Green Tea Epigallocatechin 3-Gallate Reduced Platelet Aggregation and Improved Anticoagulant Proteins in Patients with Transfusion-Dependent β-Thalassemia: A Randomized Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation and Chemical Analyses of GTE, Placebo, and GTE Tablets
2.3. Ethical Consideration
2.4. Clinical Trial Registration
2.5. In Vitro Study for GTE Treatment on PLT Aggregation
2.5.1. Blood Collection and Preparation
2.5.2. Treatment of PRP with GTE
2.5.3. Assay of PLT Aggregation Induced by Agonists
2.6. Assessment for Consumption of GTE on PLT Aggregation and Blood Coagulation in TDT
2.6.1. Patient Selection
2.6.2. Randomization and Study Design
2.6.3. Intervention
2.6.4. Patients’ Blood Collection
2.6.5. Determination of PLT Numbers
2.6.6. Measurements of Blood Coagulation
2.6.7. Determinations of Iron Status and Liver Functions
2.7. Outcomes of the Study
2.8. Statistical Analysis
3. Results
3.1. Chemical Compositions in GTE, GTE, and Placebo Tablets
3.2. Effect of GTE Treatment on PLT Aggregation In Vitro
3.2.1. Information About the Blood Donors
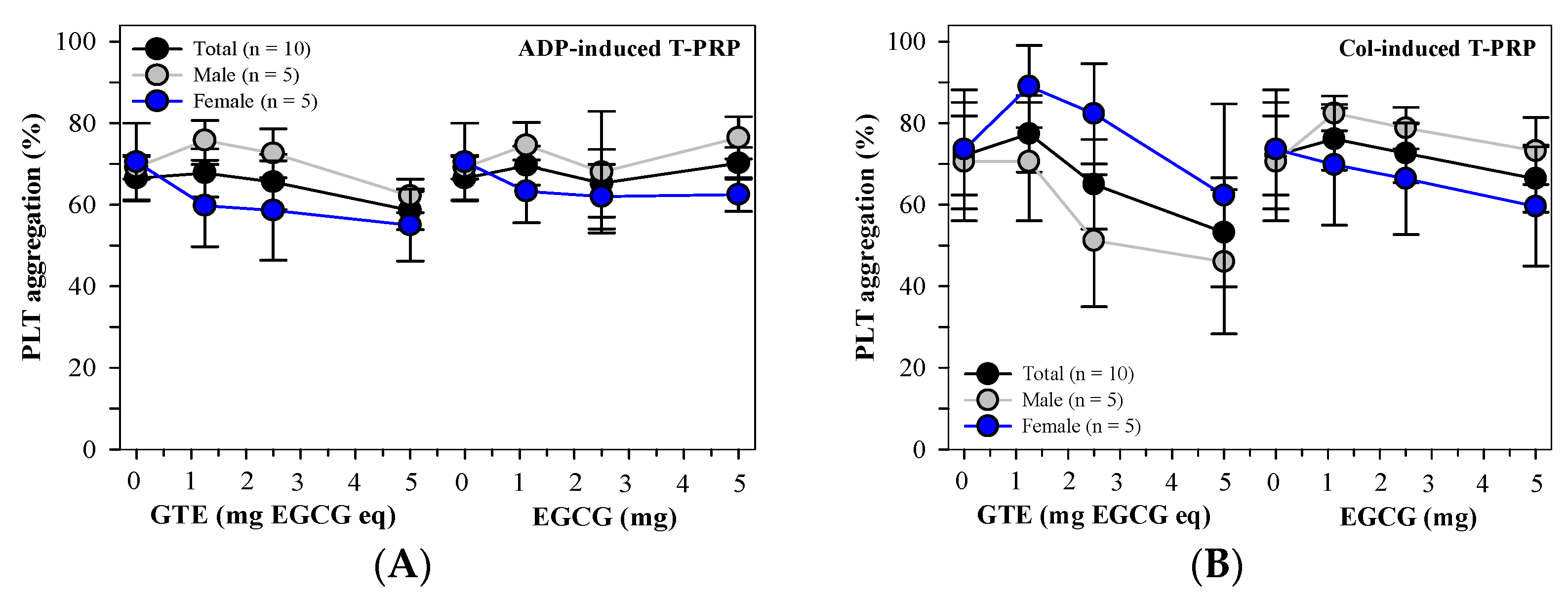
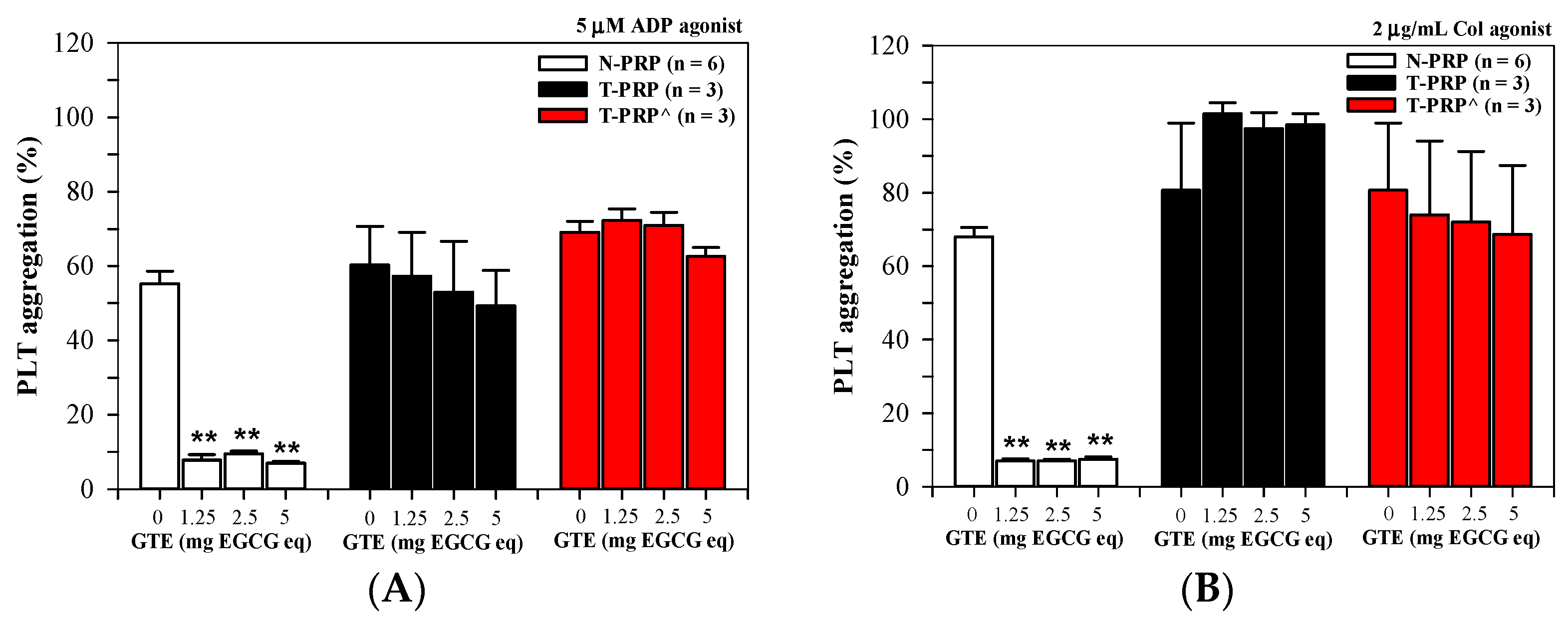
3.2.2. PLT Aggregation in N-PRP and T-PRP Treated with GTE
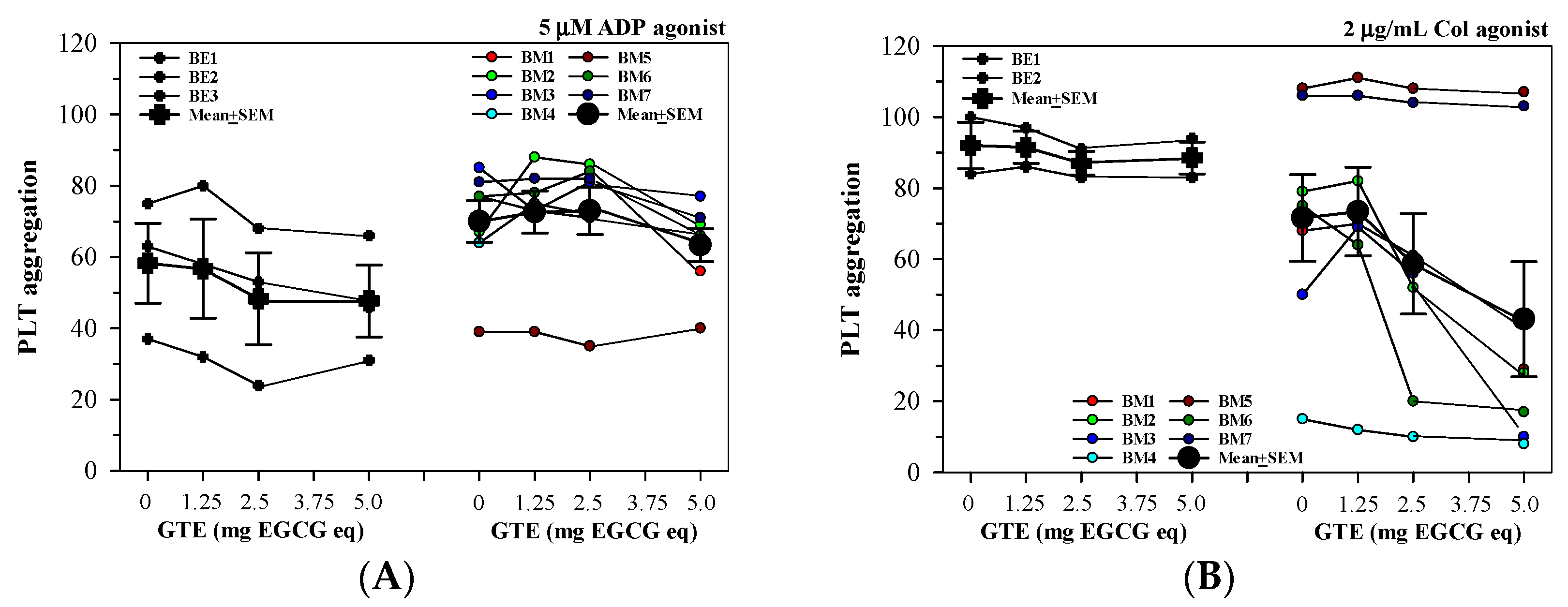
3.2.3. Effect of Splenectomy on PLT Aggregation in PRP Treated with GTE
3.2.4. Anti-PLT Activity by GTE in PRP Acquired from Different Thalassemia Types
3.3. Effects of GTE Consumption on PLT Aggregation and Blood Coagulation in Patients with TDT
3.3.1. Demographic Characteristics
3.3.2. Platelet Indices
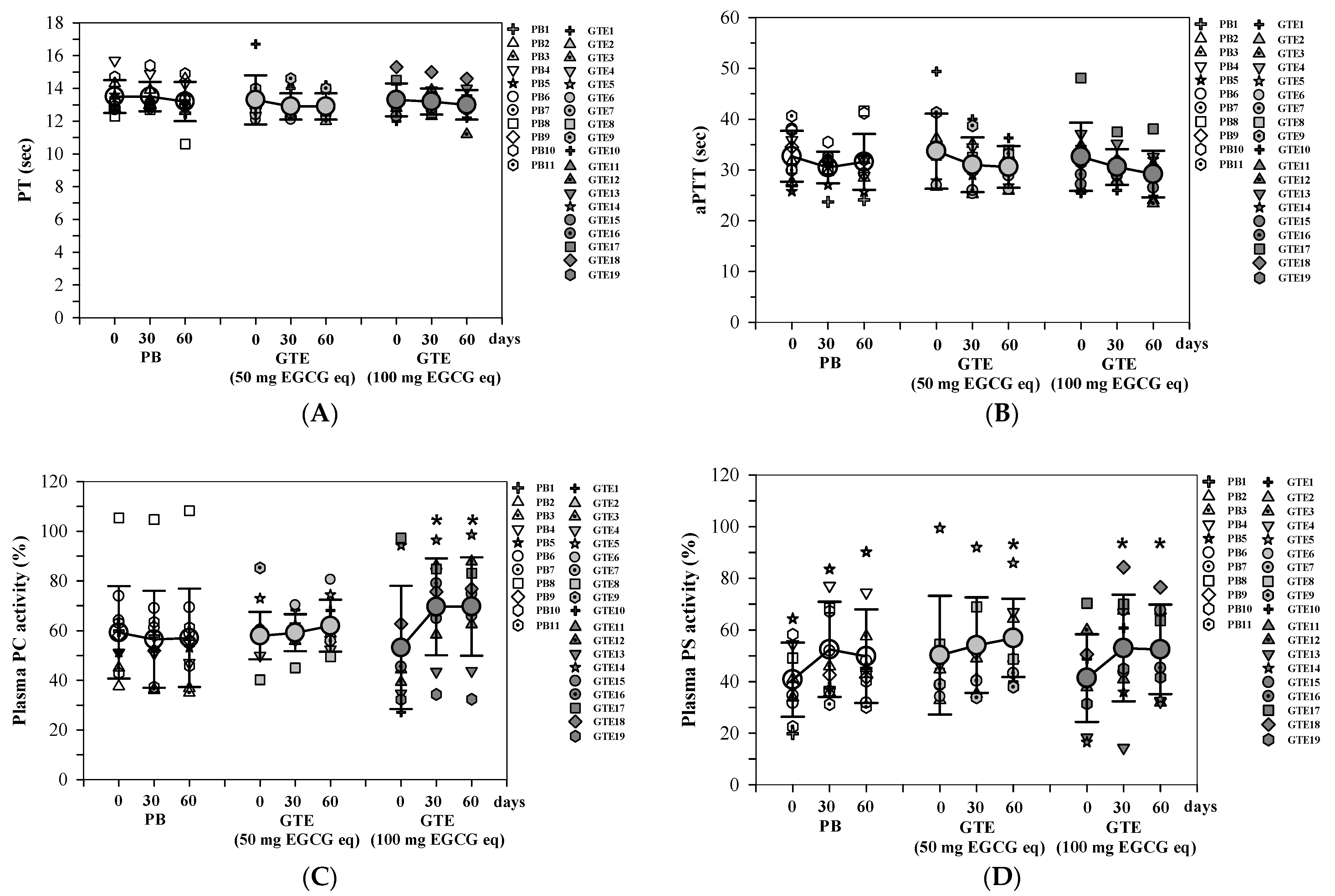
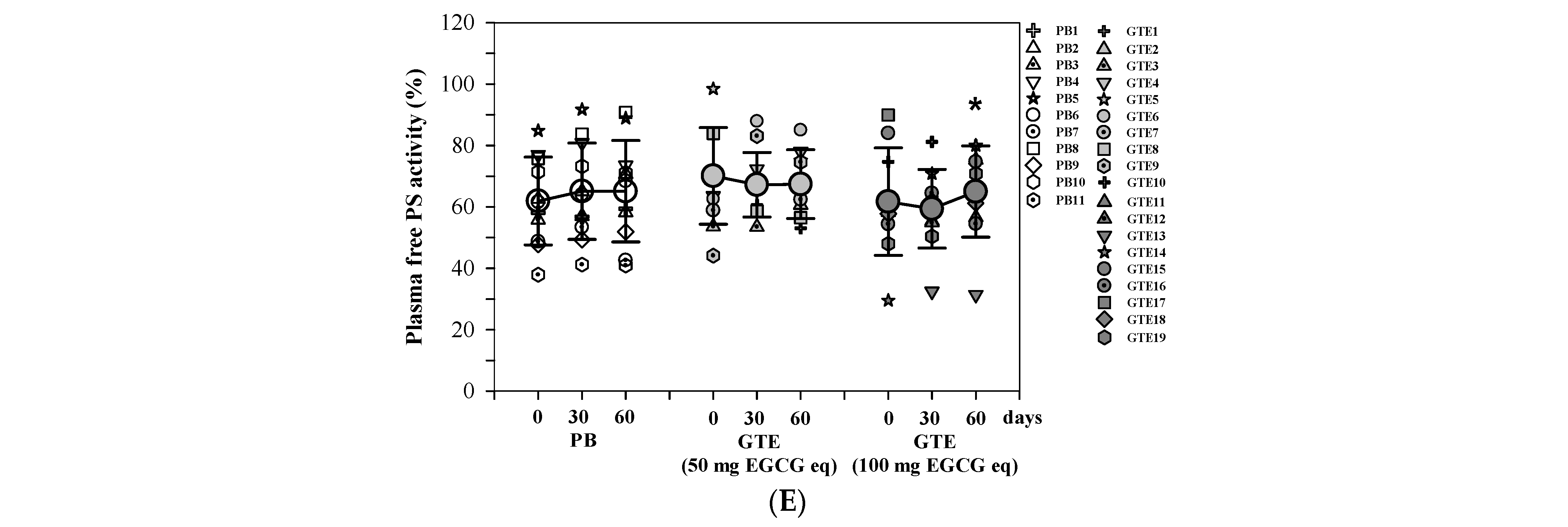
3.3.3. Coagulation Time and Anticoagulant Proteins
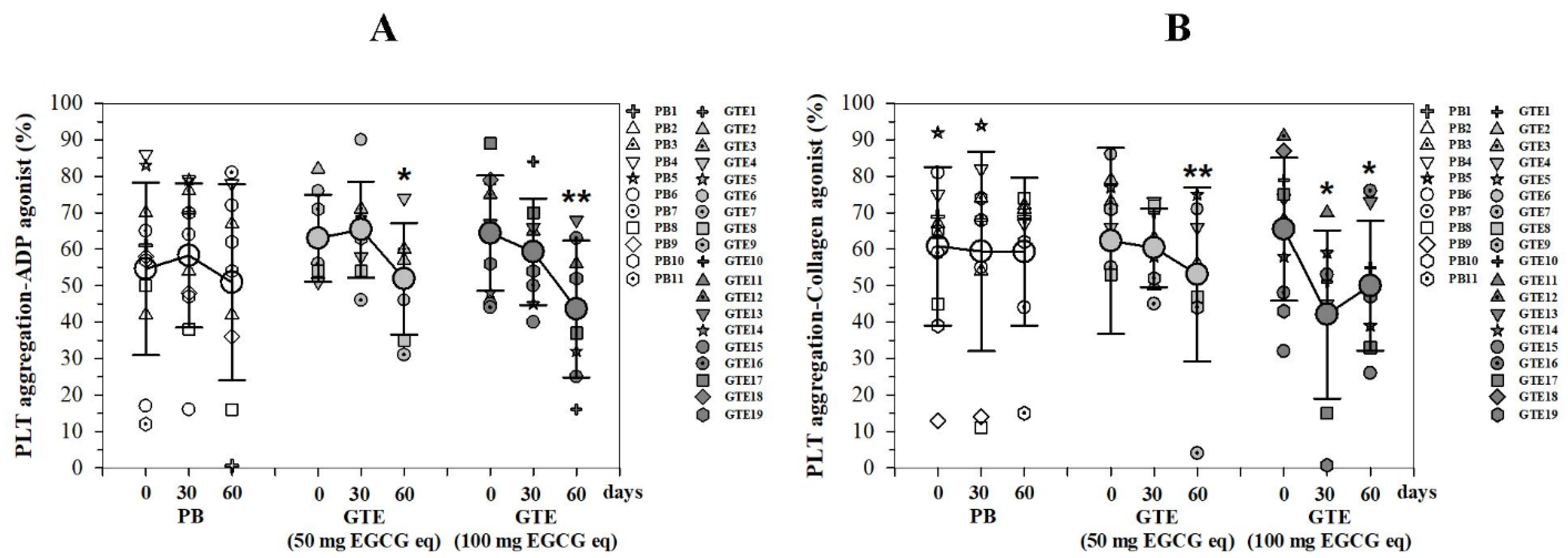
3.3.4. Platelet Aggregation
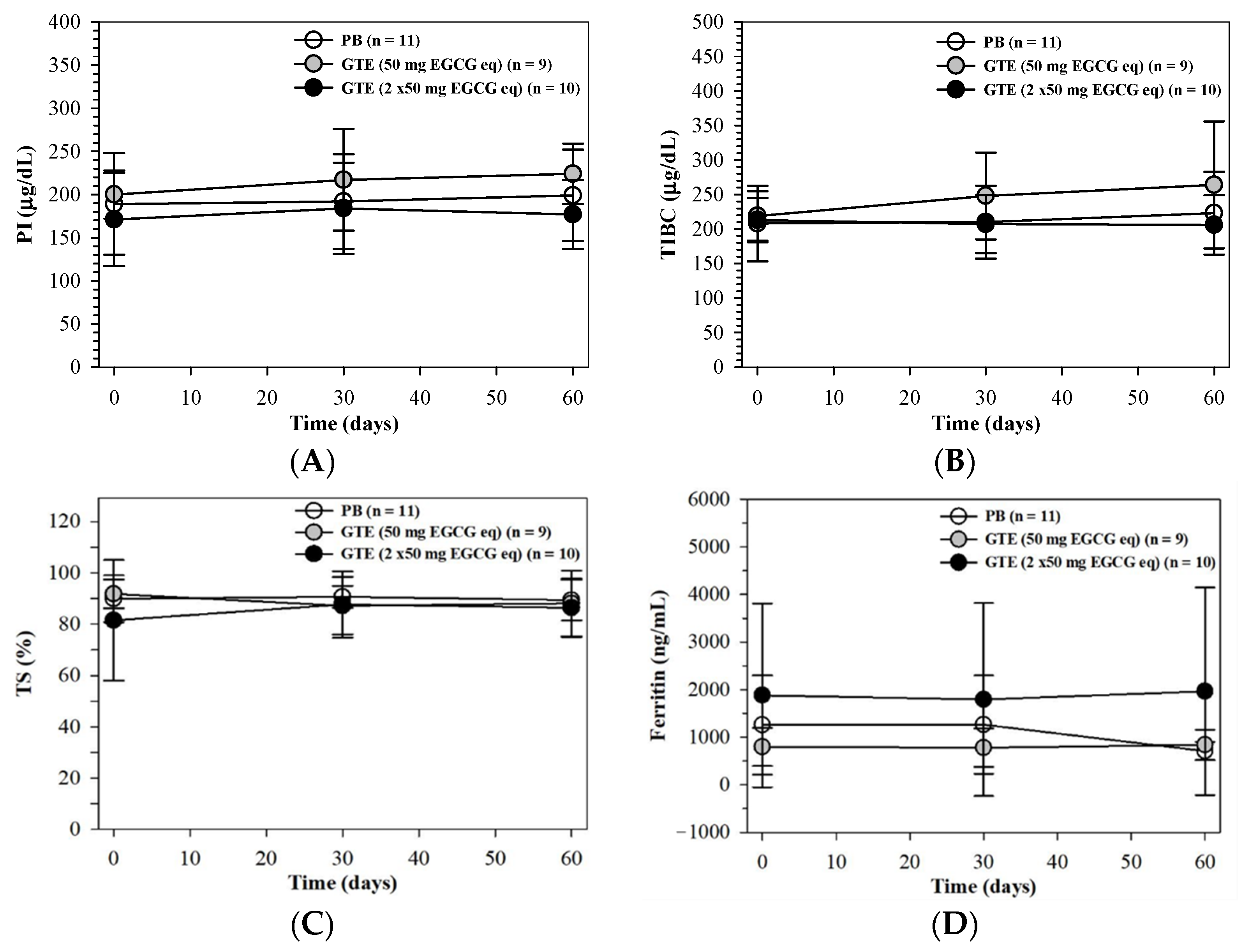
3.3.5. Body Iron and Liver Function Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Intitutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sirachainan, N. Thalassemia and the hypercoagulable state. Thromb. Res. 2013, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.A.; Elsayh, K.I.; Zahran, A.M.; Embaby, M. Splenectomy for patients with β-thalassemia major: Long-term outcomes. Egypt. J. Surg. 2014, 33, 232–236. [Google Scholar] [CrossRef]
- Cianciulli, P. Iron chelation therapy in thalassemia syndromes. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009034. [Google Scholar] [CrossRef] [PubMed]
