Lactiplantibacillus plantarum for the Preparation of Fermented Low-Bitter Enzymatic Skim Milk with Antioxidant Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparation of ESM
2.3. Determination of the Hydrolysis Degree of ESM
2.4. Sensory Evaluation of the Bitterness of ESM
2.5. Determination of the Bitterness Response Value of ESM
2.6. Preparation of LAB-Fermented ESM
2.7. Determination of Antioxidant Ability
2.7.1. Determination of the Scavenging Ability of DPPH Free Radical
2.7.2. Determination of the Inhibitory Ability Against ·OH
2.7.3. The Determination of SOD Activity
2.8. Determination of Volatile Flavor Compounds in ESM Fermented by LAB
2.9. Optimization of the Fermentation Conditions for the Fermentation of ESM by LAB
2.9.1. Single-Factor Experiment on Fermentation of ESM by LAB
- (1)
- The fermentation time is 18.0 h, the inoculum quantity of LAB is 3.0% and the pH of ESM is 6.5. The fermentation temperatures are 25.0, 29.0, 33.0, 37.0, 41.0 and 45.0 °C, respectively.
- (2)
- The fermentation temperature is 37.0 °C, the inoculum quantity of LAB is 3.0% and the pH of ESM is 6.5. The fermentation times are 10.0, 12.0, 14.0, 16.0, 18.0 and 22.0 h, respectively.
- (3)
- The fermentation temperature is 37.0 °C, the fermentation time is 18.0 h and the pH of ESM is 6.5. The inoculum quantities of LAB are 1.0%, 2.0%, 3.0%, 4.0%, 5.0% and 6.0%, respectively.
- (4)
- The fermentation temperature is 37.0 °C, the fermentation time is 18.0 h and the inoculum quantity of LAB is 3.0%. The pH values of ESM are 4.5, 5.0, 5.5, 6.0, 6.5 and 7.0, respectively.
2.9.2. Response Surface Experiment on the Fermentation of ESM by LAB
2.10. The Determination of Amino Acid Content
2.11. Statistics and Analysis
3. Results
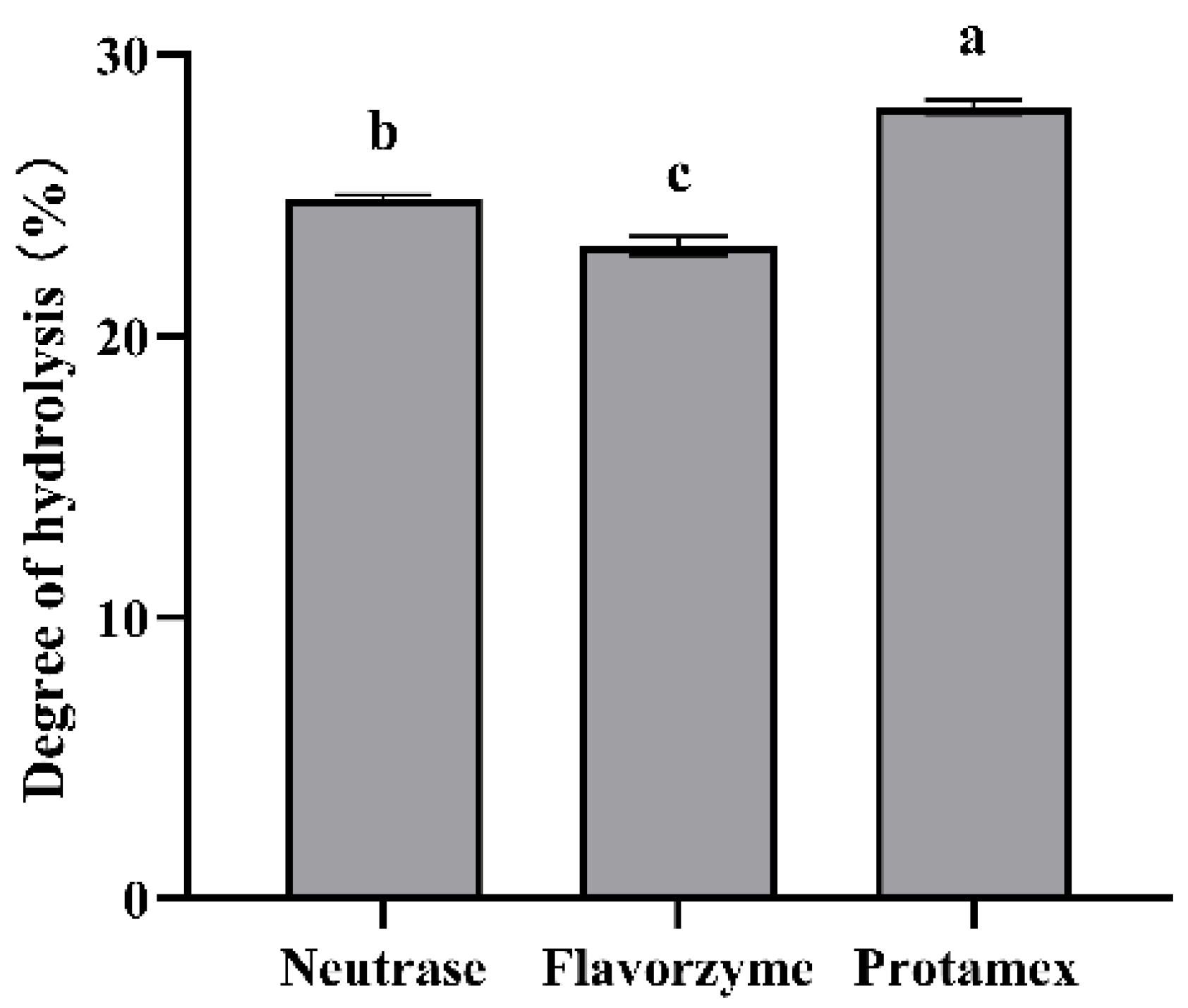
3.1. The Impact of Protease on the Degree of Hydrolysis of ESM
3.2. The Effect of Proteases on the Bitterness of Skim Milk
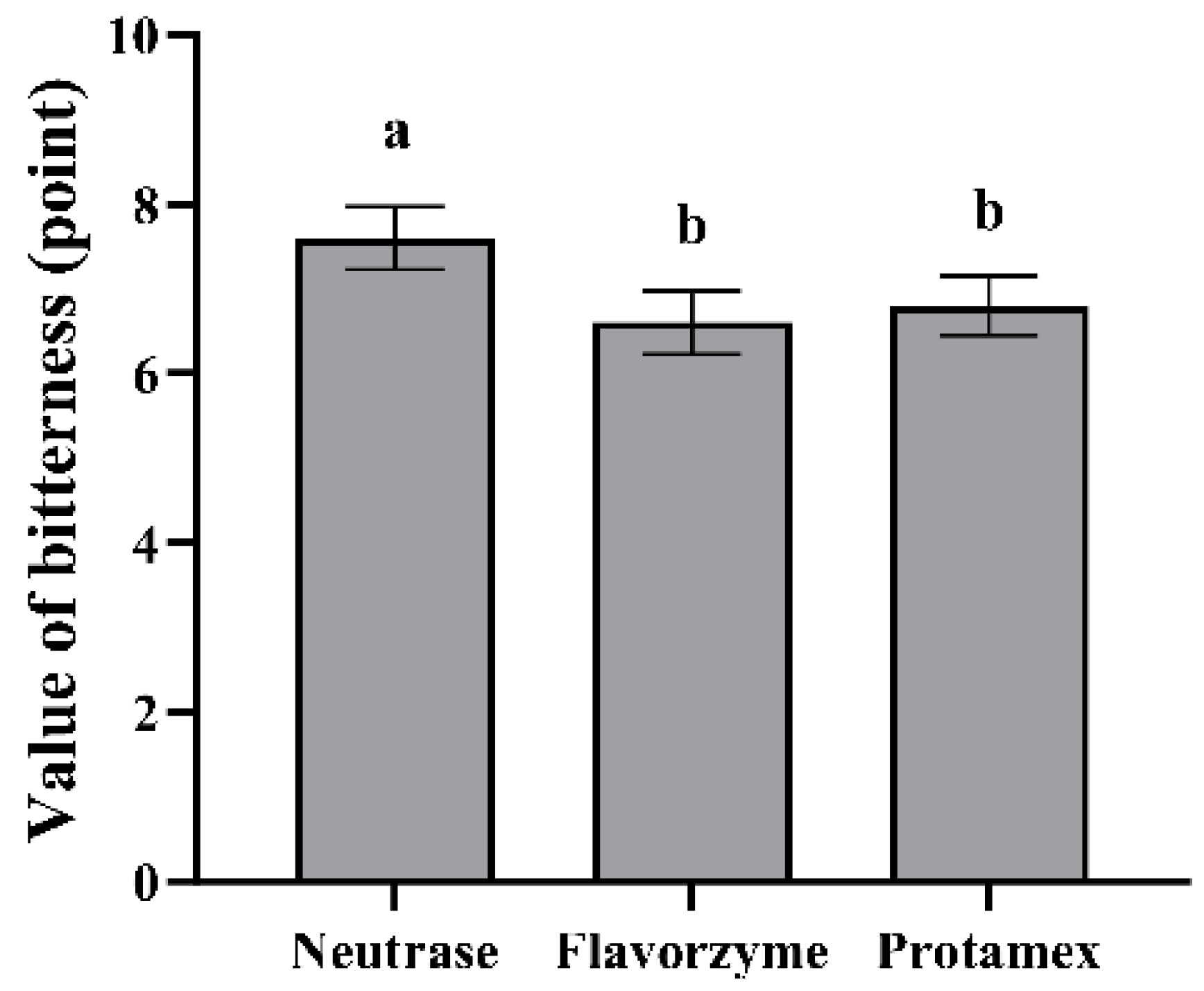
3.2.1. The Sensory Evaluation of the Bitterness of ESM
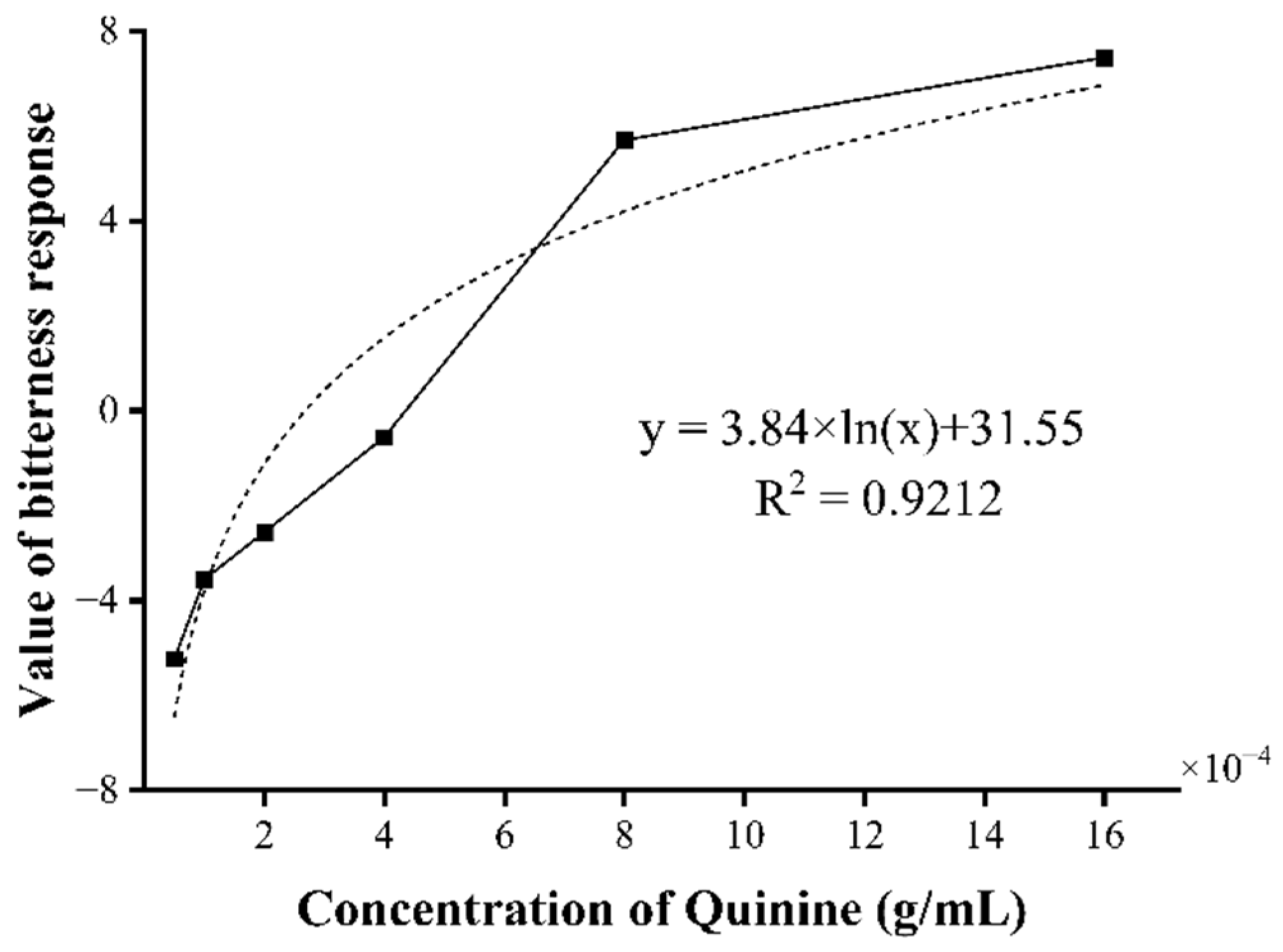
3.2.2. The Bitterness Response Value of ESM
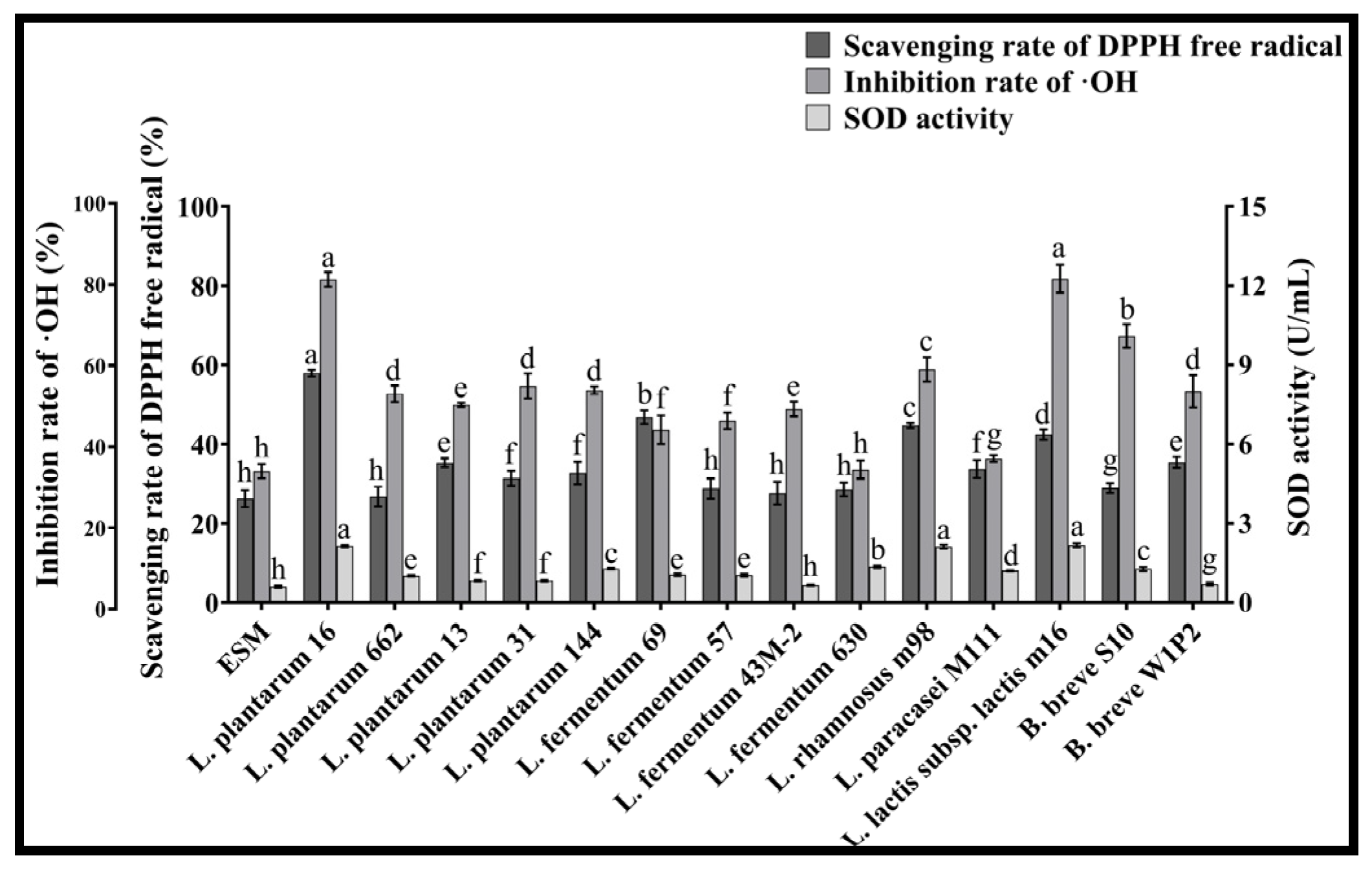
3.3. The Antioxidant Ability of LAB
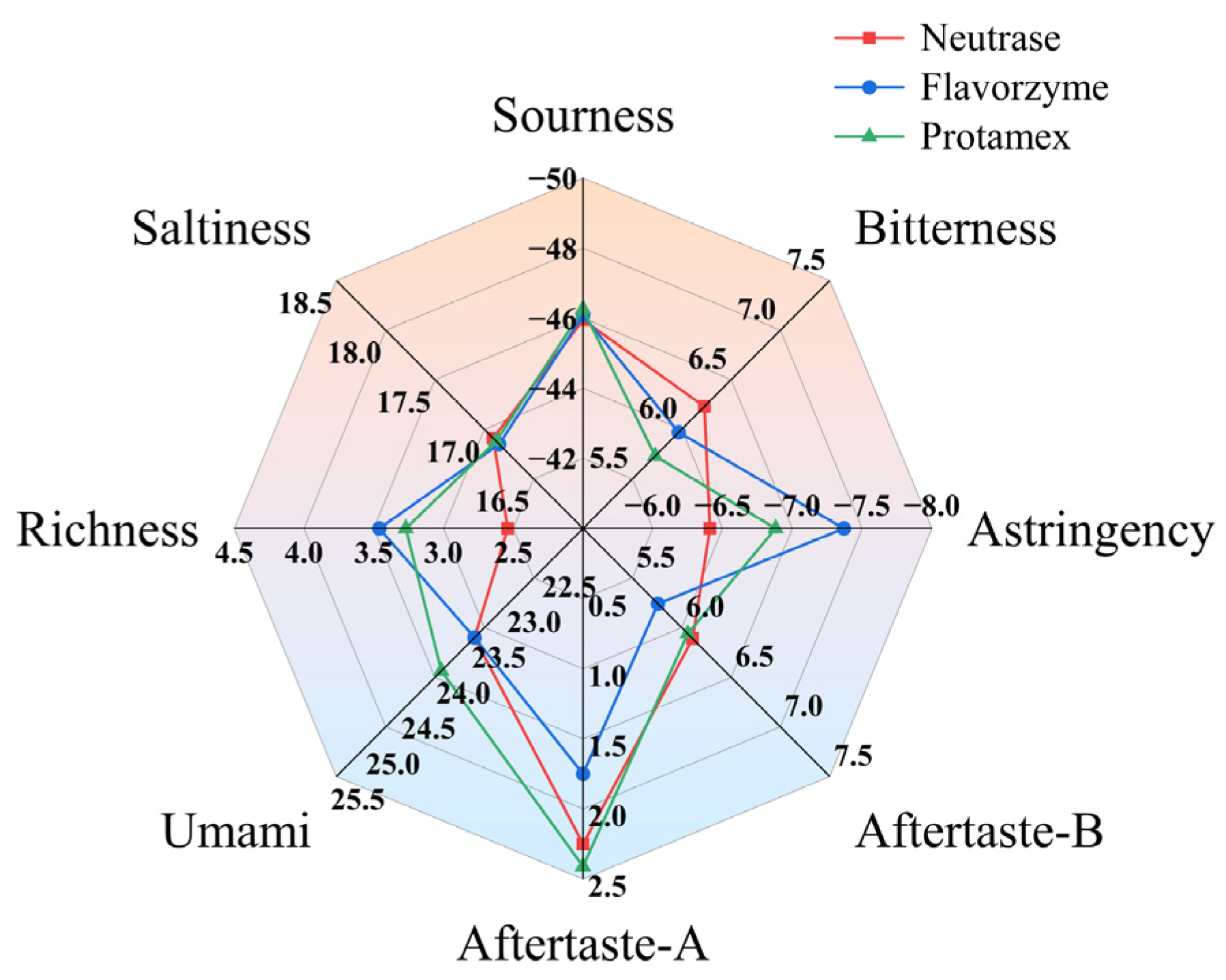
3.4. The Effect of LAB Fermentation on the Bitterness of ESM
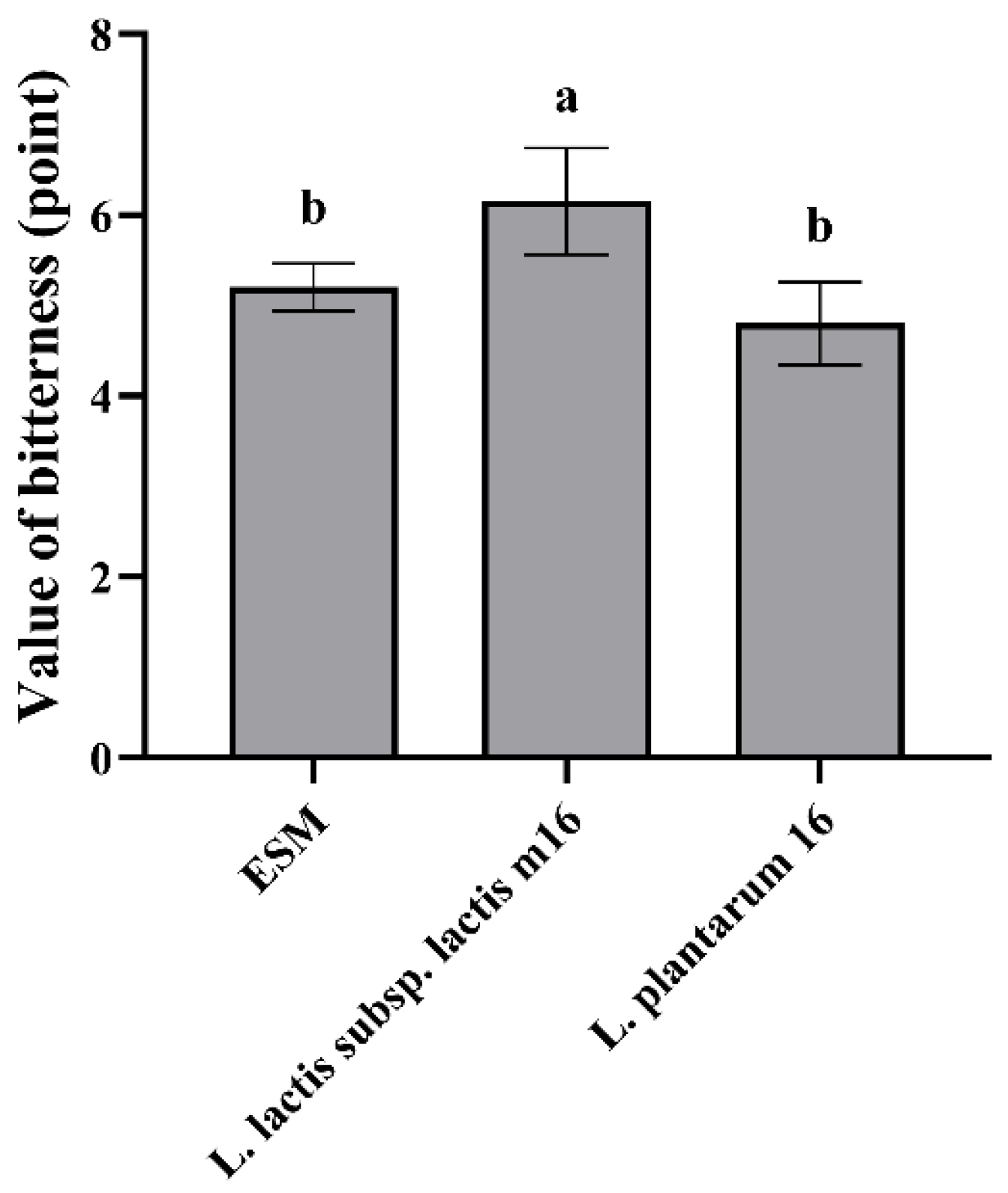
3.4.1. The Evaluation Value of Bitterness of Fermented ESM
3.4.2. The Response Value of Bitterness of Fermented ESM
3.5. The Effect of LAB Fermentation on the Volatile Flavor Substances in ESM
3.6. Optimization of the Fermentation Conditions of ESM by L. plantarum 16
3.6.1. Single-Factor Experiment
