Characterization and Potential Food Applications of Oat Flour and Husks from Differently Colored Genotypes as Novel Nutritional Sources of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Plant Material
2.3. Chemical Procedures
2.3.1. Analysis of Basic Chemical Composition
2.3.2. Analysis of the Alcohol-Soluble Protein Fraction
2.3.3. Analysis of Dietary Fibers
2.3.4. Analysis of β-Glucan
2.3.5. Analysis of Resistant Starch
2.3.6. Extraction of Total Phenolic Compounds
2.3.7. Analysis of Total Phenolic Compounds (TPCs)
2.3.8. Analysis of Phenolic Acids
2.3.9. Analysis of Total Antioxidant Capacity (TAC)
2.4. Baking Functionality Properties
2.4.1. Gelling Properties
2.4.2. Solvent Retention Capacity (SRC)
2.5. In Vitro Multistep Enzymatic Digestion Protocol
2.6. Antimicrobial Activity Evaluation
2.6.1. Extract Preparation
2.6.2. Antimicrobial Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Profile of OF and OH
3.1.1. Basic Chemical Composition
3.1.2. Dietary Fibers
3.2. Phenolic Compounds and Antioxidant Capacity
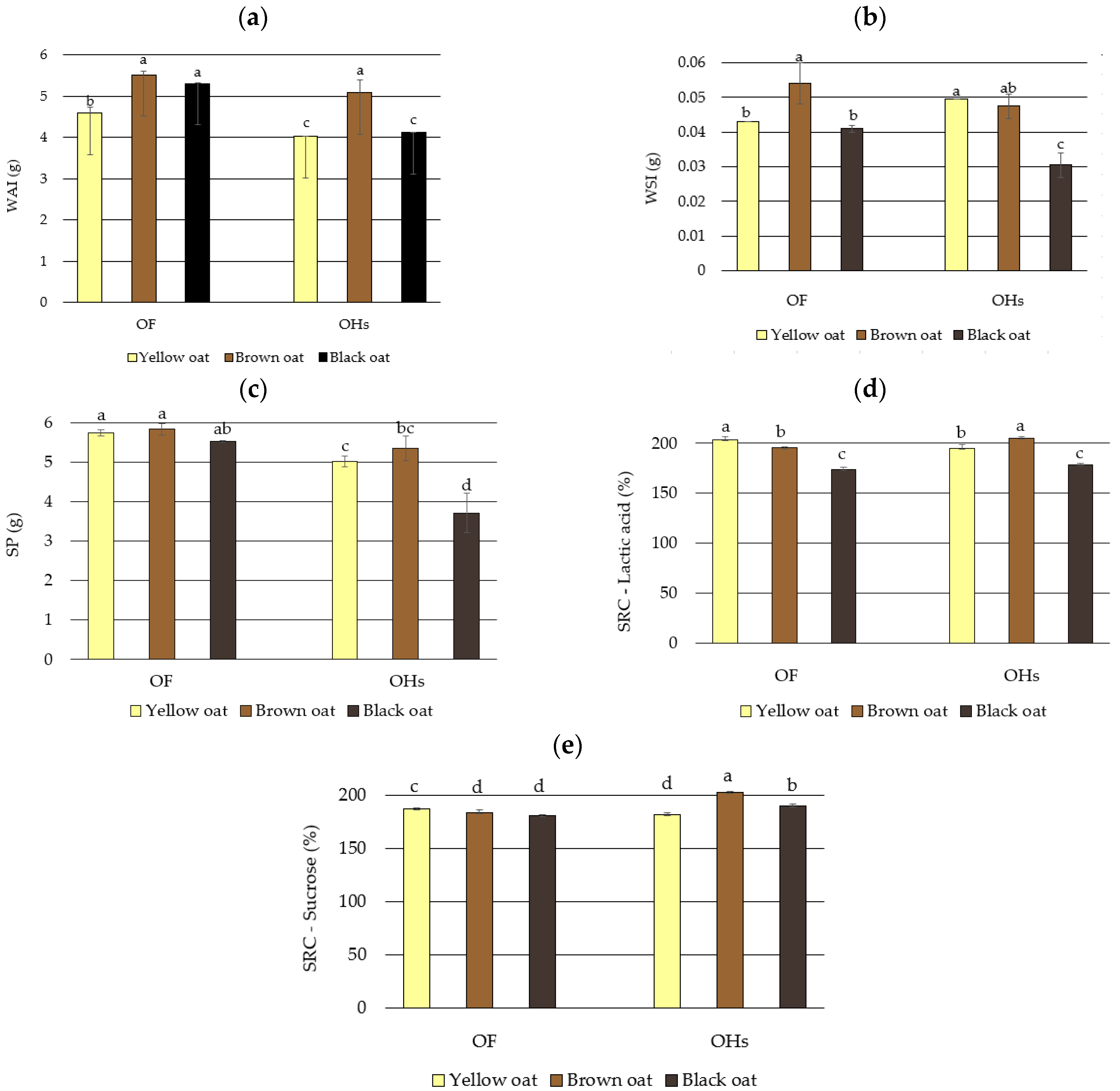
3.3. Baking Functionality
3.4. In Vitro Digestibility
3.5. Antimicrobial Activity of the Oat Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batîr Rusu, D.C.; Murariu, D.; Gheorghita, R.; Graur, M. Some nutritional value aspects of barley and oat and their impact in human nutrition and healthy life. Plants 2024, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Venn, B.J.; Mann, J.I. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W. Whole grains protect against atherosclerotic cardiovascular disease. Proc. Nutr. Soc. 2003, 62, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (Oat), A potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2012, 53, 126–144. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, W.; Zhang, T.; Miao, M. Structure and functional characteristics of starch from different hulled oats cultivated in China. Carbohydr. Polym. 2024, 330, 121791. [Google Scholar] [CrossRef]
- Jágr, M.; Hofinger-Horvath, A.; Ergang, P.; Čepková, P.H.; Schönlechner, R.; Pichler, E.C.; D’Amico, S.; Grausgruber, H.; Vagnerová, K.; Dvořáček, V. Comprehensive study of the effect of oat grain germination on the content of avenanthramides. Food Chem. 2024, 437, 137807. [Google Scholar] [CrossRef]
- Quiñones-Muñoz, T.A.; Villanueva-Rodríguez, S.J.; Torruco-Uco, J.G. Nutraceutical properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L. A review of metabolites and mechanisms. Metabolites 2022, 12, 806. [Google Scholar] [CrossRef]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs; Integrative Medicine Communications: Boston, MA, USA, 2000; p. xiii+519. [Google Scholar]