- Dissayabutra, T.; Tosukhowong, P.; Seksan, P. The benefits of vitamin C and vitamin E in children with beta-thalassemia with high oxidative stress. J. Med. Assoc. Thai 2005, 88, S317–S321. [Google Scholar] [PubMed]
- Perret-Guillaume, C.; Wahl, D.G. Low-dose warfarin in atrial fibrillation leads to more thromboembolic events without reducing major bleeding when compared to adjusted-dose—A meta-analysis. Thromb. Haemost. 2004, 91, 394–402. [Google Scholar] [CrossRef]
- Erb, L.; Weisman, G.A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 789–803. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Krumphuber, J.; Jilma, B. Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases. Br. J. Pharmacol. 2010, 159, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Hutachok, N.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Mackie, I.J.; Porter, J.B.; Srichairatanakool, S. Anti-platelet aggregation and anti-cyclooxygenase activities for a range of coffee extracts (Coffea arabica). Molecules 2020, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Koonyosying, P.; Kongkarnka, S.; Uthaipibull, C.; Svasti, S.; Fucharoen, S.; Srichairatanakool, S. Green tea extract modulates oxidative tissue injury in beta-thalassemic mice by chelation of redox iron and inhibition of lipid peroxidation. Biomed. Pharmacother. 2018, 108, 1694–1702. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Lim, I.H.; Yuk, D.Y.; Chung, K.H.; Park, J.B.; Yoo, H.S.; Yun, Y.P. Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate. Thromb. Res. 1999, 96, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wang, X.B.; Guan, R.F.; Tu, J.; Gong, Z.H.; Zheng, N.; Yang, J.H.; Zhang, Y.Y.; Ying, M.M. Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice. Food Funct. 2013, 4, 1521–1525. [Google Scholar] [CrossRef]
- Joo, H.J.; Park, J.Y.; Hong, S.J.; Kim, K.A.; Lee, S.H.; Cho, J.Y.; Park, J.H.; Yu, C.W.; Lim, D.S. Anti-platelet effects of epigallocatechin-3-gallate in addition to the concomitant aspirin, clopidogrel or ticagrelor treatment. Korean J. Intern. Med. 2018, 33, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Settakorn, K.; Hantrakool, S.; Petiwathayakorn, T.; Hutachok, N.; Tantiworawit, A.; Charoenkwan, P.; Chalortham, N.; Chompupoung, A.; Paradee, N.; Koonyosying, P.; et al. A randomized placebo-controlled clinical trial of oral green tea epigallocatechin 3-gallate on erythropoiesis and oxidative stress in transfusion-dependent beta-thalassemia patients. Front. Mol. Biosci. 2023, 10, 1248742. [Google Scholar] [CrossRef] [PubMed]