The Effect of Fermentation Temperature on the Antioxidant Ability of ESM Fermented by L. plantarum 16
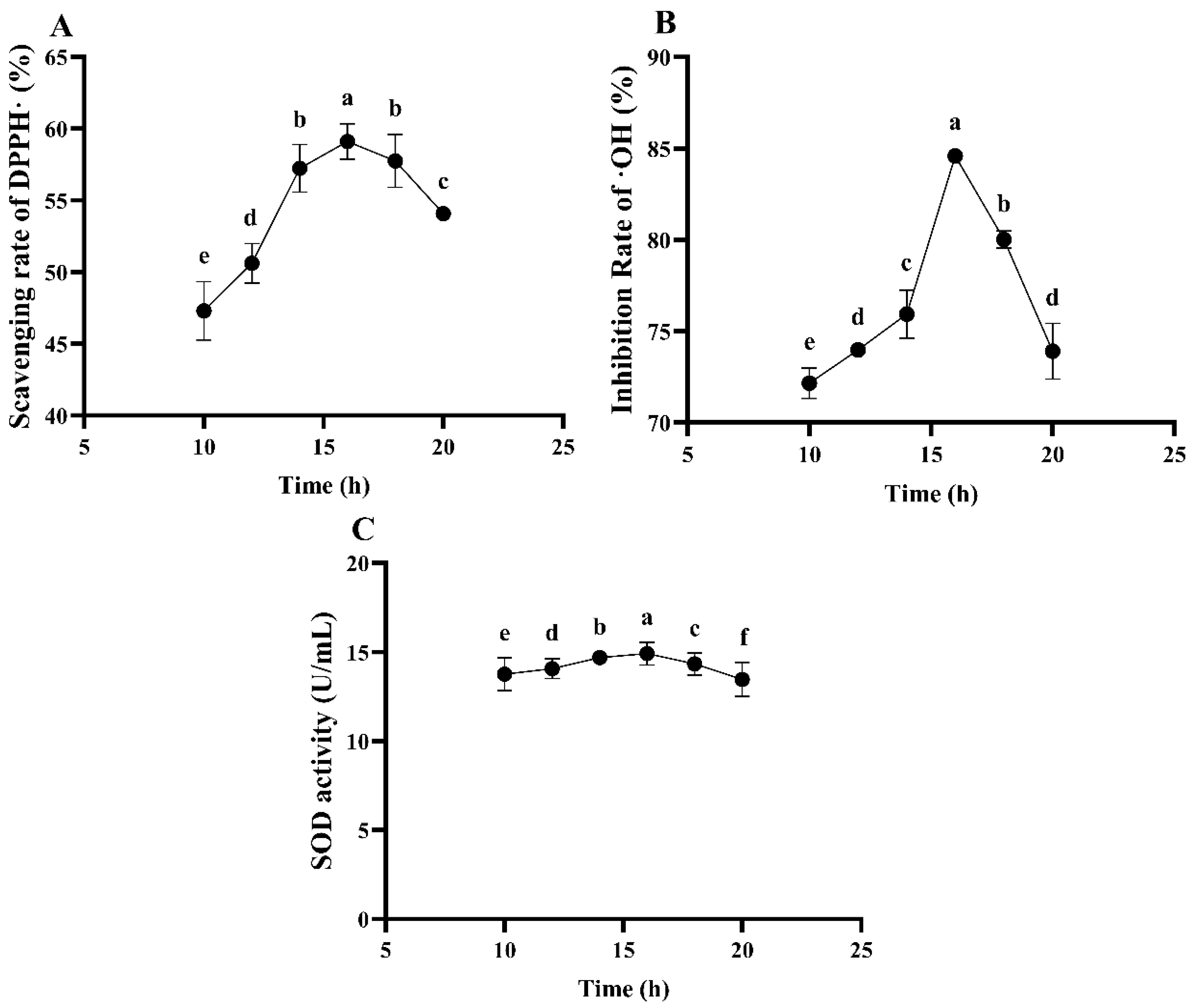
The Effect of Fermentation Time on the Antioxidant Ability of ESM Fermented by L. plantarum 16
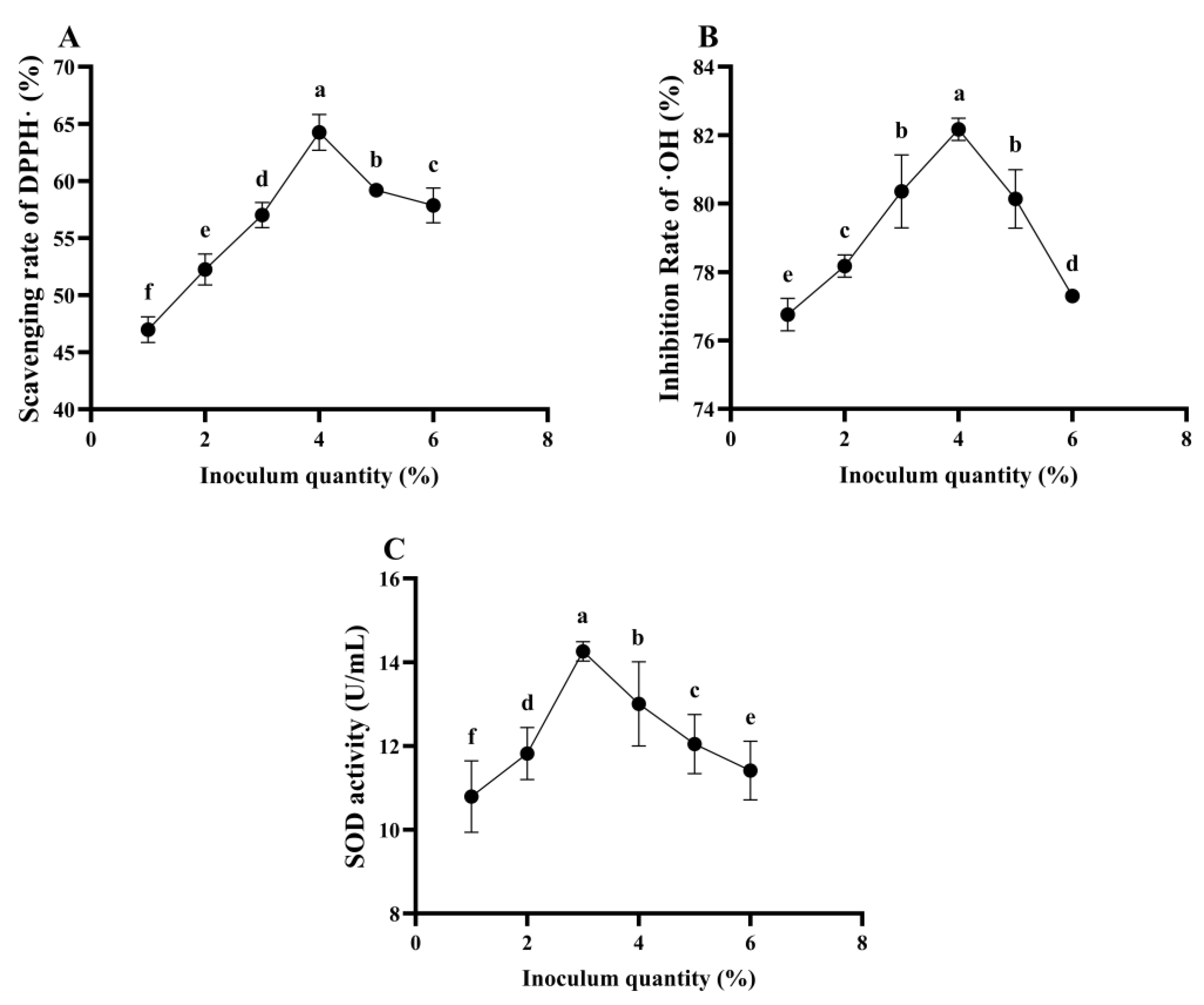
The Effect of Inoculum Quantity on the Antioxidant Ability of L. plantarum 16-Fermented ESM
The Effect of ESM pH on the Antioxidant Ability of L. plantarum 16 Fermentation
3.6.2. The Results of the Response Surface Experiment
Response Surface Optimization Scheme and Results
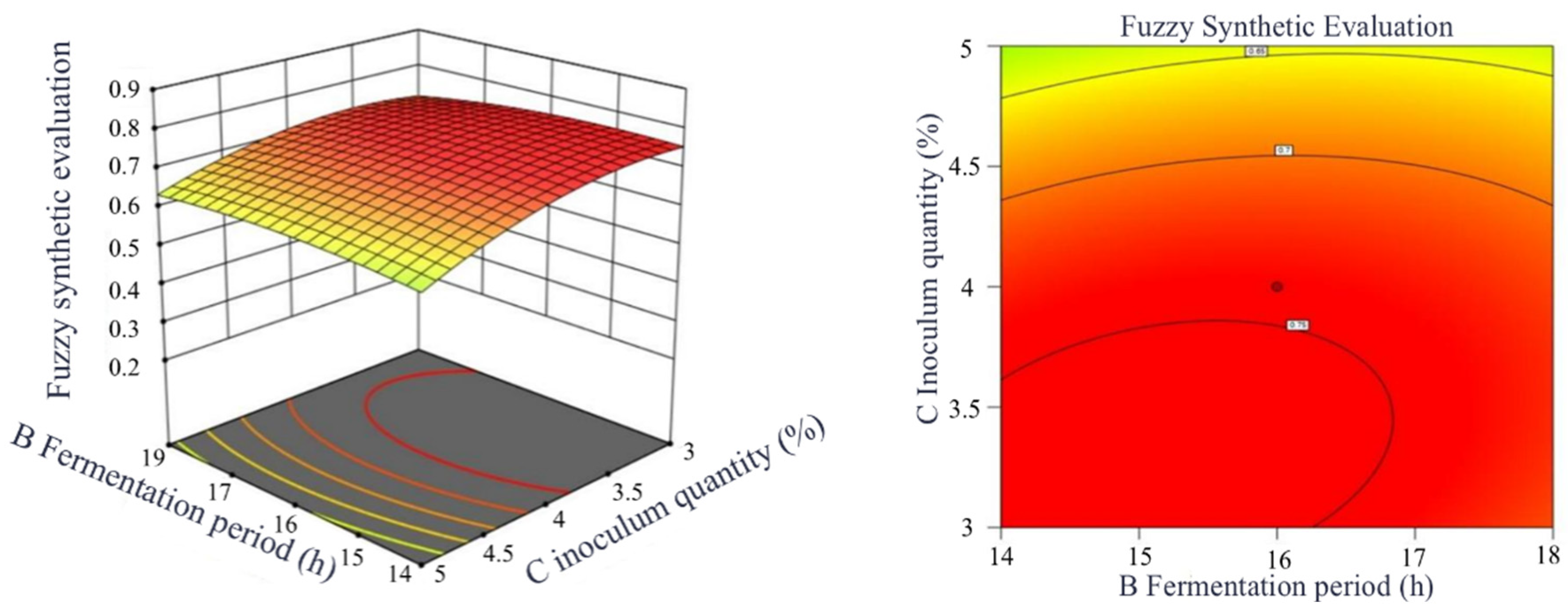
Response Surface Interaction and Contour Graph
More in Line with the Academic Context
3.7. The Content of Amino Acids in ESM Fermented by L. plantarum 16
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crittenden, R.G.; Bennett, L.E. Cow’s milk allergy: A complex disorder. J. Am. Coll. Nutr. 2005, 24, 582S–591S. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Ortega, B.; Goh, A.; Xepapadaki, P.; Sprikkelman, A.; Nicolaou, N.; Hernandez, R.E.H.; Latiff, A.H.A.; Yat, M.T.; Diab, M.; Al Hussaini, B.; et al. Strategies and Future Opportunities for the Prevention, Diagnosis, and Management of Cow Milk Allergy. Front. Immunol. 2021, 12, 608372. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Gómez, R. Peptidic profile, molecular mass distribution and immunological properties of commercial hypoallergenic infant formulas. Milchwissenschaft 2002, 57, 187–190. [Google Scholar]
- Zhou, H.; Wang, C.; Ye, J.; Tao, R.; Chen, H.; Cao, F. Effects of Enzymatic Hydrolysis Assisted by High Hydrostatic Pressure Processing on the Hydrolysis and Allergenicity of Proteins from Ginkgo Seeds. Food Bioprocess Technol. 2016, 9, 839–848. [Google Scholar] [CrossRef]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Wheatcroft, R.; Smithers, G. A review of allergic and intolerant reactions to dairy protein experienced by non-infant consumers. Aust. J. Dairy Technol. 2001, 56, 126. [Google Scholar]
- Ling, C.; Yuan, L.; Fang, Z. Enhancing bioavailability of soy protein isolate (SPI) nanoparticles through limited enzymatic hydrolysis: Modulating structural properties for improved digestion and absorption. Food Hydrocoll. 2024, 147, 109397. [Google Scholar]
- Cui, Q.; Sun, Y.X.; Cheng, J.J.; Guo, M.R. Effect of two-step enzymatic hydrolysis on the antioxidant properties and proteomics of hydrolysates of milk protein concentrate. Food Chem. 2022, 366, 130711. [Google Scholar] [CrossRef]
- Mahmoud, M.I. Physicochemical and functional properties of protein hydrosylates in nutritional products. Food Technol. 1994, 48, 89–95. [Google Scholar]