- Abascal, K.; Yarnell, E. Nervine herbs for treating anxiety. Altern. Complement. Ther. 2004, 10, 309–315. [Google Scholar] [CrossRef]
- Bi, Y.; Gao, F.; Guo, J.; Yao, X.; Wang, A.; Liu, H.; Sun, Y.; Yao, R.; Li, M. An ethnobotanical survey on the medicinal and edible plants used by the Daur people in China. J. Ethnobiol. Ethnomed. 2024, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Sargin, S.A. Plants used against obesity in Turkish folk medicine: A review. J. Ethnopharmacol. 2021, 270, 113841. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; De Marco, E.V.; Gallo, O.; Tagarelli, G. Italian folk plant-based remedies to heal headache (XIX–XX century). J. Ethnopharmacol. 2018, 210, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Tucakov, J. Ovas—Avena sativa. In Lečenje Biljem, New, Amended and Supplemented Edition in Serbian; Vulkan Izdavaštvo: Belgrade, Serbia, 2014; p. 439. [Google Scholar]
- Rodríguez, J.M.; Estévez, V.; Bascuñán, K.; Ayala, J.; Araya, M. Commercial oats in gluten-free diet: A persistent risk for celiac patients. Front. Nutr. 2022, 9, 986282. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Karaca, A.C.; Deng, L.; Colussi, R.; Narita, I.M.P.; Kaur, K.; Aaliya, B.; Sunooj, K.V.; Falsafi, S.R. Oat starch—How physical and chemical modifications affect the physicochemical attributes and digestibility? Carbohydr. Polym. 2022, 296, 119931. [Google Scholar] [CrossRef]
- Sunilkumar, B.A.; Leonova, S.; Oste, R.; Olsson, O. Identification and characterization of high protein oat lines from a mutagenized oat population. J. Cereal Sci. 2017, 75, 100–107. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, C.; Cui, J.; Fan, M.; Li, Y.; Huang, L.; Wang, L. Analysis of the effect of oat β-glucan on gut microbiota and clarification of their interaction relationship. J. Cereal Sci. 2024, 117, 103921. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.-Y.; Liu, L.; Wu, C.-Y.; Liu, H.; Zhao, L.; Zhang, Q.; Liu, Y.-T.; Li, S.-Q.; Qin, W.; et al. Effects of sulfated modification on the physicochemical properties and biological activities of β-glucans from Qingke (Tibetan hulless barley). Int. J. Biol. Macromol. 2019, 141, 41–50. [Google Scholar] [CrossRef]