- Luca, V.S.; Stan, A.M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Catechins profile, caffeine content and antioxidant activity of Camellia sinensis teas commercialized in Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi 2016, 120, 457–463. [Google Scholar] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Nelson, S.D.; Patil, B.S. Rapid simultaneous determination of amines and organic acids in citrus using high-performance liquid chromatography. Talanta 2011, 83, 948–954. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Koonyosying, P.; Tantiworawit, A.; Hantrakool, S.; Utama-Ang, N.; Cresswell, M.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S. Consumption of a green tea extract-curcumin drink decreases blood urea nitrogen and redox iron in beta-thalassemia patients. Food Funct. 2020, 11, 932–943. [Google Scholar] [CrossRef]
- Yuthavong, S.; Chatiketu, P.; Keadto, O.; Srichairatanakool, P.; Srichairatanakool, S.; Chatupos, V. Evaluating and comparing the effects of paracetamol and ibuprofen on wound healing, MMP-9, and TGF-β1 levels in patients following upper third molar tooth extraction. BMP Oral Health 2004, 16, 3821. [Google Scholar]
- Akrawinthawong, K.; Park, J.W.; Piknova, B.; Sibmooh, N.; Fucharoen, S.; Schechter, A.N. A flow cytometric analysis of the inhibition of platelet reactivity due to nitrite reduction by deoxygenated erythrocytes. PLoS ONE 2014, 9, e92435. [Google Scholar] [CrossRef] [PubMed]
- Atichartakarn, V.; Angchaisuksiri, P.; Aryurachai, K.; Chuncharunee, S.; Thakkinstian, A. In vivo platelet activation and hyperaggregation in hemoglobin E/beta-thalassemia: A consequence of splenectomy. Int. J. Hematol. 2003, 77, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Klaihmon, P.; Lertthammakiat, S.; Anurathapan, U.; Pakakasama, S.; Sirachainan, N.; Hongeng, S.; Pattanapanyasat, K. Activated platelets and leukocyte activations in young patients with beta-thalassemia/HbE following bone marrow transplantation. Thromb. Res. 2018, 169, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Day, S.M.; Duquaine, D.; Mundada, L.V.; Menon, R.G.; Khan, B.V.; Rajagopalan, S.; Fay, W.P. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation 2003, 107, 2601–2606. [Google Scholar] [CrossRef]