- Cheison, S.C.; Wang, Z.; Xu, S.Y. Use of macroporous adsorption resin for simultaneous desalting and debittering of whey protein hydrolysates. Int. J. Food Sci. Technol. 2007, 42, 1228–1239. [Google Scholar] [CrossRef]
- Spellman, D.; O’Cuinn, G.; FitzGerald, R.J. Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem. 2008, 114, 440–446. [Google Scholar] [CrossRef]
- Boye, L.; Nana, L.; Fusheng, C.; Jingsi, Z.; Xiaorui, S.; Lei, X.; Fang, F. Review on the release mechanism and debittering technology of bitter peptides from protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5153–5170. [Google Scholar]
- Ryan, G.; O’Regan, J.; FitzGerald, R.J. Foaming and sensory properties of bovine milk protein isolate and its associated enzymatic hydrolysates. Int. Dairy J. 2023, 137, 105511. [Google Scholar] [CrossRef]
- Ryan, G.; Nongonierma, A.B.; O’Regan, J.; FitzGerald, R.J. Functional properties of bovine milk protein isolate and associated enzymatic hydrolysates. Int. Dairy J. 2018, 81, 113–121. [Google Scholar] [CrossRef]
- Ryan, G.; O’Regan, J.; FitzGerald, R.J. Rehydration and water sorption behaviour of bovine milk protein isolate and its associated enzymatic hydrolysates. Int. Dairy J. 2022, 128, 105323. [Google Scholar] [CrossRef]
- Guowei, S.; Jie, H.; Chunju, B.; Jiangpeng, M.; He, C.; Jili, C. Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Converting Enzyme-Inhibitory Activity in Goat and Cow Milk. Biomolecules 2018, 8, 101. [Google Scholar] [CrossRef]
- Huang, X.Y.; Dai, H.; Xu, F.B.; Yao, L.Y.; Han, F.K. Quantitative Evaluation of Orange Juice Sensory Quality using Electronic Tongue. Mod. Food Sci. Technol. 2014, 30, 172–177. [Google Scholar]
- Fagundes, G.A.; Benedetti, S.; Pagani, M.A.; Fiorentini, A.M.; Severo, J.; Salas-Mellado, M. Electronic sensory assessment of bread enriched with cobia (Rachycentron canadum). J. Food Process Eng. 2022, 45, 13656. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Hu, C.X.; Liu, A.M.; Chen, J.J.; Gu, C.F.; Zhang, X.; You, C.P.; Tong, H.B.; Wu, M.J.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 1, 8918914. [Google Scholar] [CrossRef]
- Hao, J.X.; Qiu, S.; Li, H.Y.; Chen, T.P.; Liu, H.J.; Li, L.T. Roles of hydroxyl radicals in electrolyzed oxidizing water (EOW) for the inactivation of Escherichia coli. Int. J. Food Microbiol. 2012, 155, 99–104. [Google Scholar] [CrossRef]
- Hucheng, J.; Minghua, W.; You, Z.; Fengwei, C.; Longlong, F.; Liqiang, Z.; Xiaohui, C.; Wenji, B. Dietary laminarin administration to enhance the immune responses, promote growing and strengthen physique in Ictalurus punctatus. Aquac. Nutr. 2021, 27, 1181–1191. [Google Scholar]