- Žilić, S.; Šukalović, V.H.T.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Schmitz, E.; Karlsson, E.N.; Adlercreutz, P. Ultrasound assisted alkaline pretreatment efficiently solubilises hemicellulose from oat hulls. Waste Biomass Valorization 2021, 12, 5371–5381. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Beltrame, G.; Laaksonen, O.; Yang, B. Ultrasonication-assisted enzymatic bioprocessing as a green method for valorizing oat hulls. Food Chem. 2023, 426, 136658. [Google Scholar] [CrossRef] [PubMed]
- Savas, B.S.; Akan, E. Oat bran fortified raspberry probiotic dairy drinks: Physicochemical, textural, microbiologic properties, in vitro bioaccessibility of antioxidants and polyphenols. Food Biosci. 2021, 43, 101223. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; de Sotomayor, M.A.; Herrera, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Nemes, S.A.; Fărcas, A.C.; Ranga, F.; Teleky, B.E.; Călinoiu, L.F.; Dulf, F.V.; Vodnar, D.C. Enhancing phenolic and lipid compound production in oat bran via acid pretreatment and solid-state fermentation with Aspergillus niger. New Biotechnol. 2024, 83, 91–100. [Google Scholar] [CrossRef]
- Tian, W.; Hu, R.; Chen, G.; Zhang, Y.; Wang, W.; Li, Y. Potential bioaccessibility of phenolic acids in whole wheat products during in vitro gastrointestinal digestion and probiotic fermentation. Food Chem. 2021, 362, 130135. [Google Scholar] [CrossRef]
- Spinola, V.; Llorent-Martinez, E.J.; Castilho, P.C. Polyphenols of Myrica faya inhibit key enzymes linked to type II diabetes and obesity and formation of advanced glycation end-products (in vitro): Potential role in the prevention of diabetic complications. Food Res. Int. 2019, 116, 1229–1238. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- ISO 10520; Determination of Starch Content-Ewers Polarimetric Method. ISO—International Organization for Standardization: Geneva, Switzerland, 1997.
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lookhart, G.; Bean, S. Separation and characterization of wheat protein fractions by high-performance capillary electrophoresis. Cereal Chem. 1995, 72, 527–532. [Google Scholar]
- Žilić, S.; Milašinović, M.; Terzić, D.; Barać, M.; Ignjatović Micić, D. Grain characteristics and composition of maize specialty hybrids. Span. J. Agric. Res. 2011, 9, 230–241. [Google Scholar] [CrossRef]
- Megazyme, 2023. β-Glucan (Mixed Linkage) Assay Protocol K-BGLU. Available online: https://d1kkimny8vk5e2.cloudfront.net/documents/Assay_Protocol/K-BGLU_DATA.pdf (accessed on 1 November 2024).
- Megazyme, 2023a. Resistant Starch (Rapid) Assay Protocol K-RAPRS. Available online: https://d1kkimny8vk5e2.cloudfront.net/documents/Assay_Protocol/K-RAPRS_DATA.pdf (accessed on 1 November 2024).
