Preparation of Functional Food with Enhanced Antioxidant Properties by Adding Aronia melanocarpa Polyphenol Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Purification of Polyphenols from Aronia melanocarpa
2.3. Characterization of AMP by UPLC-TQ-MS
2.4. Preparation of AMP Honey Addition
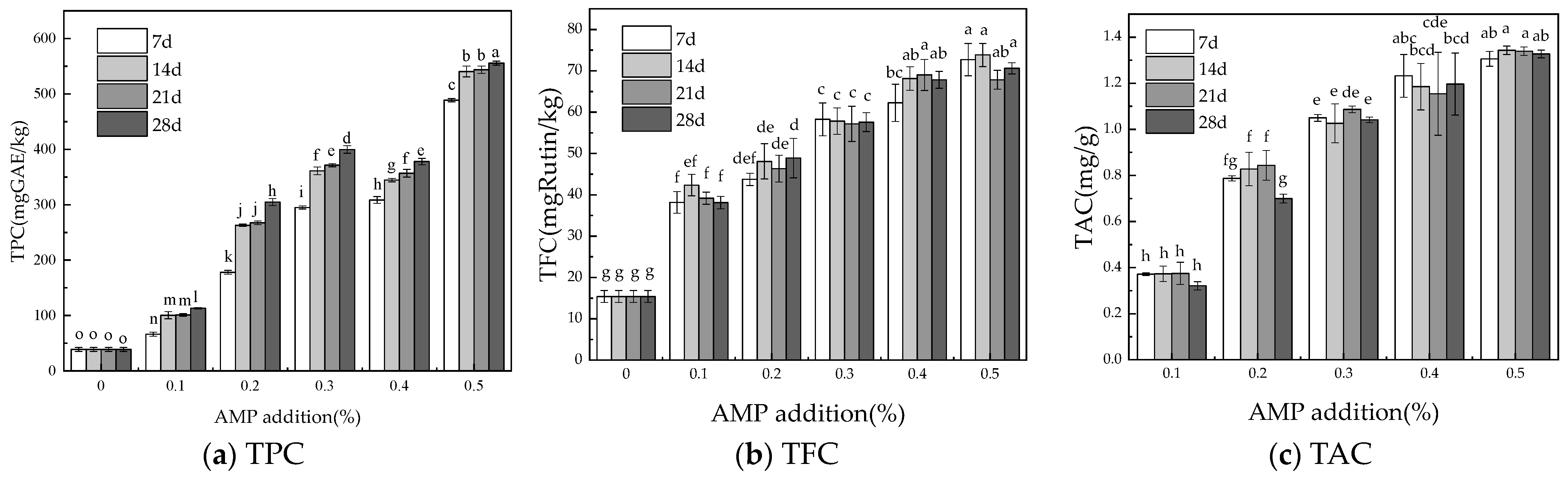
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoids Content (TFC)
2.7. Total Anthocyanin Content (TAC)
2.8. Antioxidant Activity Assay
2.8.1. DPPH Radical Scavenging Assay
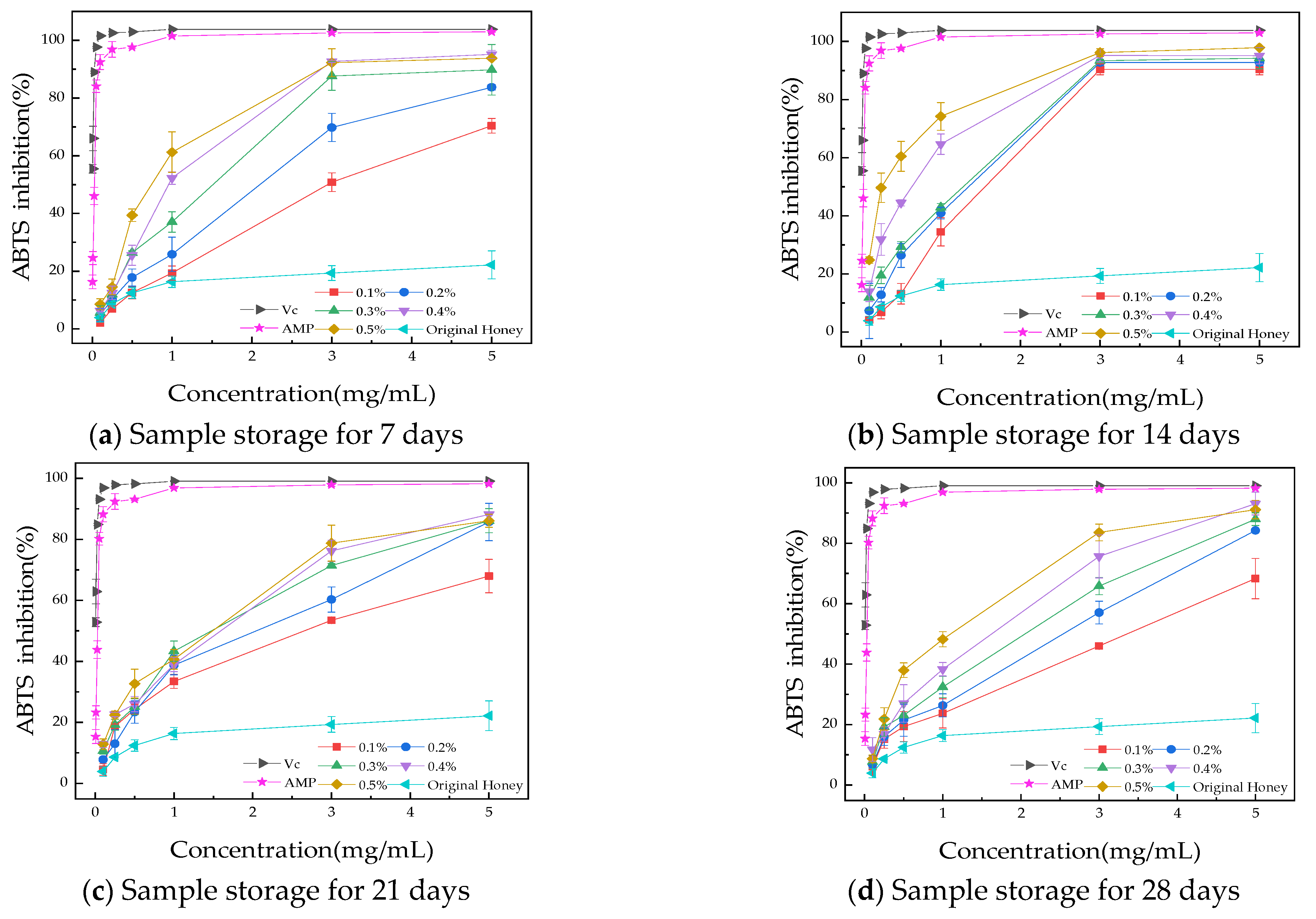
2.8.2. ABTS Radical Scavenging Assay
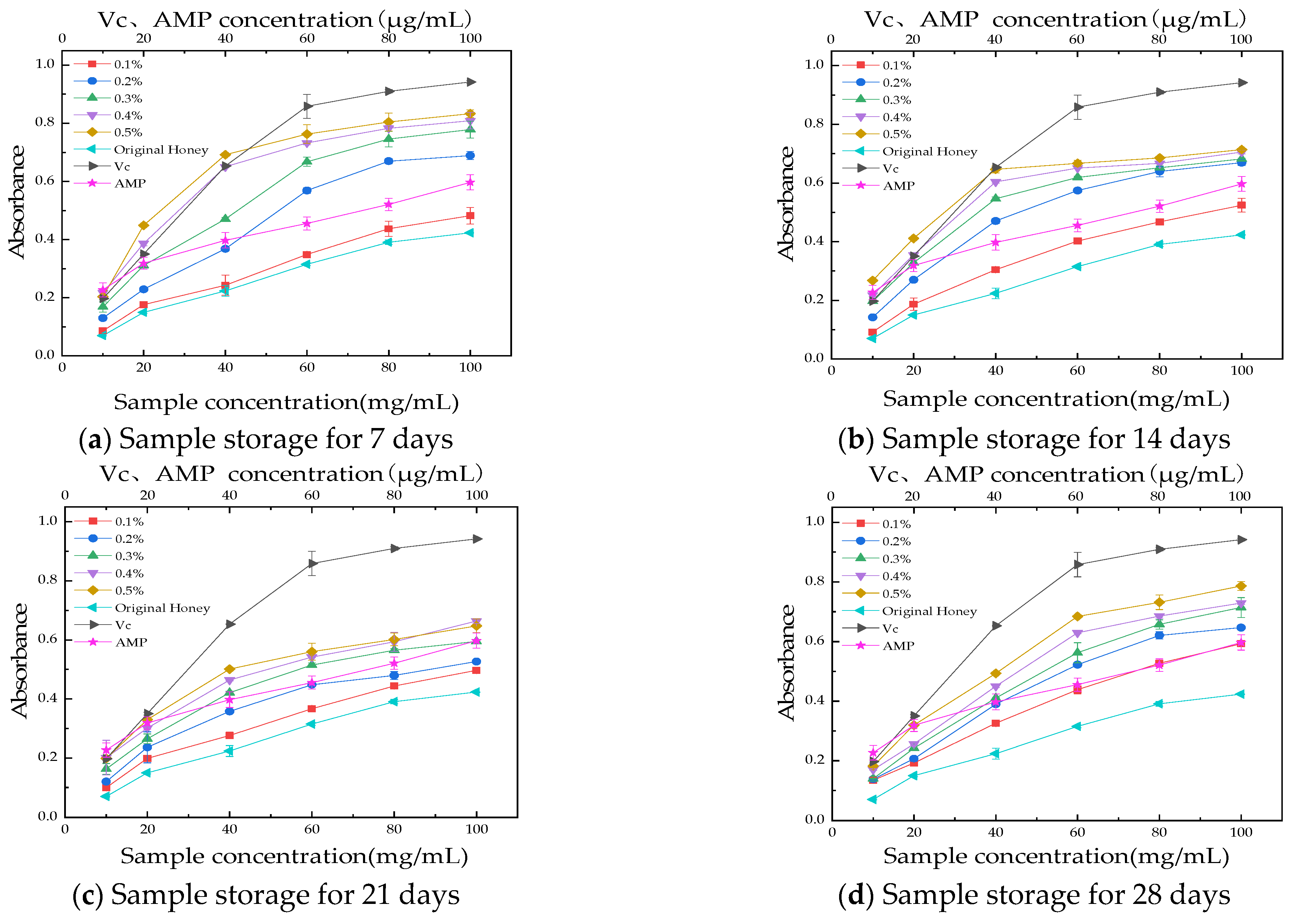
2.8.3. Reducing Power Assay
2.9. Effects on the Viability of HepG2 Liver Cancer Cells
2.9.1. Culture of HepG2 Cells
2.9.2. Determination of HepG2 Cell Viability
2.10. Determination of the Effect on Enzyme Activity in Honey
2.10.1. Sucrose Invertase Assay
2.10.2. Glucose Oxidase Assay
2.10.3. Amylase Assay
2.11. Sensory Evaluation
2.11.1. Determination of Solubility
2.11.2. Determination of Pfund Value
2.11.3. Sensory Evaluation
2.12. Statistical Analysis
3. Results
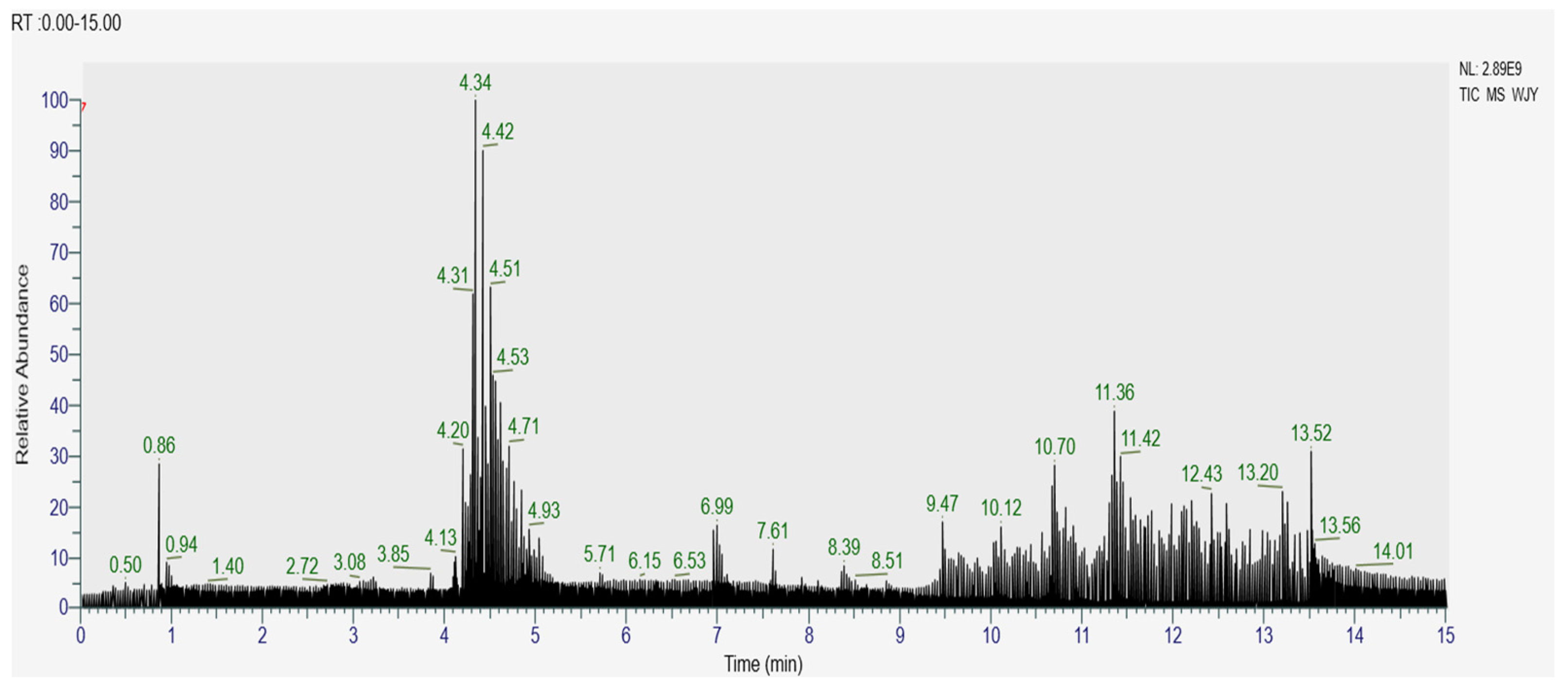
3.1. Component Analysis of AMP by UPLC-TQ-MS
3.2. Active Ingredients
3.3. Antioxidant Activity
3.3.1. DPPH Radical Scavenging Assay
3.3.2. ABTS Radical Scavenging Assay
3.3.3. Reducing Power Assay
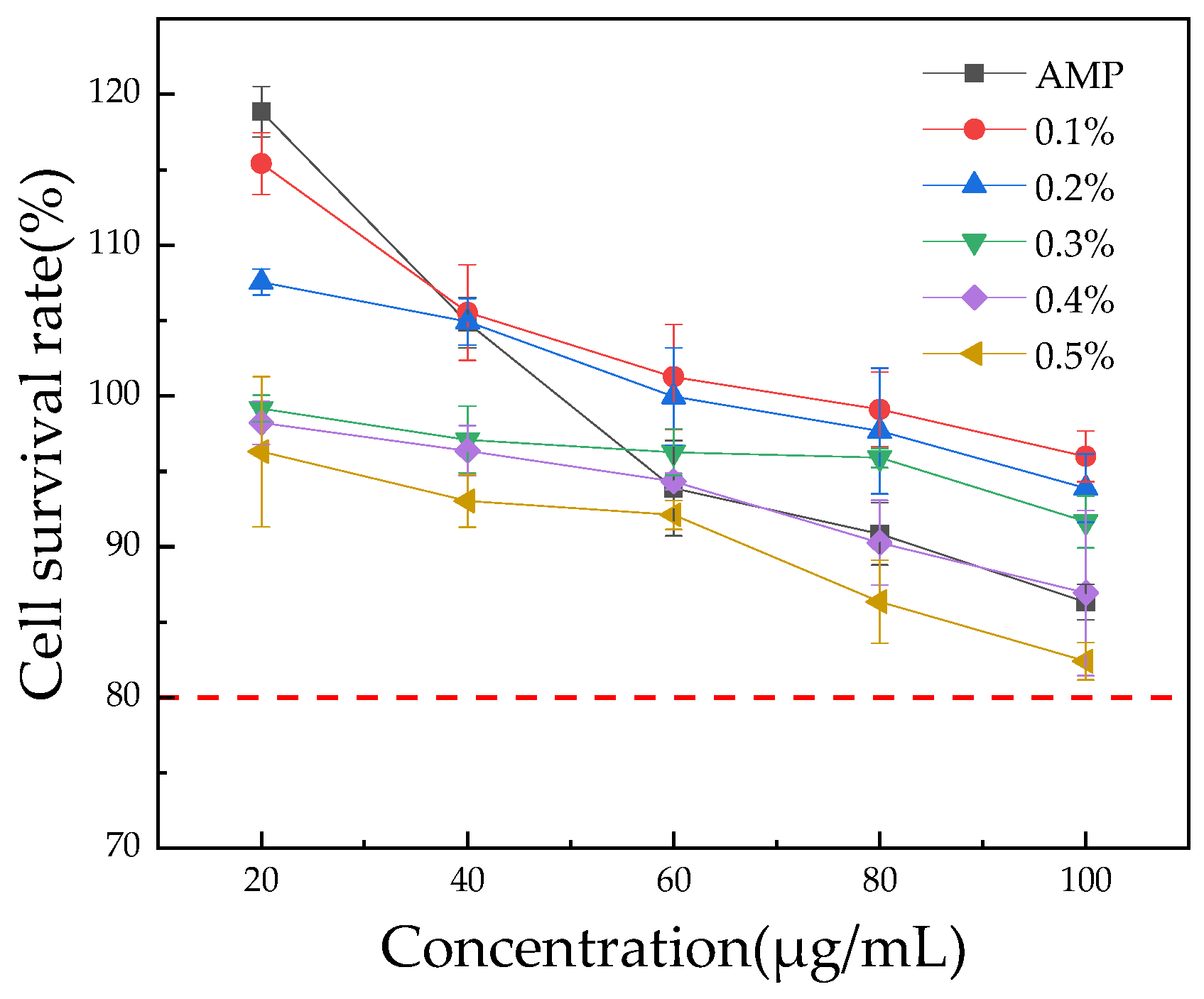
3.4. HepG2 Cell Viability
3.5. Effect on Enzyme Activity in Honey
3.6. Sensory Evaluation
3.6.1. Solubility
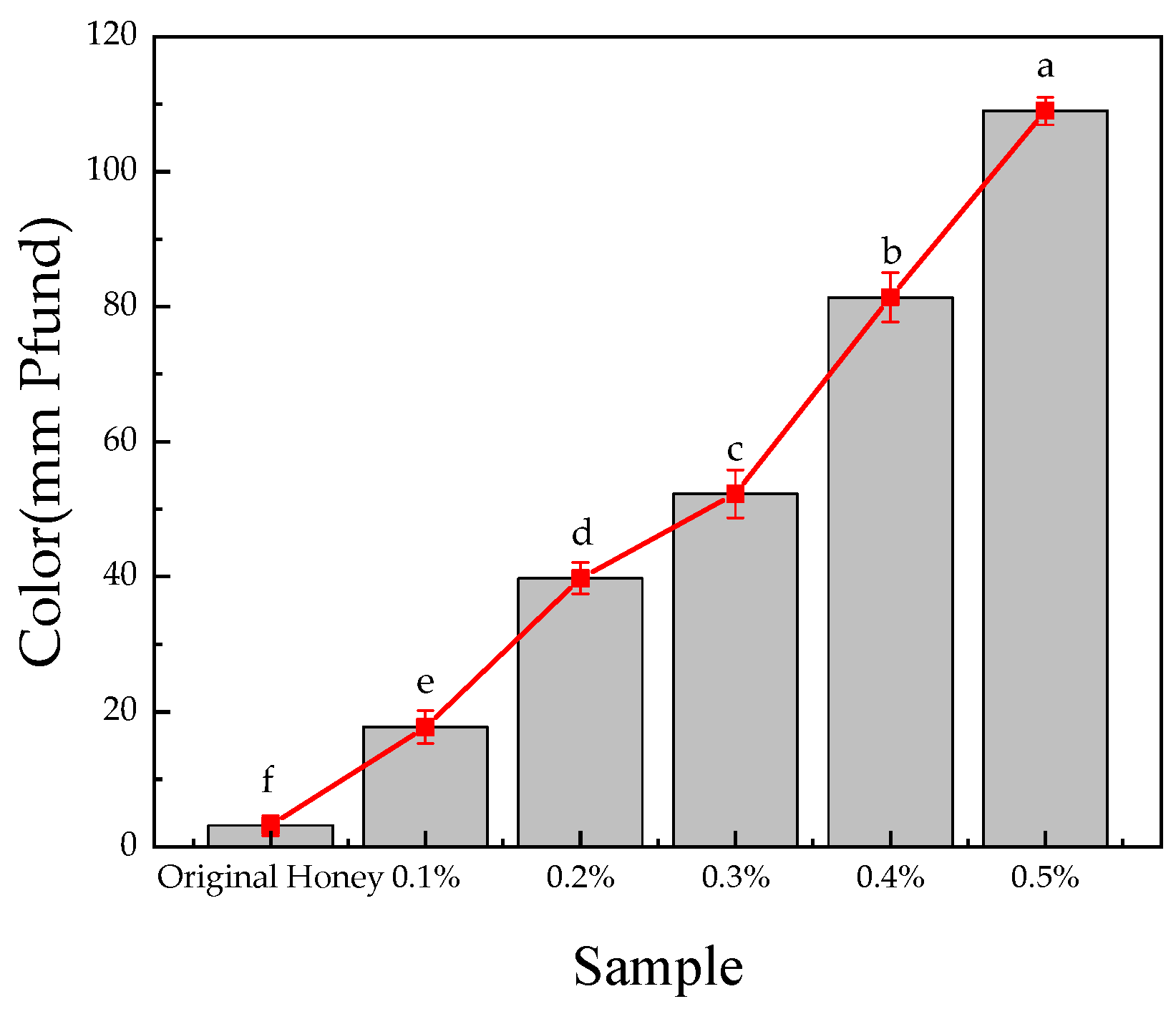
3.6.2. Pfund Value
3.6.3. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P. Sugar profile of different floral origin honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.T.; Lin, X.H.; Bai, W.D.; Xiao, G.C.; Liu, G.L. Composition, functional properties and safety of honey: A review. J. Sci. Food Agric. 2023, 103, 6767–6779. [Google Scholar] [CrossRef]
- Marta, P.M.; Jesús, R.H.; Celia, R. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef] [PubMed]
- Sadia, N.; Mohammad, F. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 2021, 282, 114614. [Google Scholar] [CrossRef]
- Guo, N.N.; Zhao, Y.Z.; Wang, K.; Peng, W.J. Research Progresson the Effect of Honey on Wound Healing and Its Mechanism. J. Agric. Sci. Technol. 2021, 23, 123–133. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, Y.; Liu, J.; Hu, Y.Q.; Gao, H.Y.; Fan, M.H.; Lv, C.Q. Effects of fermentation technology on the quality and antioxidant activities of Aroniamelanocarpa wine. China Brew. 2023, 42, 191–196. [Google Scholar]