- Dan, T.; Chen, H.Y.; Li, T.; Tian, J.L.; Ren, W.Y.; Zhang, H.P.; Sun, T.S. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef] [PubMed]
- Jaudzems, G.; Guthrie, J.; Lahrichi, S.; Fuerer, C. Total Amino Acids by UHPLC-UV in Infant Formulas and Adult Nutritionals, First Action 2018.06. J. AOAC Int. 2019, 102, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Huppertz, T.; Vasiljevic, T. Plant proteases and their application in dairy systems. Int. Dairy J. 2024, 154, 105925. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, J.; Liu, B.; Huang, Z.; Chen, F. Characteristics of the enzyme-induced release of bitter peptides from wheat gluten hydrolysates. Front. Nutr. 2022, 9, 1022257. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yixun, X.; Sicong, F.; Fang, Z. Enzymatic debittering of cheese flavoring and bitterness characterization of peptide mixture using sensory and peptidomics approach. Food Chem. 2023, 440, 138229. [Google Scholar]
- Alain, G.; Mylène, B.; Émilie, C.; Christophe, G.; Véronique, M.; Vincent, J. Mass Spectrometry Analysis of the Extracellular Peptidome of Lactococcus lactis: Lines of Evidence for the Coexistence of Extracellular Protein Hydrolysis and Intracellular Peptide Excretion. J. Proteome Res. 2016, 15, 3214–3224. [Google Scholar]
- Yawei, W.; Puying, Z.; Ying, Z.; Xiaomin, H.; Hairong, X. From bitter to delicious: Properties and uses of microbial aminopeptidases. World J. Microbiol. Biotechnol. 2023, 39, 72. [Google Scholar]
- Han, X.; Pengyan, G.; Zhen-Ming, L.; Fang-Zhou, W.; Li-Juan, C.; Jin-Song, S.; Hui-Ling, Z.; Yan, G.; Xiao-Juan, Z.; Zheng-Hong, X. Changes in physicochemical characteristics and metabolites in the fermentation of goji juice by Lactiplantibacillus plantarum. Food Biosci. 2023, 54, 102881. [Google Scholar]
- Naveed, M.; Nadeem, F.; Mehmood, T.; Bilal, M.; Anwar, Z.; Amjad, F. Protease—A Versatile and Ecofriendly Biocatalyst with Multi-Industrial Applications: An Updated Review. Catal. Lett. 2020, 151, 307–323. [Google Scholar] [CrossRef]
- Jagrani, M.; Priyanka, C.; Catherine, P.; Kumar, S.R. Bio-functional properties of probiotic Lactobacillus: Current applications and research perspectives. Crit. Rev. Food Sci. Nutr. 2020, 61, 2207–2224. [Google Scholar]
- Huang, Y.; Sun, Y.; Mehmood, A.; Lu, T.; Chen, X. Unraveling the temporal changes of Maillard reaction products and aroma profile in coffee leaves during hot-air drying. J. Food Compos. Anal. 2024, 128, 106055. [Google Scholar] [CrossRef]