- Žilić, S.; Akıllıoğlu, H.G.; Serpen, A.; Perić, V.; Gökmen, V. Comparisons of phenolic compounds, isoflavones, antioxidant capacity and oxidative enzymes in yellow and black soybeans seed coat and dehulled bean. Eur. Food Res. Technol. 2013, 237, 409–418. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioglu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Cornejo, F.; Rosell, C.M. Physicochemical properties of long rice grain varieties in relation to gluten free bread quality. LWT-Food Sci. Technol. 2015, 62, 1203–1210. [Google Scholar] [CrossRef]
- Haynes, L.C.; Bettge, A.D.; Slade, L. Soft wheat and flour products methods review: Solvent retention capacity equation correction. Cereal Foods World 2009, 54, 174–175. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Formation and elimination reactions of 5-hydroxymethylfurfural during in vitro digestion of 635 biscuits. Food Res. Int. 2017, 99, 308–314. [Google Scholar] [CrossRef]
- Mao, H.; Xu, M.; Ji, J.; Zhou, M.; Li, H.; Wen, Y.; Wang, J.; Sun, B. The utilization of oat for the production of wholegrain foods: Processing technology and products. Food Front. 2022, 3, 28–45. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, K.; Basha, S.J.; Kennedy, J.F. Current trends in the preparation, characterization and applications of oat starch—A review. Int. J. Biol. Macromol. 2022, 212, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, Y.; Zhao, Y.; Yang, Z.; Jia, Q.; Feng, B.; David, C.; Taylor, D.C.; Du, C.; Zhang, M. Lipidomics studies reveal dynamic changes in polar lipids of developing endosperm of oat and wheat varieties with differing oil contents. Food Chem. 2024, 444, 138597. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Pokhrel, K.; Božik, M.; Tilami, S.K.; Horčička, P. Fat content and fatty acid profiles of recently registered varieties of naked and hulled oats with and without husks. J. Cereal Sci. 2021, 99, 103216. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ahmad, A.; Sohail, A.; Asad, M.J. Nutritional and functional characterization of different oat (Avena sativa L.) cultivars. Int. J. Food Prop. 2020, 23, 1373–1385. [Google Scholar] [CrossRef]
- Usman, S.; Nazir, S.; Ali, S.; Nasreen, Z.; Najim, A. Determination of biochemical composition of Avena sativa (oat) and to estimate the effect of high fiber diet on hypercholesterolemic rats. Bangladesh Res. Publ. J. 2010, 4, 312–319. [Google Scholar]
- Neitzel, N.; Eder, M.; Hosseinpourpia, R.; Walther, T.; Adamopoulos, S. Chemical composition, particle geometry, and micro-mechanical strength of barley husks, oat husks, and wheat bran as alternative raw materials for particleboards. Mater. Today Commun. 2023, 36, 106602. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, L.; Huang, L.; Shi, Z.; Dong, J.; Yao, Y.; Shen, R. Effects of oat β-glucan, oat resistant starch, and the whole oat flour on insulin resistance, inflammation, and gut microbiota in high-fat-diet-induced type 2 diabetic rats. J. Funct. Foods 2020, 69, 103939. [Google Scholar] [CrossRef]
- Ingvordsen, C.H.; Gislum, R.; Jørgensen, J.R.; Mikkelsen, T.N.; Stockmarr, A.; Jørgensen, R.B. Grain protein concentration and harvestable protein under future climate conditions. A Study of 108 Spring Barley Accessions. J. Exp. Bot. 2016, 67, 2151–2158. [Google Scholar] [CrossRef]
- Miller, S.S.; Fulcher, R.G. Microstructure and chemistry of the oat kernel, In Oats: Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2011; pp. 77–94. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Ercili-Cura, D.; Poutanen, K.; Holopainen-Mantila, U.; Lehtinen, P.; Sozer, N. Protein from oat: Structure, processes, functionality, and nutrition. In Sustainable Protein Sources; Nadathur, S., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2024; pp. 121–141. [Google Scholar] [CrossRef]