- Shi, D.F.; Xu, J.; Sheng, L.; Song, K. Comprehensive Utilization Technology of Aronia melanocarpa. Molecules 2024, 29, 1388. [Google Scholar] [CrossRef]
- Jang, Y.B.; Koh, E. Characterisation and storage stability of aronia anthocyanins encapsulated with combinations of maltodextrin with carboxymethyl cellulose, gum Arabic, and xanthan gum. Food Chem. 2023, 405, 135002. [Google Scholar] [CrossRef]
- Chen, L.M.; Chen, W.X.; Li, D.M.; Liu, X.M. Anthocyanin and proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, fractionation, and enzyme inhibition. Food Sci. Nutr. 2023, 11, 3911–3922. [Google Scholar] [CrossRef]
- Li, J.P.; Gao, J.; Wang, Z.Y.; Zhao, Z.X.; Zhang, Y.K.; Cui, Z.F.; Sun, X.Y.; Ma, T.T. Analysis on current situation of industrial development of Aronia melanocarpa based on big data of Tianyancha. Food Ferment. Ind. 2024, 50, 408–416. [Google Scholar] [CrossRef]
- Young, G.W.Z.; Blundell, R. A review on the phytochemical composition and health applications of honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.P.; Lakhsmi, S.; Nagesvari, R.; Hua, G.S. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Małgorzata, D.; Patrycja, S.; Monika, K.; Monika, W.; Maria, C. Physicochemical Parameters and Antioxidant Activity of Bee Honey Enriched with Herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Milek, M.; Sidor, E.; Puchalski, C.; Dzugan, M. Antiviral and Antibacterial Effect of Honey Enriched with Rubus spp. as a Functional Food with Enhanced Antioxidant Properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwinczuk, W.; Puchalski, C.; Dzugan, M. The Effect of Adding the Leaves and Fruits of Morus alba to Rape Honey on Its Antioxidant Properties, Polyphenolic Profile, and Amylase Activity. Molecules 2019, 25, 84. [Google Scholar] [CrossRef]
- Fan, Z.L.; Wang, Z.Y.; Zuo, L.L.; Tian, S.Q. Separation and Functionalities of Anthocyanins from Vaccinium vitis-idaea. For. By-Prod. Spec. China 2011, 5, 1–4. [Google Scholar] [CrossRef]
- Gao, P.; Zhao, M.; Ma, Z.J.; Jiang, T.; Liu, L.D.; Wen, C.X. Effects of Different Drying Methods on Total Phenolic Content and Antioxidant Capacity of Dandelion Leaf. Mod. Food 2023, 29, 157–159. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xu, J.; Liu, C.T.; Huang, J.H.; Ceng, J.R. Determination of Antioxidant Activity, Total Polyphenol and Total Flavonoid Content of Seven Hainan Specialty Tea. Chin. J. Trop. Crops 2024, 45, 1244–1251. [Google Scholar]