- Tangyu, M.; Fritz, M.; Tan, J.P.; Ye, L.; Bolten, C.J.; Bogicevic, B.; Wittmann, C. Flavour by design: Food-grade lactic acid bacteria improve the volatile aroma spectrum of oat milk, sunflower seed milk, pea milk, and faba milk towards improved flavour and sensory perception. Microb. Cell Factories 2023, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Panpan, C.; Yue, L.; Jihong, W.; Bing, Y.; Hu, Z.; Mingquan, H.; Fuping, Z. Sensory-directed decoding of key aroma compounds from Jiugui-series Baijiu, the representative of Fuyu-flavor-type Baijiu (FFTB). J. Food Compos. Anal. 2022, 114, 104799. [Google Scholar]
- Zhang, Y.; Wang, X.; Lin, J.; Liu, J.; Wang, K.; Nie, Q.; Ye, C.; Sun, L.; Ma, Y.; Qu, R.; et al. A microbial metabolite inhibits the HIF-2α-ceramide pathway to mediate the beneficial effects of time-restricted feeding on MASH. Cell Metab. 2024, 36, 1823–1838. [Google Scholar] [CrossRef]
- Nisa, K.; Rosyida, V.T.; Nurhayati, S.; Indrianingsih, A.W.; Darsih, C.; Apriyana, W. Total phenolic contents and antioxidant activity of rice bran fermented with lactic acid bacteria. In Proceedings of the 2nd International Conference on Natural Products and Bioresource Sciences—2018, Tangerang, Indonesia, 1–2 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 251, p. 012020. [Google Scholar]
- Saritas, S.; Portocarrero, A.C.M.; López, J.M.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; Alves, J.L.D.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J.S. Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Achim, L.; Astrid, S.; Gerald, W.; Bernd, M.; Friedrich, B. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol. Pharmacol. 2002, 61, 1081–1088. [Google Scholar]
- Ahmad, A.; Mina, M.-Y.; Mehdi, S.; Hossein, E. Influence of different amino acid groups on the free radical scavenging capability of multi walled carbon nanotubes. J. Biomed. Mater. Res. Part A 2013, 101, 2219–2228. [Google Scholar]
- Yao, L.; Zhimei, Z.; Liangliang, B.; Fang, W.; Hehe, Y. The preparation and antioxidant activities of four 2-aminoacyl-chitooligosaccharides. Carbohydr. Res. 2022, 521, 108667. [Google Scholar]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Neto, J.H.P.L.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]



| Proteinase | Enzymolysis Temperature/(°C) | Enzyme pH | Enzyme Dosage/(U/g) |
|---|---|---|---|
| Neutrase | 50 | 7.0 | 10,000 |
| Flavorzyme | 50 | 7.0 | 6000 |
| Protamex | 55 | 7.0 | 10,000 |
| Coding | Factor | |||
|---|---|---|---|---|
| Inoculum Quantity/(%) | Fermentation Temperature/(°C) | Fermentation Time/(h) | ESM pH | |
| −1 | 3.0 | 33.0 | 14.0 | 5.5 |