- Polette, A.; Blache, D. Effect of vitamin E on acute iron load-potentiated aggregation, secretion, calcium uptake and thromboxane biosynthesis in rat platelets. Atherosclerosis 1992, 96, 171–179. [Google Scholar] [CrossRef]
- Lee, D.H.; Barmparas, G.; Fierro, N.; Sun, B.J.; Ashrafian, S.; Li, T.; Ley, E.J. Splenectomy is associated with a higher risk for venous thromboembolism: A prospective cohort study. Int. J. Surg. 2015, 24, 27–32. [Google Scholar] [CrossRef]
- Boyle, S.; White, R.H.; Brunson, A.; Wun, T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood 2013, 121, 4782–4790. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Role of red blood cells in haemostasis and thrombosis. ISBT Sci. Ser. 2017, 12, 176–183. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Atichartakarn, V.; Angchaisuksiri, P.; Aryurachai, K.; Onpun, S.; Chuncharunee, S.; Thakkinstian, A.; Atamasirikul, K. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/beta-thalassaemic patients. Br. J. Haematol. 2002, 118, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Atichartakarn, V.; Chuncharunee, S.; Chandanamattha, P.; Likittanasombat, K.; Aryurachai, K. Correction of hypercoagulability and amelioration of pulmonary arterial hypertension by chronic blood transfusion in an asplenic hemoglobin E/beta-thalassemia patient. Blood 2004, 103, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Robbiolo, L.; Bottasso, B.M.; Coppola, R.; Fiorelli, G.; Mannucci, A.P. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br. J. Haematol. 2000, 111, 467–473. [Google Scholar] [CrossRef]
- Hadi, T.K.; Mohammad, N.S.; Nooruldin, S.A. Protein C and protein S levels in β-thalassemia major patients in Erbil, Kurdistan Region. Cell Mol. Biol. 2020, 66, 25–28. [Google Scholar] [CrossRef]
- Shirahata, A.; Funahara, Y.; Opartkiattikul, N.; Fucharoen, S.; Laosombat, V.; Yamada, K. Protein C and protein S deficiency in thalassemic patients. Southeast Asian J. Trop. Med. Public Health 1992, 23, 65–73. [Google Scholar] [PubMed]
- Hooper, S.; Hausenblas, H.A.; Winters, C. Efficacy of MitoHeal((R)) supplementation on adult skin quality and patient satisfaction: A randomized, double-blind, placebo-controlled, pilot study. Clin. Exp. Dermatol. 2022, 47, 2269–2272. [Google Scholar] [CrossRef]