- Shewry, P.R. Avenins: The prolamins of oats. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherland, 1999; pp. 79–92. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Konitzer, K. Gluten–the precipitating factor. In Celiac Disease and Gluten: Multidisciplinary Challenges and Opportunities, 1st ed.; Wieser, H., Koehler, P., Konitzer, K., Eds.; Elsevier: Amsterdam, The Netherland, 2014; pp. 97–148. [Google Scholar] [CrossRef]
- Kosová, K.; Leišová-Svobodová, L.; Dvořáček, V. Oats as a safe alternative to Triticeae cereals for people suffering from celiac disease? A review. Plant Foods Hum. Nutr. 2020, 75, 131–141. [Google Scholar] [CrossRef]
- Ahola, H.G.; Sontag-Strohm, T.S.; Schulman, A.H.; Tanhuanpää, P.; Viitala, S.; Huang, X. Immunochemical analysis of oat avenins in an oat cultivar and landrace collection. J. Cereal Sci. 2020, 95, 103053. [Google Scholar] [CrossRef]
- Sha, X.; Sun, H.; Li, Y.; Wu, J.; Zhang, H.; Yang, R. The prolamins, from structure, property, to the function in encapsulation and delivery of bioactive compounds. Food Hydrocoll. 2023, 149, 109508. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanin in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosad, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Žilić, S.; Dodig, D.; Milašinović-Šeremešić, M.; Kandić, V.; Kostadinović, M.; Prodanović, S.; Savić, Đ. Small grain cereals compared for dietary fibre and protein contents. Genetika 2011, 43, 381–395. [Google Scholar] [CrossRef]
- Nikolić, V.; Žilić, S.; Radosavljević, M.; Vančetović, J.; Božinović, S. Properties of different silage maize hybrids. Food Feed Res. 2020, 47, 139–147. [Google Scholar] [CrossRef]
- Pichler, E.C.; Schönlechner, R.; Różyło, R.; Dziki, D.; Świeca, M. Addition of micronized oat husk fiber to gluten-free bread-Effects on chemical, physical and physiological properties. J. Cereal Sci. 2024, 118, 103981. [Google Scholar] [CrossRef]
- Majumdar, A.; Gil-González, A.B.; Grau, A.B.; Sardari, R.R.; Larsson, O.; Thyagarajan, A.; Hansson, A.; Hernández-Hernández, O.; Olsson, O.; Zambrano, J.A. Macromolecular characterization of high β-glucan oat lines. Heliyon 2024, 10, e24552. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, R.; Hu, X.; Ren, C.; Li, Y. Oat-based foods: Chemical constituents, glycemic index, and the effect of processing. Foods 2021, 10, 1304. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Xia, J.; Zhang, Y.; Huang, K.; Cao, H.; Sun, Q.; Wang, M.; Zhang, S.; Sun, Z.; Guan, X. Different multi-scale structural features of oat resistant starch prepared by ultrasound combined enzymatic hydrolysis affect its digestive properties. Ultrason. Sonochem. 2023, 96, 106419. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Amare, E. Nutritional and phytochemical composition and associated health benefits of oat (Avena sativa) grains and oat-based fermented food products. Sci. World J. 2023, 1, 2730175. [Google Scholar] [CrossRef]
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chem. 1999, 76, 902–906. [Google Scholar] [CrossRef]
- Hodzic, Z.; Pasalic, H.; Memisevic, A.; Srabovic, M.; Saletovic, M.; Poljakovic, M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur. J. Sci. Res. 2009, 28, 471–477. [Google Scholar]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci. 2001, 41, 1676–1681. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, B.; Luo, S.; Wu, G.; Hui, X. Inducing apoptosis in human hepatocellular carcinoma cell lines via Nrf2/HO-1 signalling pathway of blueberry and blackcurrant powder manipulated oat bran paste extracts. J. Funct. Foods 2022, 89, 104967. [Google Scholar] [CrossRef]