| 0 | 4.0 | 35.0 | 16.0 | 6.0 |
| 1 | 5.0 | 37.0 | 18.0 | 6.5 |
| Species | Material | Relative Abundance/(%) | ||
|---|---|---|---|---|
| Control | L. Plantarum 16 | L. lactis Subsp. lactis m16 | ||
| Aldehydes | Tridecanal | 0.15 | ||
| Heptanal | 0.27 | 0.09 | ||
| Isoamyl aldehyde | 0.25 | |||
| Decanal | 0.22 | 0.12 | 0.34 | |
| Natural nonanal | 1.07 | 0.12 | 0.35 | |
| Phenylacetaldehyde | 0.78 | |||
| 2,4-Dimethylbenzaldehyde | 1.29 | 2.96 | ||
| Benzaldehyde | 5.68 | 2.59 | ||
| Subtotal | 8.53 | 1.27 | 6.48 | |
| Alcohols | 2-Decanol | 0.11 | ||
| 1-Octen-3-ol | ||||
| Hexadecanol | ||||
| cis-3-Decanol | 0.07 | |||
| (E)-Dec-3-en-1-ol | 0.15 | |||
| β-Phenylethanol | ||||
| 2-Decen-1-ol | 0.09 | 0.34 | ||
| n-Hexanol | 0.33 | |||
| 3,4-Dimethylbenzyl alcohol | 0.18 | 0.21 | ||
| (2S,3S)-(+)-2,3-Butanediol | 1.08 | |||
| Undecyl alcohol | 0.55 | 0.51 | ||
| Isopentyl alcohol | 1.50 | |||
| 1-Decanol | 1.66 | |||
| 1-Heptanol | 0.86 | 0.63 | ||
| 1-Pentanol | ||||
| Nonyl alcohol | 1.90 | 1.92 | ||
| Octanol | 1.34 | 4.52 | 3.11 | |
| Subtotal | 1.34 | 13.00 | 6.72 | |
| Ketones | 2-Tridecanone | 0.18 | ||
| 2-Nonanone | 0.34 | 0.17 | 0.49 | |
| 2,3-Heptanedione | ||||
| 2-Heptanone | 0.30 | 0.33 | 0.61 | |
| 2-Decanone | 1.19 | 0.23 | 0.34 | |
| Isophorone | 0.26 | 2.14 | 3.39 | |
| Subtotal | 2.09 | 2.87 | 5.01 | |
| Esters | Ethyl octanoate | |||
| Methyl 8,11-octadecadienoate | 0.25 | |||
| Methyl 12,15-octadecadienoate | 0.36 | |||
| Isobutyl 2,2,4-trimethyl-3-hydroxyvalerate | 0.35 | |||
| Heptyl formate | ||||
| Methyl 7,10-octadecadienoate | 0.90 | |||
| Methyl palmitate | 2.53 | 0.14 | 0.32 | |
| Methyl (Z, Z)-9,12-octadecadienoate | 0.77 | 1.74 | ||
| Methyl stearate | 4.90 | |||
| 2,2,4-Trimethyl-1,3-pentanediol di isobutyrate | 5.13 | 1.54 | 1.03 | |
| Methyl linoleate | 33.90 | |||
| Subtotal | 47.61 | 2.81 | 3.44 | |
| Acids | 2-Hydroxy-4-methyl pentanoic acid | 0.28 | ||
| Caprylic acid | ||||
| Subtotal | 0.00 | 0.28 | 0.00 | |
| Heterocyclic compounds | Octane | |||
| Octadecane | 0.47 | |||
| Longifolene | 0.14 | 0.10 | 0.21 | |
| m-Isopropyltoluene | ||||
| Dodecane | 0.38 | 0.21 | ||
| 2-Undecane | 0.13 | 0.33 | ||
| 2,4-Di-tert-butylphenol | 0.16 | 0.36 | ||
| Azulene | 0.19 | 0.30 | ||
| Tetradecane | 0.24 | 0.47 | ||
| 1,3,5-Trimethylbenzene | ||||
| Butylated hydroxytoluene | 2.68 | 1.38 | 2.23 | |
| Subtotal | 3.01 | 2.69 | 4.28 | |
| Experiment Number | Influencing Factors | Sensory Assessment | DPPH Free Radical Scavenging Rate/(%) | Inhibition Rate of ·OH/(%) | SOD Activity/(%) | Fuzzy Synthetic Evaluation | |||