- Zhang, S.; Xing, Z.Y.; Jiao, X.Y. Study on Extraction of Anthocyanins from Chinese Herbal Medicine Cornus Officinalis. Mod. Chem. Res. 2024, 06, 168–170. [Google Scholar] [CrossRef]
- Miłek, M.; Grabek-Lejko, D.; Stȩpień, K.; Sidor, E.; Mołoń, M.; Dżugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Cheng, Y.; Wang, J.Y.; Ding, M.; Fan, Z.L. Antioxidant Activity, Formulation, Optimization and Characterization of an Oil-in-Water Nanoemulsion Loaded with Lingonberry (Vaccinium vitis-idaea L.) Leaves Polyphenol Extract. Foods 2023, 12, 4256. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D. Component Analysis of Linden Honey at Different Maturity Stages and Its Antioxidant and Antibacterial Effects In Vitro. Master’s Thesis, Yanbian University, Yanbian, China, 2022. [Google Scholar] [CrossRef]
- Tian, J. Preliminary Study on the Evaluation Index of Maturity in Natural Ripening Process of Rape Honey. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar] [CrossRef]
- Yi, Z.L.; Zhang, X.Q.; Zhang, M.; Han, B.G.; Lou, W.; Luo, M.; Xu, X.J.; Xiong, Z.L.; Liu, Y.; Zhang, C.L.; et al. Study on the Physicochemical Indicators and Quality Analysis Four Kinds of Apiscerana Honey in Jiangxi Province. J. Bee 2024, 44, 1–6. [Google Scholar]
- Wei, L.Y. Study on the Key Processing Techniques of Puerariae Powderand the Compound Beverage and Its Functional Evaluation. Master’s Thesis, Guizhou University, Guiyang, China, 2016. [Google Scholar]
- Kate, N.; Kaitlyn, B.; Michael, C.G. Profiling of the Polyphenol Content of Honey from Different Geographical Origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, L.G.; Mucignat-Caretta, C.; Marchese, M.C.; Piana, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2018, 57, 75–87. [Google Scholar] [CrossRef]
- Kardas, M.; Bartecka, S.W.; Sołtys, K.; Dul, L.; Sapała, A.M.; Kiciak, A.; Bielaszka, A.; Kardas, J. The quality of selected raw and pasteurized honeys based on their sensory profiles and consumer preferences. Front. Nutr. 2024, 10, 1330307. [Google Scholar] [CrossRef] [PubMed]
- Mongi, J.R.; Ruhembe, C.C. Sugar profile and sensory properties of honey from different geographical zones and botanical origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Sasmaz, H.K.; Kilic-Buyukkurt, O.; Selli, S.; Bouaziz, M.; Kelebek, H. Antioxidant Capacity, Sugar Content, and Tandem HPLC-DAD-ESI/MS Profiling of Phenolic Compounds from Aronia melanocarpa Fruits and Leaves (Nero and Viking Cultivars). ACS Omega 2024, 9, 14963–14976. [Google Scholar] [CrossRef]