- Audomkasok, S.; Singpha, W.; Chachiyo, S.; Somsak, V. Antihemolytic Activities of green tea, safflower, and mulberry extracts during Plasmodium berghei infection in mice. J. Pathog. 2014, 2014, 203154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heptinstall, S.; May, J.; Fox, S.; Kwik-Uribe, C.; Zhao, L. Cocoa flavanols and platelet and leukocyte function: Recent in vitro and ex vivo studies in healthy adults. J. Cardiovasc. Pharmacol. 2006, 47, S197–S205, discussion S206–S209. [Google Scholar] [CrossRef]
- Elnour Elkhalifa, A.; Yassin, N.; Tabash, M.; Tom, M.A.; Msahad, E.; Alnor, H.L.; Mohajar, M.; Mahdi, N.; Elnour, S.; AL-Mohaithef, M. Green tea consumption effects on coagulation profile. J. Appl. Hematol. 2020, 11, 191–194. [Google Scholar] [CrossRef]
- Lill, G.; Voit, S.; Schrör, K.; Weber, A.-A. Complex effects of different green tea catechins on human platelets. FEBS Lett. 2003, 546, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.V.; Chen, M.H.; Thibord, F.; Nkambule, B.B.; Lachapelle, A.R.; Grech, J.; Schneider, Z.E.; Wallace de Melendez, C.; Huffman, J.E.; Hayman, M.A.; et al. Factors that modulate platelet reactivity as measured by 5 assay platforms in 3429 individuals. Res Pr. Thromb Haemost 2024, 8, 102406. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, T.; Dolai, T.K.; Mandal, P.K.; Karthik, S.; Bandyopadhyay, A. platelet aggregation study in patients with hemoglobin E/beta thalassemia in india. Clin. Appl. Thromb. Hemost. 2016, 22, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.; Cox, A.C.; Gerrard, J.M.; White, J.G. Effects of 2,2′-dipyridyl and related compounds on platelet prostaglandin synthesis and platelet function. Biochim. Biophys. Acta 1980, 628, 468–479. [Google Scholar] [CrossRef]
- Petiwathayakorn, T.; Hantrakool, S.; Settakorn, K.; Hutachok, N.; Tantiworawit, A.; Chalortham, N.; Koonyosying, P.; Srichairatanak, S. Consumption of green tea extract tablets improved anticoagulant proteins and reduced platelet aggregation in transfusion-dependent β-thalassemia patients. Research Square 2023. [Google Scholar] [CrossRef]
- van het Hof, K.H.; Kivits, G.A.; Weststrate, J.A.; Tijburg, L.B. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar] [CrossRef]