|---|---|---|---|---|---|---|---|---|---|
| A Fermentation Temperature/(°C) | B Fermentation Time/(h) | C Inoculum Quantity/ (%) | D ESM pH | ||||||
| 1 | 33 | 14 | 4 | 6 | 3.75 | 58.88 | 48.36 | 12.22 | 0.40 |
| 2 | 37 | 14 | 4 | 6 | 5.00 | 59.65 | 46.86 | 11.52 | 0.64 |
| 3 | 33 | 18 | 4 | 6 | 4.75 | 60.5 | 77.45 | 13.32 | 0.51 |
| 4 | 37 | 18 | 4 | 6 | 6.00 | 54.65 | 53.81 | 12.17 | 0.54 |
| 5 | 35 | 16 | 3 | 5.5 | 7.25 | 60.01 | 81.6 | 12.97 | 0.44 |
| 6 | 35 | 16 | 5 | 5.5 | 6.50 | 59.71 | 47.66 | 11.49 | 0.47 |
| 7 | 35 | 16 | 3 | 6.5 | 5.00 | 59.51 | 52.01 | 10.50 | 0.51 |
| 8 | 35 | 16 | 5 | 6.5 | 5.50 | 58.59 | 40.93 | 11.75 | 0.45 |
| 9 | 33 | 16 | 4 | 5.5 | 4.75 | 59.25 | 46.75 | 12.39 | 0.55 |
| 10 | 37 | 16 | 4 | 5.5 | 6.75 | 60.18 | 39.91 | 9.23 | 0.58 |
| 11 | 33 | 16 | 4 | 6.5 | 3.75 | 56.05 | 73.57 | 12.32 | 0.51 |
| 12 | 37 | 16 | 4 | 6.5 | 3.75 | 56.27 | 46.08 | 12.66 | 0.74 |
| 13 | 35 | 14 | 3 | 6 | 5.50 | 59.49 | 49.01 | 11.50 | 0.45 |
| 14 | 35 | 18 | 3 | 6 | 6.50 | 59.61 | 40.87 | 9.13 | 0.48 |
| 15 | 35 | 14 | 5 | 6 | 5.75 | 54.06 | 42.57 | 10.51 | 0.38 |
| 16 | 35 | 18 | 5 | 6 | 6.75 | 58.39 | 78.76 | 14.77 | 0.46 |
| 17 | 33 | 16 | 3 | 6 | 4.75 | 55.48 | 44.08 | 10.86 | 0.41 |
| 18 | 37 | 16 | 3 | 6 | 4.75 | 56.97 | 46.43 | 10.25 | 0.64 |
| 19 | 33 | 16 | 5 | 6 | 4.15 | 52.42 | 53.53 | 12.57 | 0.46 |
| 20 | 37 | 16 | 5 | 6 | 5.75 | 62.19 | 62.68 | 11.97 | 0.60 |
| 21 | 35 | 14 | 4 | 5.5 | 6.25 | 55.69 | 44.46 | 12.18 | 0.49 |
| 22 | 35 | 18 | 4 | 5.5 | 7.75 | 48.5 | 52.01 | 10.25 | 0.50 |
| 23 | 35 | 14 | 4 | 6.5 | 4.25 | 59.44 | 47.43 | 12.72 | 0.51 |
| 24 | 35 | 18 | 4 | 6.5 | 5.50 | 60.82 | 38.51 | 12.44 | 0.62 |
| 25 | 35 | 16 | 4 | 6 | 5.75 | 61.07 | 44.72 | 12.27 | 0.47 |
| 26 | 35 | 16 | 4 | 6 | 5.50 | 62.31 | 41.93 | 9.96 | 0.50 |
| 27 | 35 | 16 | 4 | 6 | 5.25 | 57.59 | 43.77 | 11.38 | 0.51 |
| 28 | 35 | 16 | 4 | 6 | 5.25 | 61.05 | 85.59 | 14.04 | 0.50 |
| 29 | 35 | 16 | 4 | 6 | 5.50 | 57.35 | 43.88 | 14.17 | 0.52 |
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.1623 | 14 | 0.0116 | 12.75 | <0.0001 | Statistical significance |
| A—Fermentation Temperature | 0.0695 | 1 | 0.0695 | 76.44 | <0.0001 | ** |
| B—Fermentation Duration | 0.0045 | 1 | 0.0045 | 4.9 | 0.044 | ** |
| C—Inoculum Quantity | 0.0007 | 1 | 0.0007 | 0.7485 | 0.4015 | |
| D—pH of ESM | 0.0083 | 1 | 0.0083 | 9.09 | 0.0093 | ** |
| AB | 0.0101 | 1 | 0.0101 | 11.08 | 0.005 | ** |
| AC | 0.0023 | 1 | 0.0023 | 2.56 | 0.1319 | |
| AD | 0.0102 | 1 | 0.0102 | 11.23 | 0.0048 | ** |
| BC | 0.0008 | 1 | 0.0008 | 0.8772 | 0.3649 | |
| BD | 0.0025 | 1 | 0.0025 | 2.76 | 0.1189 | |