- Long, W.J.; Lin, Y.W.; Lv, C.X.; Dong, J.L.; Lv, M.L.; Lou, X.H. High-compatibility properties of Aronia melanocarpa extracts cross-linked chitosan/polyvinyl alcohol composite film for intelligent food packaging. Int. J. Biol. Macromol. 2024, 270, 132305. [Google Scholar] [CrossRef]
- Dorneanu, R.; Cioanca, O.; Chifiriuc, O.; Albu, E.; Tuchilus, C.; Mircea, C.; Salamon, I.; Hăncianu, M. Synergic benefits of Aronia melanocarpa anthocyanin-rich extracts and antibiotics used for urinary tract infections. Farmacia 2017, 65, 778–783. [Google Scholar]
- Chomphen, L.; Yamanont, P.; Morales, P.N. Flavonoid Metabolites in Serum and Urine after the Ingestion of Selected Tropical Fruits. Nutrients 2024, 16, 161. [Google Scholar] [CrossRef]
- Jacobsen, S.S.; Knob, C.F.; Simon, P.A.; Oldoni, T.L.C. Selective Extraction Process and Characterization of Antioxidant Phenolic Compounds from Pereskia aculeata Leaves Using UPLC-ESI-Q-TOF-MS/MS. ACS Omega 2024, 9, 37374–37385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, X.Y.; Wang, H.Y.; Pi, M.T.; Hu, J.G.; Zhu, Z.Q.; Zeng, J.G.; Li, B.; Xu, Z.Y. Identification and quantification of flavonoids in edible dock based on UPLC-qTOF MS/MS and molecular networking. J. Food Compos. Anal. 2024, 133, 106399. [Google Scholar] [CrossRef]
- Hernández, M.; Castañeta, G.; Simirgiotis, J.M.; Sepulveda, B.; Areche, C. Comprehensive phytochemical profile of leaves, stems and fruits from Orthopterygium huaucui (A. Gray) Hemsl. and their antioxidant activities. Chem. Biodivers. 2024, 21, e202400746. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.N. Analysis of Flavonoids and Fatty Acids in Qinghai Specialty Food Resources Seabuckthorn, Black Barley, Lycium chinensis, Lycium ruthenicum murr. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of Bioactive Components and Antioxidant Activity of Aronia melanocarpa Fruits at Various Stages of Their Growth, Using Chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef]
- Marinaccio, L.; Gentile, G.; Martínez, L.J.E.; Zengin, G.; Masci, D.; Flamminii, F.; Stefanucci, A.; Mollica, A. Valorization of grape pomace extracts against cranberry, elderberry, rose hip berry, goji berry and raisin extracts: Phytochemical profile and in vitro biological activity. Food Chem. 2025, 463, 141323. [Google Scholar] [CrossRef] [PubMed]
