Physiol. Biochem. 2000, 38, 37–55. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 20, 923–929. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.; Lin, J.; Liu, X.Y.; Qi, D.; Luo, Y.; Aheyeli-Kai, Y.; Ma, H. Separation, structural identification and antibacterial activity of pectin oligosaccharides derived from seed melon. Food Biosci. 2023, 53, 102616. [Google Scholar] [CrossRef]
- Martínez-Gómez, S.; Fernández-Bautista, M.; Rivas, S.; Yáñez, R.; Alonso, J.L. Recent advances in the production of oligogalacturonides and their biological properties. Food Funct. 2023, 14, 4507–4521. [Google Scholar] [CrossRef]
- Wang, T.; Tao, Y.; Lai, C.; Huang, C.; Ling, Z.; Yong, Q. Influence of glycosyl composition on the immunological activity of pectin and pectin-derived oligosaccharide. Int. J. Biol. Macromol. 2022, 222 Pt A, 671–679. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno Palop, C.; Tuohy, K.; Gibson, G.R.; Bennett, R.N.; Waldron, K.W.; Bisignano, G.; Narbad, A.; Faulds, C.B. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2007, 73, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.T.; Nunez, A.; Gibson, G.; Rastall, R.A. Methods of Promoting the Growth of Beneficial Bacteria in the Gut. U.S. Patent 8,313,789, 20 November 2012. [Google Scholar]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Tingirikari, J.M.R. Agro waste derived pectin poly and oligosaccharides: Synthesis and functional characterization. Biocatal. Agric. Biotechnol. 2021, 31, 101910. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Rimbach, G.H.; Gibson, G.R.; Rastall, R.A. Pectin and pectic oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2003, 23, 341–346. [Google Scholar]
- Yan, J.; Katz, A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic effects of modified citrus pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Watts, P.; Smith, A. PecSys: In situ gelling system for optimised nasal drug delivery. Expert Opin. Drug Deliv. 2009, 6, 543–552. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Cervone, F.; Hahn, M.G.; De Lorenzo, G.; Darvill, A.; Albersheim, P. Host-pathogen interactions: XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol. 1989, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Patel, D. Production, Purification, and Characterization of Carbohydrate Elicitor: Pectic Oligomers. In Biotic Elicitors; Amin, D., Amaresan, N., Ray, S., Eds.; Springer Protocols Handbooks; Humana: New York, NY, USA, 2022; pp. 79–86. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Uttapap, D.; Wandee, Y.; Kotatha, D.; Rungsardthong, V. Promoting effect of pectic-oligosaccharides produced from pomelo peel on rice seed germination and early seedling growth. J. Plant Growth Regul. 2023, 42, 2176–2188. [Google Scholar] [CrossRef]
- Cano, M.E.; García-Martin, A.; Comendador Morales, P.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Manthey, J.A. Continuous process for enhanced release and recovery of pectic hydrocolloids and phenolics from citrus biomass. J. Chem. Technol. Biotechnol. 2016, 91, 2597–2606. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Manthey, J.A.; Bai, J.; Ferguson, K.L. Analysis and potential value of compounds extracted from Star Ruby, Rio Red, and Ruby Red grapefruit, and grapefruit juice processing residues via steam explosion. Front. Nutr. 2021, 8, 691663. [Google Scholar] [CrossRef]
- Cameron, R.G.; Branca, E.; Dorado, C.; Kim, Y. Pectic hydrocolloids from steam-exploded lime pectin peel: Effect of temperature and time on macromolecular and functional properties. Food Sci. Nutr. 2021, 9, 1939–1948. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. 24th Rev. The National Formulary, 19th ed.; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1995. [Google Scholar]
- Yuliarti, O.; Goh, K.K.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef]
- Noguchi, M.; Hasegawa, Y.; Suzuki, S.; Nakazawa, M.; Ueda, M.; Sakamoto, T. Determination of chemical structure of pea pectin by using pectinolytic enzymes. Carbohydr. Polym. 2020, 231, 115738. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Fishman, M.L.; Walker, P.N.; Chau, H.K.; Hotchkiss, A.T. Flash extraction of pectin from orange albedo by steam injection. Biomacromolecules 2003, 4, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.-F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crépeau, M.-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M. The effect of high pressure homogenization on pectin: Importance of pectin source and pH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Yi, J.; Peng, J.; Ning, C.; Wellala, C.K.D.; Zhang, B. Effects of high pressure homogenization on pectin structural characteristics and carotenoid bioaccessibility of carrot juice. Carbohydr. Polym. 2019, 203, 176–184. [Google Scholar] [CrossRef]
- Harding, S.E. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef]
- Tanford, C. Physical Chemistry of Macromolecules; Wiley: New York, NY, USA, 1961; pp. 407–411. [Google Scholar]
- Lin, Z.; Pattathil, S.; Hahn, M.G.; Wicker, L. Blueberry cell wall fractionation, characterization and glycome profiling. Food Hydrocoll. 2019, 90, 385–393. [Google Scholar] [CrossRef]
- Hellin, P.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Homogalacturonans from lime pectins exhibit homogeneous charge density and molar mass distribution. Carbohydr. Polym. 2005, 60, 307–317. [Google Scholar] [CrossRef]
- Hotchkiss, A.T., Jr.; Hicks, K.B. Analysis of oligogalacturonic acids with 50 or fewer residues by high-performance anion-exchange chromatography and pulsed amperometric detection. Anal. Biochem. 1990, 184, 200–206. [Google Scholar] [CrossRef]
- Wong, K.S.; Jane, J. Quantitative analysis of debranched amylopectin by HPAEC-PAD with a postcolumn enzyme reactor. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 297–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).