| CD | 0.002 | 1 | 0.002 | 2.21 | 0.1593 | |
| A2 | 0.0217 | 1 | 0.0217 | 23.88 | 0.0002 | ** |
| B2 | 0.0021 | 1 | 0.0021 | 2.34 | 0.1483 | |
| C2 | 0.0123 | 1 | 0.0123 | 13.55 | 0.0025 | ** |
| D2 | 0.0061 | 1 | 0.0061 | 6.71 | 0.0214 | ** |
| Residual | 0.0127 | 14 | 0.0009 | |||
| Lack of fit | 0.0111 | 10 | 0.0011 | 2.8 | 0.1665 | Insignificant |
| Pure error | 0.0016 | 4 | 0.0004 | |||
| Sum total | 0.1751 | 28 |
| Amino Acid | ESM (g/100 g) | ESM Fermented by L. plantarum 16 (g/100 g) |
|---|---|---|
| Aspartic acid | 0.270 ± 0.017 a | 0.270 ± 0.017 a |
| Threonine | 0.150 ± 0.017 a | 0.150 ± 0.017 a |
| Serine | 0.180 ± 0.004 a | 0.170 ± 0.005 b |
| Glutamic acid | 0.670 ± 0.010 b | 0.690 ± 0.005 a |
| Proline | 0.300 ± 0 a | 0.300 ± 0.010 a |
| Glycine | 0.063 ± 0 b | 0.064 ± 0.001 a |
| Alanine | 0.104 ± 0.001 a | 0.112 ± 0 b |
| Valine | 0.200 ± 0.017 a | 0.200 ± 0.017 a |
| Methionine | 0.073 ± 0.001 b | 0.077± 0.001 a |
| Isoleucine | 0.165 ± 0.015 a | 0.165 ± 0.015 a |
| Leucine | 0.305 ± 0.045 a | 0.305 ± 0.045 a |
| Tyrosine | 0.160 ± 0.003 a | 0.150 ± 0.005 b |
| Phenylalanine | 0.180 ± 0.004 a | 0.160 ± 0.010 b |
| Histidine | 0.104 ± 0.002 a | 0.099 ± 0 b |
| Lysine | 0.275 ± 0.005 b | 0.286 ± 0.003 a |
| Arginine | 0.115 ± 0.005 b | 0.125 ± 0.003 a |
| Total amount | 3.314 ± 0.329 a | 3.323 ± 0.135 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, L.; Jin, Y.; Xu, H.; Liang, Y.; Xia, Z.; Zhang, C.; Guan, C.; Qu, H.; Wa, Y.; et al. Lactiplantibacillus plantarum for the Preparation of Fermented Low-Bitter Enzymatic Skim Milk with Antioxidant Ability. Foods 2024, 13, 3828. https://doi.org/10.3390/foods13233828
Jiang Y, Zhang L, Jin Y, Xu H, Liang Y, Xia Z, Zhang C, Guan C, Qu H, Wa Y, et al. Lactiplantibacillus plantarum for the Preparation of Fermented Low-Bitter Enzymatic Skim Milk with Antioxidant Ability. Foods. 2024; 13(23):3828. https://doi.org/10.3390/foods13233828
Chicago/Turabian StyleJiang, Yi, Longfei Zhang, Yushi Jin, Haiyan Xu, Yating Liang, Zihan Xia, Chenchen Zhang, Chengran Guan, Hengxian Qu, Yunchao Wa, and et al. 2024. "Lactiplantibacillus plantarum for the Preparation of Fermented Low-Bitter Enzymatic Skim Milk with Antioxidant Ability" Foods 13, no. 23: 3828. https://doi.org/10.3390/foods13233828
APA StyleJiang, Y., Zhang, L., Jin, Y., Xu, H., Liang, Y., Xia, Z., Zhang, C., Guan, C., Qu, H., Wa, Y., Wang, W., Huang, Y., Gu, R., & Chen, D. (2024). Lactiplantibacillus plantarum for the Preparation of Fermented Low-Bitter Enzymatic Skim Milk with Antioxidant Ability. Foods, 13(23), 3828. https://doi.org/10.3390/foods13233828