- Unno, T.; Sagesaka, Y.M.; Kakuda, T. Analysis of tea catechins in human plasma by high-performance liquid chromatography with solid-phase extraction. J. Agric. Food Chem. 2005, 53, 9885–9889. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.; Marmet, C.; Longet, K.; Fraering, A.L.; Moulin, J.; Barron, D.; Dionisi, F.; Cavin, C.; Steiling, H.; et al. Plasma appearance and correlation between coffee and green tea metabolites in human subjects. Br. J. Nutr. 2010, 104, 1635–1640. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Saewong, T.; Ounjaijean, S.; Mundee, Y.; Pattanapanyasat, K.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S. Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice. Med. Chem. 2010, 6, 57–64. [Google Scholar] [CrossRef]


| Information | Healthy Subjects | Patients with Thalassemia |
|---|---|---|
| Gender: | 6 (3 male, 3 female) | 10 (5 male, 5 female) |
| Age (years): | 26.8 ± 1.0 (25–28) | 32.6 ± 7.2 (20–46) |
| BW (kg): | 63.0 ± 8.9 | 52.6 ± 7.0 |
| BMI (kg/m2): | 33.1 ± 7.2 | 32.9 ± 3.3 |
| Type of thalassemia: n (%) | ||
| BM | 0 | 7 (70.0) |
| BE | 0 | 3 (30.0) |
| Iron chelator: n (%) | ||
| DFP | 0 | 7 (70.0) |
| DFX | 0 | 1 (10.0) |
| DFP + DFO | 0 | 1 (10.0) |
| DFO + DFX | 0 | 1 (10.0) |
| ASA: n (%) | ||
| Taking | 0 | 7 (70.0) |
| Not taking | 6 (100.0) | 3 (30.0) |
| Splenectomy: n (%) | ||
| Performed | 0 | 7 (70.0) |
| Not performed | 6 (100.0) | 3 (30.0) |
| Liver span (cm): | 0 | 12.1 ± 1.8 |
| PLT numbers (×105 cells/mm3) | 2.36 ± 0.28 | 5.36 ± 2.74 |
| Ft (ng/mL) | 99 ± 9 | 1098 ± 484 |
| Code | Sex | Type | Age (y) | IC | ASA | SPX | BW (kg) | Ht (cm) | BMI (kg/m2) | Liver Span (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| PB1 | F | BE | 40 | DFP | Y | Y | 46 | 145 | 31.7 | 10 |
| PB2 | M | BE | 38 | DFP | Y | Y | 47 | 168 | 28.0 | 10 |
| PB3 | M | BM | 31 | DFP | Y | Y | 42 | 158 | 26.6 | 13 |
| PB4 | F | BM | 31 | DFX | Y | Y | 34 | 140 | 24.3 | 12 |
| PB5 | M | BM | 30 | DFP | Y | Y | 43 | 160 | 26.9 | 12 |
| PB6 | M | BM | 25 | DFP | Y | Y | 54 | 160 | 33.8 | 16 |
| PB7 | F | BE | 47 | DFP | N | N | 47 | 160 | 29.4 | 12 |
| PB8 | F | AEBart | 54 | DFP | N | N | 46 | 148 | 31.1 | 10 |
| PB9 | M | BE | 54 | DFP | N | N | 53 | 160 | 33.1 | 15 |
| PB10 | M | BE | 39 | DFP | N | N | 58 | 170 | 34.1 | 13 |
| PB11 | F | BE | 49 | DFP | N | N | 47 | 156 | 30.1 | 17 |
| 6M, 5F | 4BM, 6BE, 1AEBart | 39.8 ± 10.1 | 10DFP, 1DFX | 6/11 | 6/11 | 47.0 ± 6.5 | 157 ± 9 | 29.9 ± 3.2 | 12.7 ± 2.4 | |
| GTE1 | F | AE Bart | 62 | DFP | Y | Y | 30 | 135 | 22.2 | 10 |