- Majzoobi, M.; Raiss Jalali, A.; Farahnaky, A. Impact of whole oat flour on dough properties and quality of fresh and stored part baked bread. J. Food Qual. 2016, 39, 620–626. [Google Scholar] [CrossRef]
- Smuda, S.S.; Mohsen, S.M.; Olsen, K.; Aly, M.H. Bioactive compounds and antioxidant activities of some cereal milling by-products. J. Food Sci. Technol. 2018, 55, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Simić, M.; Kandić, V.; Dodevska, M.; Titan, P.; Dodig, D.; Žilić, S. Pasting properties and the baking functionality of whole-grain wheat flour with different amylose and dietary fibers content. J. Food Process. Preserv. 2022, 46, e15805. [Google Scholar] [CrossRef]
- Hammed, A.M.; Ozsisli, B.; Ohm, J.; Simsek, S. Relationship between solvent retention capacity and protein molecular weight distribution, quality characteristics, and breadmaking functionality of hard red spring wheat flour. Cereal Chem. 2016, 92, 466–474. [Google Scholar] [CrossRef]
- Pasha, I.; Anjum, F.M.; Butt, M.S. Genotypic variation of spring wheats for solvent retention capacities in relation to end-use quality. LWT-Food Sci. Technol. 2009, 42, 418–423. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins, and carbohydrates-influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical profile, bioactivity, and prebiotic potential of bound phenolics released from rice bran dietary fiber during in vitro gastrointestinal digestion and colonic fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Wirkijowska, A.; Gasiński, A.; Średnicka-Tober, D.; Trafiałek, J.; Kazimierczak, R. Oat and oat processed products—Technology, composition, nutritional value, and health. Appl. Sci. 2023, 13, 11267. [Google Scholar] [CrossRef]
- Alrahmany, R.; Avis, T.J.; Tsopmo, A. Treatment of oat bran with carbohydrases increases soluble phenolic acid content and influences antioxidant and antimicrobial activities. Food Res. Int. 2013, 52, 568–574. [Google Scholar] [CrossRef]
- Lim, S.; Yun, C.-I.; Song, D.; Kang, S.-S.; Kim, Y.-J. Validation of avenanthramide and other phenolic compounds in oats and sprouted oats and their antimicrobial properties against Escherichia coli O157:H7. Food Sci. Biotechnol. 2022, 31, 1145–1155. [Google Scholar] [CrossRef]
- Verardo, V.; Serea, C.; Segal, R.; Caboni, M.F. Free and bound minor polar compounds in oats: Different extraction methods and analytical determinations. J. Cereal Sci. 2011, 54, 211–217. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Hu, X. Enhancement of polyphenol content and antioxidant capacity of oat (Avena nuda L.) bran by cellulase treatment. Appl. Biol. Chem. 2016, 59, 397–403. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D. Honey melanoidins: Analysis of the compositions of the high molecular weight melanoidins exhibiting radical-scavenging activity. Food Chem. 2011, 127, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Kukuminato, S.; Koyama, K.; Koseki, S. Antibacterial properties of melanoidins produced from various combinations of Maillard reaction against pathogenic bacteria. Microbiol. Spectr. 2021, 9, e01142-21. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Verma, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef]