- Atiq, S.; Ibrahim, M.; Khan, C.; Ali, A.; Qadir, R.; Khan, A.; Salahi, R.A.; Abuelizz, H.A.; Medeiros, P.S.; Sampaio, O.M.; et al. Evaluation of Antimicrobial and Antioxidant Potential of Oxalis corymbosa Extracts. Chem. Biodivers. 2024, 21, e202400883. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Alongi, M.; Lanza, U.; Gorassini, A.; Verardo, G.; Comuzzi, C.; Anese, M.; Manzocco, L.; Nicoli, M.C. The role of processing on phenolic bioaccessibility and antioxidant capacity of apple derivatives. Food Chem. 2025, 436, 141402. [Google Scholar] [CrossRef]
- Cong, L.J.; Shi, R.; Wu, P.; Liu, M.M.; Liu, S.W.; Huang, X.T. Determination of Total Polyphenols and Total Flavonoids in Fruits of Heiguoxianleihuaqiu (Aronia melanocarpa) from Different Habitats. J. Liaoning Univ. Tcm 2021, 23, 31–34. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as Insecticides in Crop Protection—A Review of Current Research and Future Prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-obesity effects of polyphenol intake: Current status and future possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Gralec, I.W.M. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar] [CrossRef]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ongyanov, M.; Yanakieva, I. Black chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Valle-Sánchez, S.L.; Rodriguez-Ramirez, R.; Avila-Villa, L.A.; Villa-Lerma, A.G.; Wall-Medrano, A.; de la Rosa, L.A.; Muñoz-Bernal, O.A.; González-Córdova, A.F.; Arellano-Gil, M. Phenolic compounds profile in extracts of Smilax spp., antioxidant activity, and inhibition of advanced glycation end products. Food Chem. 2025, 463, 141389. [Google Scholar] [CrossRef]
- Li, J.; Fang, X.; Cui, D.; Ma, Z.Y.; Yang, J.; Niu, Y.Y.; Liu, H.; Xiang, P. Mechanistic insights into cadmium exacerbating 2-Ethylhexyl diphenyl phosphate-induced human keratinocyte toxicity: Oxidative damage, cell apoptosis, and tight junction disruption. Ecotoxicol. Environ. Saf. 2024, 283, 116858. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical Pathways of Caspase Activation during Apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef]
- Sun, M.; Lei, X.; Lan, X.; Li, Z.T.; Xu, H.B.; Chen, S.Z. Online identification of potential antioxidant components and evaluation of DNA oxidative damage protection ability in Prunus persica flowers. Talanta 2024, 280, 126702. [Google Scholar] [CrossRef]