| GTE2 | M | BM | 35 | DFP + DFO | Y | Y | 44 | 150 | 29.3 | 12 |
| GTE3 | F | BE | 27 | DFP | Y | Y | 48 | 158 | 30.4 | 13 |
| GTE4 | F | BM | 28 | DFP | Y | Y | 38 | 145 | 26.2 | 10 |
| GTE5 | F | BM | 22 | DFO + DFX | Y | Y | 45 | 145 | 31.0 | 10 |
| GTE6 | M | BE | 22 | DFP | Y | Y | 55 | 161 | 34.2 | 14 |
| GTE7 | F | BE | 39 | DFP | Y | Y | 39 | 152 | 25.7 | 10 |
| GTE8 | F | BM | 24 | DFP | Y | Y | 40 | 156 | 25.6 | 10 |
| GTE9 | M | BM | 34 | DFP | N | N | 42 | 151 | 27.8 | 10 |
| 3M, 6F | 5BM, 3BE, 1AEBart | 32.6 ± 12.6 | 7DFP, 1DFO + DFP, 1DFO + DFX | 8/9 | 8/9 | 42.3 ± 7.0 | 150 ± 8 | 28.1 ± 3.6 | 11.0 ± 1.6 | |
| GTE10 | F | BM | 36 | DFO + DFX | Y | Y | 48 | 149 | 32.2 | 12 |
| GTE11 | F | BE | 33 | DFP | Y | Y | 46 | 150 | 30.7 | 13 |
| GTE12 | F | BM | 27 | DFP | Y | Y | 48 | 150 | 32.0 | 10 |
| GTE13 | M | BM | 25 | DFO + DFX | Y | Y | 70 | 175 | 40.0 | 13 |
| GTE14 | M | BE | 30 | DFP | Y | Y | 55 | 153 | 35.9 | 10 |
| GTE15 | M | BE | 29 | DFP | Y | Y | 54 | 175 | 30.9 | 10 |
| GTE16 | M | BE | 47 | DFP | Y | Y | 58 | 175 | 33.1 | 18 |
| GTE17 | F | BE | 21 | DFP | N | N | 55 | 156 | 35.3 | 10 |
| GTE18 | F | BM | 37 | DFO + DFP | N | N | 60 | 165 | 36.4 | 13 |
| GTE19 | M | BE | 30 | DFP | N | N | 50 | 166 | 30.1 | 10 |
| 5M, 5F | 4BM, 6BE | 31.5 ± 7.3 | 7DFP, 2DFO + DFX, 1DFO + DFP | 7/10 | 7/10 | 54.4 ± 7.2 | 161 ± 11 | 33.7 ± 3.2 | 11.9 ± 2.6 |
| PLT Indices | PB (n = 11) | GTE (50 mg EGCG eq) (n = 9) | GTE (100 mg EGCG eq) (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | |
| Number (×105/mm3) | 5.97 ± 0.66 | 5.92 ± 0.68 | 5.90 ± 0.94 | 5.97 ± 1.08 | 6.05 ± 1.37 | 6.07 ± 1.49 | 5.77 ± 0.89 | 5.11 ± 1.21 | 4.78 ± 1.44 |
| MPV (fL) | 10.00 ± 0.42 | 10.26 ± 1.95 | 10.30 ± 0.40 | 10.00 ± 5.72 | 9.95 ± 0.37 | 9.90 ± 0.42 | 9.95 ± 0.56 | 9.86 ± 0.52 | 9.64 ± 0.43 |
| PDW (%) | 10.88 ± 1.42 | 10.80 ± 1.44 | 10.93 ± 1.67 | 11.00 ± 2.85 | 10.94 ± 2.46 | 11.10 ± 1.73 | 11.20 ± 1.40 | 10.60 ± 1.08 | 10.85 ± 1.17 |
| Pct (%) | 0.69 ± 0.10 | 0.63 ± 0.09 | 0.60 ± 0.06 | 0.67 ± 0.13 | 0.62 ± 0.12 | 0.64 ± 0.19 | 0.66 ± 0.14 | 0.62 ± 0.14 | 0.64 ± 0.10 |
| IPF (%) | 1.83 ± 0.86 | 1.86 ± 0.69 | 1.74 ± 0.93 | 1.80 ± 0.85 | 1.81 ± 0.61 | 1.88 ± 0.82 | 1.70 ± 0.71 | 1.58 ± 0.48 | 1.60 ± 0.32 |
| Parameter | PB (n = 11) | GTE (50 mg EGCG eq) (n = 9) | GTE (100 mg EGCG eq) (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | |
| TP (g/dL) | 8.1 ± 0.6 | 8.1 ± 0.7 | 8.0 ± 0.6 | 8.6 ± 0.6 | 8.8 ± 0.7 | 8.5 ± 0.9 | 8.5 ± 0.6 | 8.4 ± 0.6 | 8.7 ± 0.7 |
| Alb (g/dL) | 4.4 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.4 | 4.6 ± 0.2 | 4.5 ± 0.6 | 4.3 ± 0.7 | 4.4 ± 0.4 | 4.4 ± 0.1 | 4.6 ± 0.2 |
| Glo (g/dL) | 3.70.6 | 3.80.6 | 3.60.6 | 4.10.7 | 4.31.1 | 4.30.9 | 4.10.6 | 4.00.7 | 4.00.7 |
| AST (U/L) | 87 ± 34 | 83 ± 25 | 86 ± 28 | 105 ± 42 | 97 ± 37 | 116 ± 56 | 89 ± 24 | 87 ± 24 | 96 ± 32 |
| ALT (U/L) | 27 ± 9 | 26 ± 11 | 11 ± 3 | 46 ± 28 | 46 ± 33 | 58 ± 48 | 28 ± 12 | 32 ± 15 | 32 ± 14 |
| ALP (U/L) | 20 ± 13 | 15 ± 6 | 8 ± 2 | 39 ± 21 | 37 ± 27 | 48 ± 46 | 24 ± 11 | 31 ± 20 | 30 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petiwathayakorn, T.; Hantrakool, S.; Settakorn, K.; Hutachok, N.; Tantiworawit, A.; Chalortham, N.; Koonyosying, P.; Srichairatanakool, S. Green Tea Epigallocatechin 3-Gallate Reduced Platelet Aggregation and Improved Anticoagulant Proteins in Patients with Transfusion-Dependent β-Thalassemia: A Randomized Placebo-Controlled Clinical Trial. Foods 2024, 13, 3864. https://doi.org/10.3390/foods13233864
Petiwathayakorn T, Hantrakool S, Settakorn K, Hutachok N, Tantiworawit A, Chalortham N, Koonyosying P, Srichairatanakool S. Green Tea Epigallocatechin 3-Gallate Reduced Platelet Aggregation and Improved Anticoagulant Proteins in Patients with Transfusion-Dependent β-Thalassemia: A Randomized Placebo-Controlled Clinical Trial. Foods. 2024; 13(23):3864. https://doi.org/10.3390/foods13233864
Chicago/Turabian StylePetiwathayakorn, Touchwin, Sasinee Hantrakool, Kornvipa Settakorn, Nuntouchaporn Hutachok, Adisak Tantiworawit, Nopphadol Chalortham, Pimpisid Koonyosying, and Somdet Srichairatanakool. 2024. "Green Tea Epigallocatechin 3-Gallate Reduced Platelet Aggregation and Improved Anticoagulant Proteins in Patients with Transfusion-Dependent β-Thalassemia: A Randomized Placebo-Controlled Clinical Trial" Foods 13, no. 23: 3864. https://doi.org/10.3390/foods13233864
APA StylePetiwathayakorn, T., Hantrakool, S., Settakorn, K., Hutachok, N., Tantiworawit, A., Chalortham, N., Koonyosying, P., & Srichairatanakool, S. (2024). Green Tea Epigallocatechin 3-Gallate Reduced Platelet Aggregation and Improved Anticoagulant Proteins in Patients with Transfusion-Dependent β-Thalassemia: A Randomized Placebo-Controlled Clinical Trial. Foods, 13(23), 3864. https://doi.org/10.3390/foods13233864