- Rurian-Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Toth, I.V.; Rangel, A.O.S.S.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Sshmamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Cueva, C.; Victoria Moreno-Arribas, M.; Martin-Alvarez, P.J.; Bills, G.; Francisca Vicente, M.; Basilio, A.; López Rivas, C.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. J. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Ojha, D.; Patil, K.N. p-Coumaric acid inhibits the Listeria monocytogenes RecA protein functions and SOS response: An antimicrobial target. Biochem. Biophys. Res. Commun. 2019, 517, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Derebasi, B.; Bulut, S.; Erden, B.; Sadeghian, N.; Taslimi, P.; Celebioglu, H. Effects of p-coumaric acid on probiotic properties of Lactobacillus acidophilus LA-5 and Lacticaseibacillus rhamnosus GG. Arch. Microbiol 2024, 206, 223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chellappan, D.; Chinnaswamy, P.; Nagarajan, S. Protective effect of p-coumaric acid against 1, 2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017, 94, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Syfani, N.; Prima Putra, D.; Arbain, D. 3,4-dihydroxybenzaldehyde from the fern Trichomanes chinense L.; isolation antimicrobial and antioxidant properties. Indones. J. Chem. 2012, 12, 273–278. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Juszczuk-Kubiak, E.; Świsłocka, R.; Shing, W.L.; Kesari, K.K.; Prakash, B.; Lewandowski, W. Biological effects of vanillic acid, iso-vanillic acid, and orto-vanillic acid as environmental pollutants. Ecotoxicol. Environ. Saf. 2024, 277, 116383. [Google Scholar] [CrossRef]
- Duskaev, D.; Kurilkina, M.; Zavyalov, O. Growth-stimulating and antioxidant effects of vanillic acid on healthy broiler chickens. Vet. World 2023, 16, 518–525. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Patil, P.A. A review of the pharmacological characteristics of vanillic acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.; Govender, A.; Olagunju, O.; Oyedeji, A. The antimicrobial activity of two phenolic acids against foodborne Escherichia coli and Listeria monocytogenes and their effectiveness in a meat system. Ital. J. Food Sci. 2021, 33, 39–45. [Google Scholar] [CrossRef]
- Kepa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Vaquero, M.; Aredes, P.; Nadra, M. Effect of phenolic compound mixtures on the viability of Listeria monocytogenes in meat model. Food Technol. Biotechnol. 2011, 49, 83–88. [Google Scholar]
- Seether, K.A.; Miya, T.S.; Perry, T.W.; Boehm, P.N. Extraction of an ulcer-preventing principle from oat hulls. J. Anim. Sci. 1971, 32, 1160–1163. [Google Scholar] [CrossRef]

| Sample | Dry Matter | Total Starch | Total Protein | Alcohol-Soluble Proteins | Fat | Ash | |
|---|---|---|---|---|---|---|---|
| (%) | (% d.m.) | (% d.m.) | (% d.m.) | (% t.p.) | (% d.m.) | (% d.m.) | |
| OF | |||||||
| Yellow | 90.60 e | 55.71 ± 0.01 b | 17.38 ± 0.10 a | 2.04 ± 0.05 b | 11.73 | 5.45 ± 0.16 c | 2.44 ± 0.01 d |
| Brown | 90.92 d | 57.38 ± 0.54 a | 16.67 ± 0.09 b | 2.31 ± 0.11 a | 13.86 | 7.20 ± 0.14 a | 2.30 ± 0.02 e |
| Black | 90.99 d | 57.58 ± 1.15 a | 13.43 ± 0.01 c | 1.60 ± 0.01 c | 11.91 | 6.15 ± 0.21 b | 2.19 ± 0.04 f |
| OHs | |||||||
| Yellow | 96.06 a | n.d. | 4.79 ± 0.06 d | 0.64 ± 0.09 d | 13.36 | 1.23 ± 0.18 d | 5.85 ± 0.02 a |
| Brown | 94.69 b | n.d. | 4.54 ± 0.02 e | 0.64 ± 0.10 d | 14.10 | 1.02 ± 0.05 d | 4.64 ± 0.00 b |
| Black | 94.51 c | n.d. | 3.09 ± 0.02 f | 0.56 ± 0.01 d | 18.12 | 1.32 ± 0.06 d | 5.00 ± 0.00 b |
| Sample | NDF | ADF | ADL | Hemicellulose | Cellulose | β-Glucan | Resistant Starch |
|---|---|---|---|---|---|---|---|
| OF | |||||||
| Yellow | 15.83 ± 0.31 f | 3.11 ± 0.08 d | 0.89 ± 0.20 d | 12.72 ± 0.40 e | 2.22 ± 0.28 d | 5.33 ± 0.01 a | 2.95 ± 0.39 b |
| Brown | 32.36 ± 0.25 d | 3.65 ± 0.12 d | 1.29 ± 0.47 cd | 28.71 ± 0.35 c | 2.36 ± 0.38 d | 4.07 ± 0.16 c | 2.43 ± 1.03 a |
| Black | 17.22 ± 0.04 e | 3.34 ± 0.26 d | 0.89 ± 0.17 d | 13.88 ± 0.23 d | 2.45 ± 0.09 d | 4.66 ± 0.00 b | 2.91 ± 0.50 b |
| OHs | |||||||
| Yellow | 80.27 ± 0.08 b | 38.96 ± 0.54 a | 6.00 ± 0.11 a | 41.31 ± 0.62 b | 32.96 ± 0.43 c | 0.06 ± 0.00 d | n.d. |