- He, L.L. Physicochemical Properties and Antioxidantspectrum-Effect Relationship of Medlar Honey. Master’s Thesis, Northwest University, Xi’an, China, 2019. [Google Scholar]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. J. Impact Factor 2017, 12, e0180022. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.S. Study on the Extraction of antioxidant Components and the Digestion and Absorption of Phenolic Components in the Fruit of Aronia melanocarpa. Master’s Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, F.; Wu, W.; Jie, B.P. Effect on the cellular oxidative stress with HepG2 cellular model by AAPH of Rose hip. Food Sci. Technol. 2012, 37, 181–186. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef]
- Tasić, A.; Pezo, L.; Lončar, B.; Pešić, M.B.; Tešić, Ž.; Kalaba, M. Assessing the Impact of Botanical Origins, Harvest Years, and Geographical Variability on the Physicochemical Quality of Serbian Honey. Foods 2024, 13, 1530. [Google Scholar] [CrossRef]
- Devarajan, S.; Venugopal, S. Antioxidant and α-amylase inhibition activities of phenolic compounds in the extracts of Indian honey. Chin. J. Nat. Med. 2012, 10, 255–259. [Google Scholar] [CrossRef]
- Chu, Y.X. Study on a Method for Evaluating the Activity of Sucrose Invertasein Honey. Master’s Thesis, Harbin University of Commerce, Harbin, China, 2019. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa oncardiometabolic diseases: Asystematic review ofquasi-design studies andrandomized controlled trials. Rev. Diabet. Stud. RDS 2022, 18, 76–92. [Google Scholar] [CrossRef]
- Augustin, C.L.; Rahoveanu, M.M.T.; Zugravu, G.A. The comparative sensory analysis of mint honey. SHS Web Conf. 2021, 95, 01015. [Google Scholar] [CrossRef]


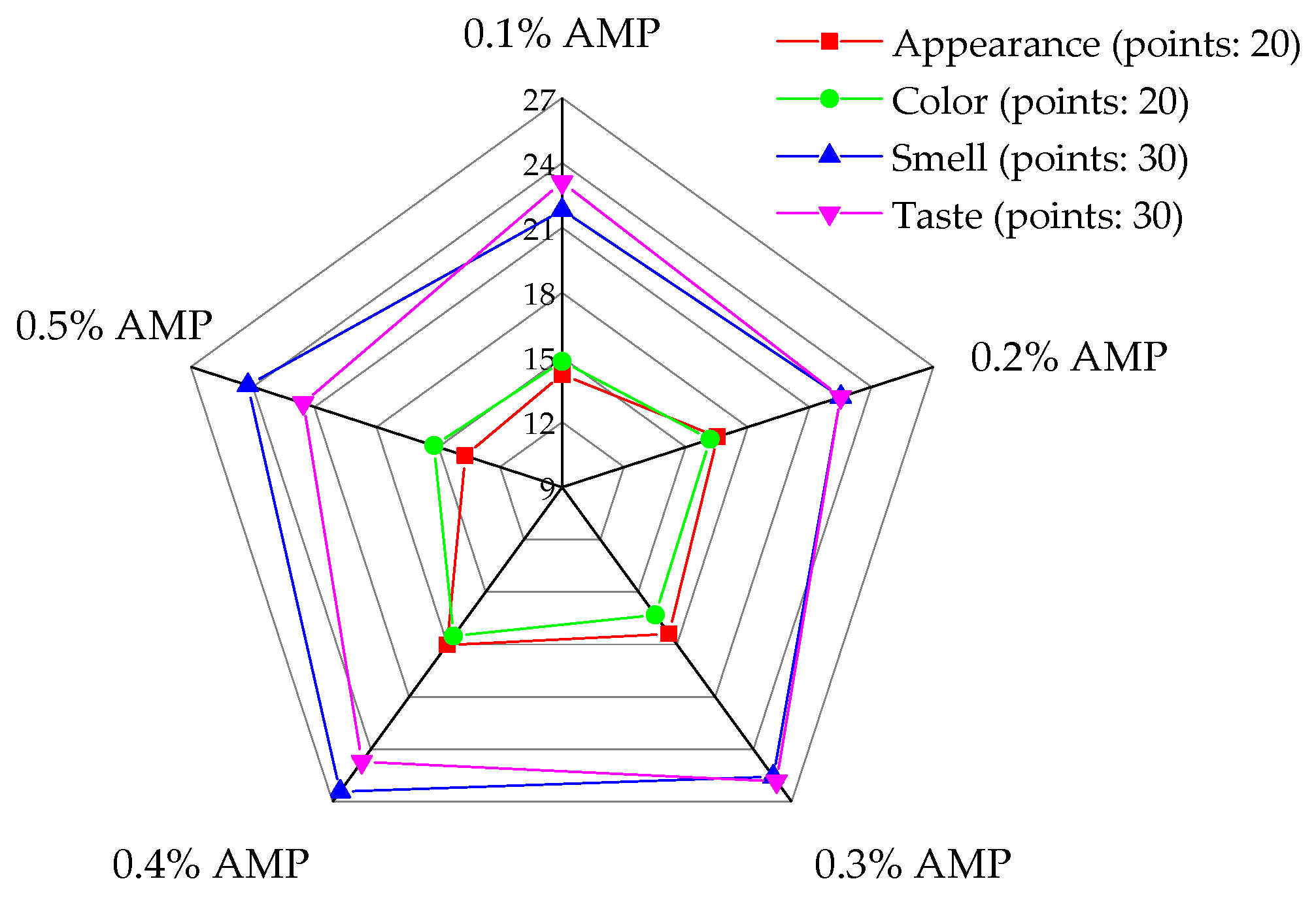
| Index | Score (Points) | Evaluation Project |
|---|---|---|
| Appearance (20 points) | 16~20 | At room temperature, the product is in paste form, with crystalline solids possibly present. AMP powder may also be observed and uniformly distributed. |
| 11~15 | The product is in a paste state at room temperature, with crystalline solids potentially present. AMP is uniformly distributed throughout. | |
| <11 | The product is in paste form at room temperature, with some or all crystalline solids potentially present. AMP is unevenly distributed. | |
| Color (20 points) | 16~20 | It has a deep purple color characteristic of AMP and is uniformly distributed. |
| 11~15 | The color is pale, and the distribution is more uniform. | |
| <11 | The color is too light, and the distribution is uneven. | |
| Smell (30 points) | 21~30 | It has the aroma of nectar-producing flowers, with the inclusion of Aronia melanocarpa, and lacks any undesirable odor. |
| 11~20 | The honey flavor and Aronia melanocarpa fruit flavor are strong or light, without any undesirable odor. | |
| <11 | The honey flavor and the Aronia melanocarpa fruit flavor are too strong or too weak, accompanied by a distinct off-putting odor. | |
| Taste (30 points) | 21~30 | It has a viscous mouthfeel, a distinct berry flavor, and a rich taste. |
| 11~20 | It has a thick, sticky mouthfeel, a subtle berry flavor, and a rough flavor. | |
| <11 | There is an absence of sticky mouthfeel, berry flavor, and bitter or sour flavor, along with a rough texture. |
| Peak No. | Chemical Name | Formula | Exact Mass (m/z) | RT (min) | Characteristic MS/MS Ions (m/z) |
|---|---|---|---|---|---|
| 1 | Cyanidin-3- glucoside | C21H21O11 | 449.10541 [M+H]+1 | 4.236 | 287.05408 |
| 2 | Cyanidin-3- O-arabinoside | C20H19O10 | 419.09497 [M+H]+1 | 4.318 | 287.05396 |
| 3 | Cyanidin-3- galactoside | C21H21O11 | 449.16202 [M+H]+1 | 3.439 | 287.05490 |
| 4 | Cyanidin-3- galactoside | C20H19O10 | 418.38831 [M+H]+1 | 8.172 | 287.05353 |
| 5 | Kaempferol | C15H10O6 | 287.05383 [M+H]+1 | 4.289 | 241.04889, 213.05402 |
| 6 | Quercetin | C15H10O7 | 303.04919 [M+H]+1 | 4.880 | 285.02867, 257.04352, 229.04895 |
| 7 | Quercetin-3-D- xyloside | C20H18O11 | 433.20703 [M-H]−1 | 5.066 | 300.02686, 255.02942, 136.35802 |
| 8 | Quercetin-3- arabinoside | C20H18O11 | 433.07681 [M-H]−1 | 5.066 | 300.02686, 255.02942, 136.35802 |
| 9 | Quercetin-3β- D-glucoside | C21H20O12 | 463.08563 [M-H]−1 | 4.840 | 301.03455, 255.02928, 227.03438, 151.00238 |
| 10 | Quercetin-3-O-sangbu disaccharide | C26H28O16 | 595.12933 [M-H]−1 | 4.671 | 301.03452, 255.02934 |
| 11 | Catechins | C15H14O6 | 289.07138 [M-H]−1 | 4.557 | 245.07950, 136.50128 |
| 12 | Rutin | C27H30O16 | 609.14508 [M-H]−1 | 4.784 | 301.0388, 255.02943 |
| 13 | Chlorogenic acid | C16H18O9 | 353.08636 [M-H]−1 | 4.390 | 191.05478, 135.04340 |
| 14 | Chlorogenic acid | C16H18O9 | 353.08636 [M-H]−1 | 4.582 | 191.05478, 135.04340 |
| 15 | Neochlorogenic acid | C16H18O9 | 353.08643 [M-H]−1 | 4.132 | 191.05502, 135.04367 |
| 16 | Gallic acid | C7H6O5 | 171.99263 [M+H]+1 | 14.740 | 153.88593, 130.96609, 107.95045, 97.34501 |
| 17 | Caffeic acid | C9H8O4 | 163.03850 [M+H-H2O]+1 | 4.403 | 135.04376, 117.03347, 89.03881 |