| Brown | 77.91 ± 0.24 c | 36.04 ± 0.69 c | 1.86 ± 0.28 b,c | 41.87 ± 0.45 b | 34.18 ± 0.42 b | 0.06 ± 0.00 d | n.d. |
| Black | 83.82 ± 0.22 a | 37.71 ± 0.08 b | 2.39 ± 0.26 b | 46.11 ± 0.13 a | 35.32 ± 0.18 a | 0.03 ± 0.01 d | n.d. |
| Sample | TPC (μg GAE/g) | p-Coumaric Acid (μg/g) | Ferulic Acid (μg/g) | Isoferulic Acid (μg/g) | Vanillic Acid (μg/g) | Syringic Acid (μg/g) | TAC (mmol Trolox Eq/kg) |
|---|---|---|---|---|---|---|---|
| OF | |||||||
| Yellow | 875.92 ± 11.73 d | 90.96 ± 8.98 c | 395.88 ± 13.72 d | n.d. | 23.05 ± 0.48 d | 18.14 ± 0.83 c | 23.85 ± 1.46 cd |
| Brown | 841.89 ± 54.16 d | 82.1 ± 8.67 c | 448.72 ± 20.65 d | 8.35 ± 1.07 a | 22.74 ± 0.95 d | 19.76 ± 1.34 c | 24.28 ± 1.36 c |
| Black | 982.08 ± 43.69 d | 84.40 ± 5.10 c | 589.14 ± 13.01 d | 10.96 ± 0.67 a | 16.66 ± 0.72 d | 19.47 ± 0.79 c | 22.05 ± 1.01 d |
| OHs | |||||||
| Yellow | 12,086.76 ± 259.41 b | 6732.36 ± 325.83 a | 4987.02 ± 110.36 c | 29.11 ± 0.94 a | 101.44 ± 5.53 c | 65.54 ± 0.73 b | 37.32 ± 2.03 b |
| Brown | 21,971.52 ± 890.64 a | 2749.63 ± 30.12 b | 13,794.82 ± 122.57 a | 49.85 ± 57.29 a | 442.62 ± 0.97 b | 92.79 ± 5.36 a | 48.19 ± 0.11 a |
| Black | 24,352.48 ± 528.41 c | 2726.76 ± 60.14 b | 13,271.2 ± 59.1 b | 70.59 ± 6.55 a | 619.38 ± 8.26 a | 63.25 ± 6.85 b | 46.64 ± 1.01 a |
| Strain | Yellow | Brown | Black | |||
|---|---|---|---|---|---|---|
| OF | OHs | OF | OHs | OF | OHs | |
| Hydrolyzed samples | ||||||
| Listeria monocytogenes | <140 | <140 | 200 | 160 | 180 | 140 |
| Staphylococcus haemolyticus | 140 | 140 | >200 | >200 | >200 | >200 |
| Salmonella typhimurium | >200 | 180 | >200 | 200 | >200 | >200 |
| Enterococcus faecalis | 140 | 140 | >200 | 180 | 180 | >200 |
| Escherichia coli | 140 | 140 | 200 | 140 | 140 | 140 |
| Shigella flexneri | 160 | 150 | >200 | 180 | >200 | >200 |
| Traditionally prepared samples | ||||||
| Listeria monocytogenes | >200 | >200 | >200 | 160 | 220 | 150 |
| Staphylococcus haemolyticus | 180 | 150 | >200 | 150 | >200 | 150 |
| Salmonella typhimurium | >200 | >200 | >200 | 200 | >200 | 200 |
| Enterococcus faecalis | >200 | 200 | >200 | 180 | >200 | 180 |
| Escherichia coli | 180 | 180 | 220 | 160 | 180 | 160 |
| Shigella flexneri | 220 | 180 | >200 | 180 | >200 | 160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, V.; Žilić, S.; Simić, M.; Šavikin, K.; Stević, T.; Živković, J.; Sarić, B.; Milovanović, D.; Kandić Raftery, V. Characterization and Potential Food Applications of Oat Flour and Husks from Differently Colored Genotypes as Novel Nutritional Sources of Bioactive Compounds. Foods 2024, 13, 3853. https://doi.org/10.3390/foods13233853
Nikolić V, Žilić S, Simić M, Šavikin K, Stević T, Živković J, Sarić B, Milovanović D, Kandić Raftery V. Characterization and Potential Food Applications of Oat Flour and Husks from Differently Colored Genotypes as Novel Nutritional Sources of Bioactive Compounds. Foods. 2024; 13(23):3853. https://doi.org/10.3390/foods13233853
Chicago/Turabian StyleNikolić, Valentina, Slađana Žilić, Marijana Simić, Katarina Šavikin, Tatjana Stević, Jelena Živković, Beka Sarić, Danka Milovanović, and Vesna Kandić Raftery. 2024. "Characterization and Potential Food Applications of Oat Flour and Husks from Differently Colored Genotypes as Novel Nutritional Sources of Bioactive Compounds" Foods 13, no. 23: 3853. https://doi.org/10.3390/foods13233853
APA StyleNikolić, V., Žilić, S., Simić, M., Šavikin, K., Stević, T., Živković, J., Sarić, B., Milovanović, D., & Kandić Raftery, V. (2024). Characterization and Potential Food Applications of Oat Flour and Husks from Differently Colored Genotypes as Novel Nutritional Sources of Bioactive Compounds. Foods, 13(23), 3853. https://doi.org/10.3390/foods13233853