| Sample Name | Amylase (mL/g·h) | Glucose Oxidase (μg/g·0.5 h) | Sucrase (mg/g·h) |
|---|---|---|---|
| Original Honey | 8.58 ± 0.15 a | 89.52 ± 2.69 a | 43.74 ± 2.70 a |
| 0.1% AMP Honey | 7.74 ± 0.27 b | 83.12 ± 1.56 b | 41.84 ± 1.66 ab |
| 0.2% AMP Honey | 7.11 ± 0.44 bc | 78.79 ± 2.19 b | 39.21 ± 1.72 bc |
| 0.3% AMP Honey | 6.94 ± 0.35 c | 71.99 ± 1.32 c | 36.56 ± 1.43 cd |
| 0.4% AMP Honey | 6.58 ± 0.31 c | 67.61 ± 2.34 c | 33.49 ± 1.47 de |
| 0.5% AMP Honey | 5.21 ± 0.26 d | 57.81 ± 1.99 d | 29.47 ± 1.09 e |
| Sample Name | Solubility Time(s) | Solubility Situation |
|---|---|---|
| Original Honey | 49.67 ± 2.08 d | Clear and transparent, without impurities, with the smell of honey water. |
| 0.1% AMP Honey | 71.00 ± 2.65 ab | Clear and transparent, with a very light pink color and no impurities. It has the scent of honey water and a very light berry aroma. |
| 0.2% AMP Honey | 66.67 ± 4.16 bc | Clear and transparent, light pink, free of impurities, with the smell of honey water and a light berry smell. |
| 0.3% AMP Honey | 63.00 ± 3.61 c | Clear, pink, free of impurities, with the smell of honey water and a certain berry smell. |
| 0.4% AMP Honey | 77.67 ± 1.15 a | Clear and transparent, dark pink, without impurities, with the smell of honey water and an obvious berry smell. |
| 0.5% AMP Honey | 74.67 ± 6.81 a | Clear and transparent, pink-purple, with trace amounts of undissolved AMP particles, with the smell of honey water and a strong berry smell. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hao, J.; Wang, J.; Wang, S.; Fan, Z. Preparation of Functional Food with Enhanced Antioxidant Properties by Adding Aronia melanocarpa Polyphenol Honey. Foods 2024, 13, 3852. https://doi.org/10.3390/foods13233852
Wang J, Hao J, Wang J, Wang S, Fan Z. Preparation of Functional Food with Enhanced Antioxidant Properties by Adding Aronia melanocarpa Polyphenol Honey. Foods. 2024; 13(23):3852. https://doi.org/10.3390/foods13233852
Chicago/Turabian StyleWang, Jingyi, Jiahui Hao, Jie Wang, Siyu Wang, and Ziluan Fan. 2024. "Preparation of Functional Food with Enhanced Antioxidant Properties by Adding Aronia melanocarpa Polyphenol Honey" Foods 13, no. 23: 3852. https://doi.org/10.3390/foods13233852
APA StyleWang, J., Hao, J., Wang, J., Wang, S., & Fan, Z. (2024). Preparation of Functional Food with Enhanced Antioxidant Properties by Adding Aronia melanocarpa Polyphenol Honey. Foods, 13(23), 3852. https://doi.org/10.3390/foods13233852






