Pot-Pollen Volatiles, Bioactivity, Synergism with Antibiotics, and Bibliometrics Overview, Including Direct Injection in Food Flavor
Abstract
1. Introduction
2. Botanical Diversity of Stingless Bee Pollen
3. Chemical Diversity of Volatile Metabolites in Stingless Bee Pollen
3.1. Chemical Classes of Pot-Pollen Metabolites
3.2. Odorants of Pot-Pollen
4. Nutritional Composition and Biological Activities of Pot-Pollen
4.1. Nutritional Facts of Pot-Pollen, Still Named Stingless Bee Bread, Which It Is Not
4.2. Biological Activities of Most Abundant VOCs in Pot-Pollen
4.3. Promising Stingless Bee Resources with Biological Activities
4.4. Pot-Pollen Is Emerging as a Potential Synergistic Agent Boosting the Efficacy of Conventional Antibiotics to Overcome Antimicrobial Resistance
5. Bibliometrics on Pot-Pollen and Direct Injection in Food Flavor Research
5.1. Bibliometrics on Pot-Pollen Research
5.1.1. Most Productive Authors in Pot-Pollen Research
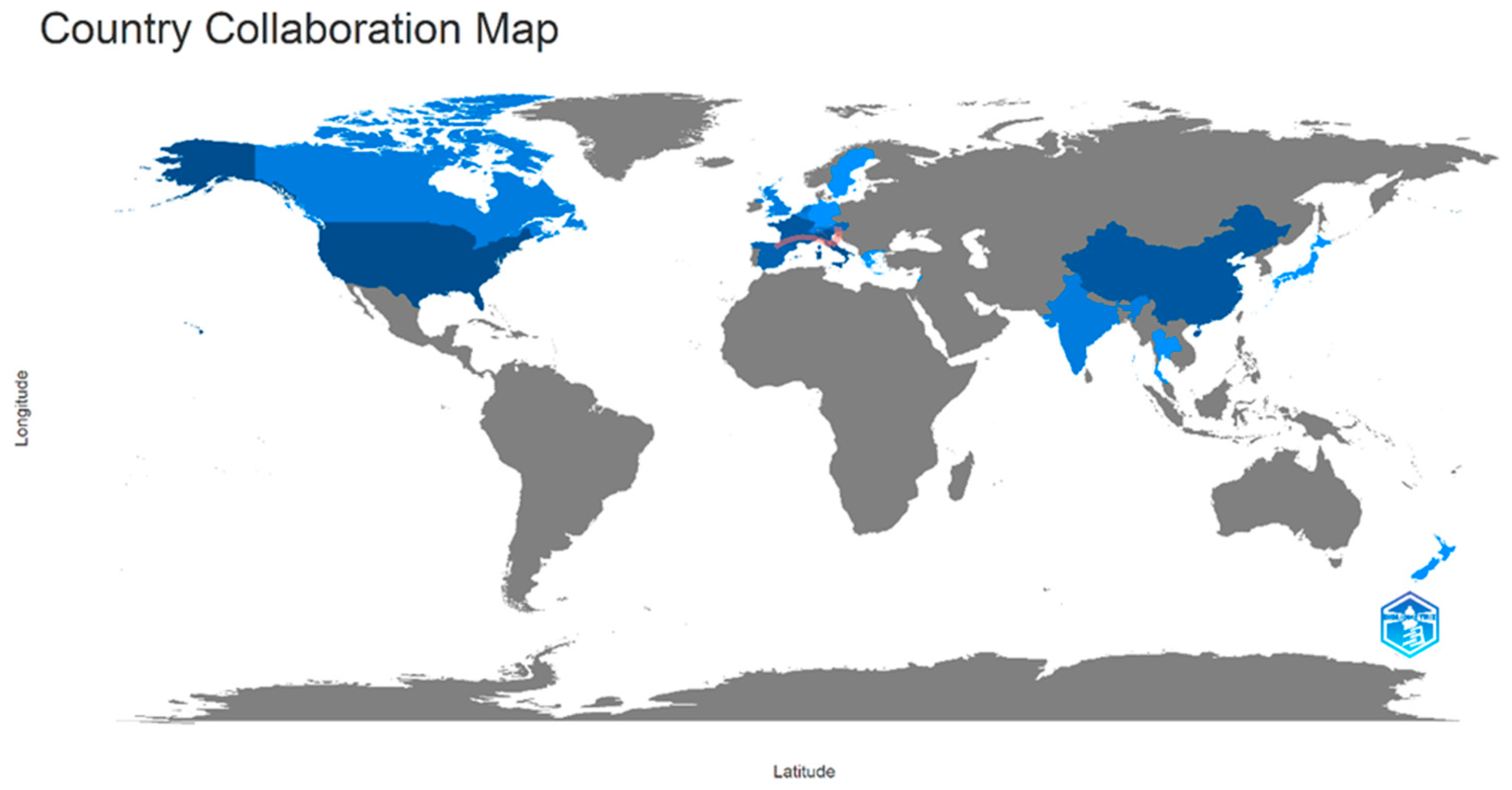
5.1.2. Geographical Distribution of Productive Institutions and Countries in Pot-Pollen Research
5.1.3. Most Frequently Used Sources for the Dissemination of Research in Pot-Pollen Research
5.1.4. Main Funding Sources in Pot-Pollen Research
5.1.5. Main Subject Areas of Research in Pot-Pollen Research
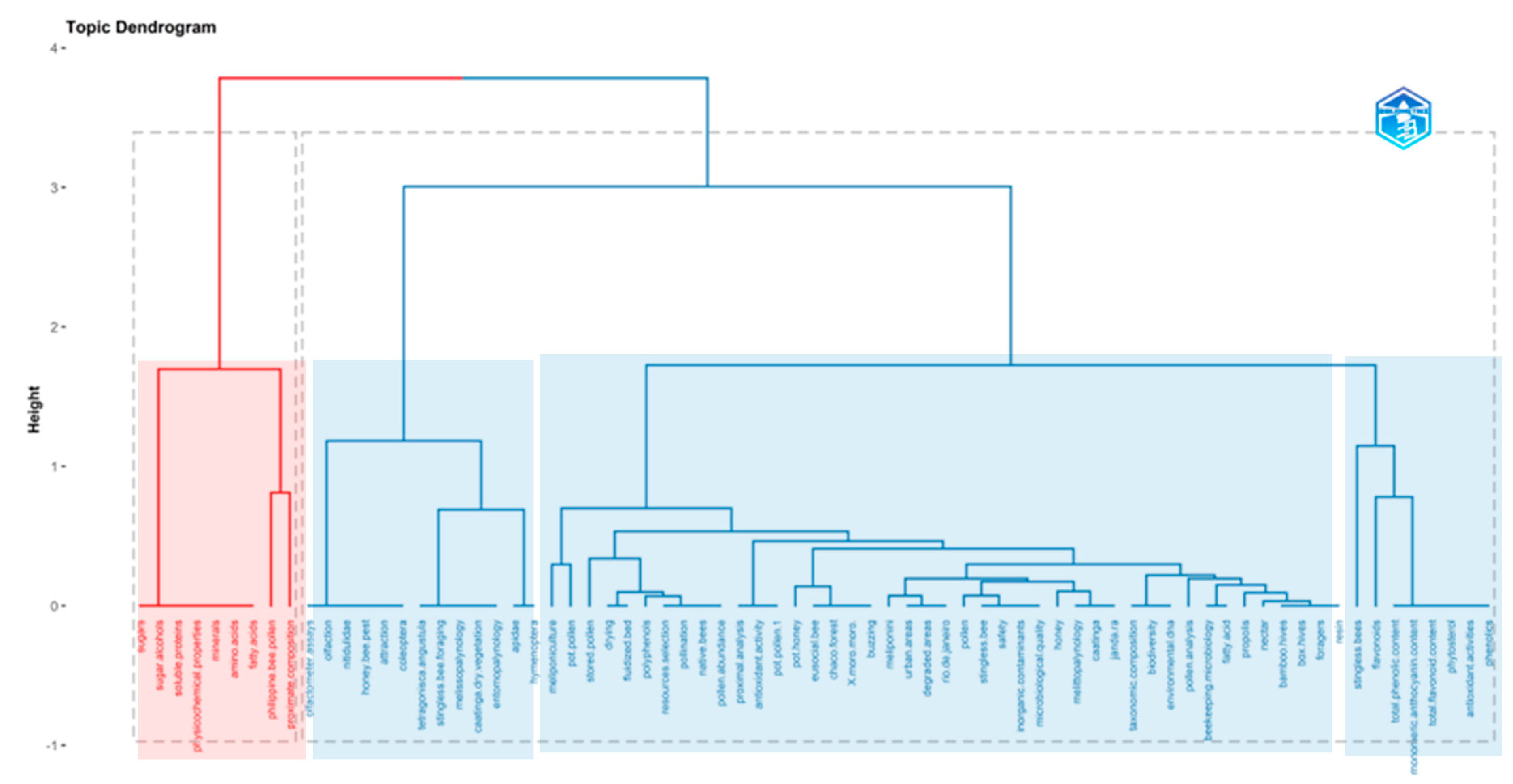
5.1.6. Authors’ Keywords and Keywords Plus: Most Relevant Words and Dendrogram in Pot-Pollen Research
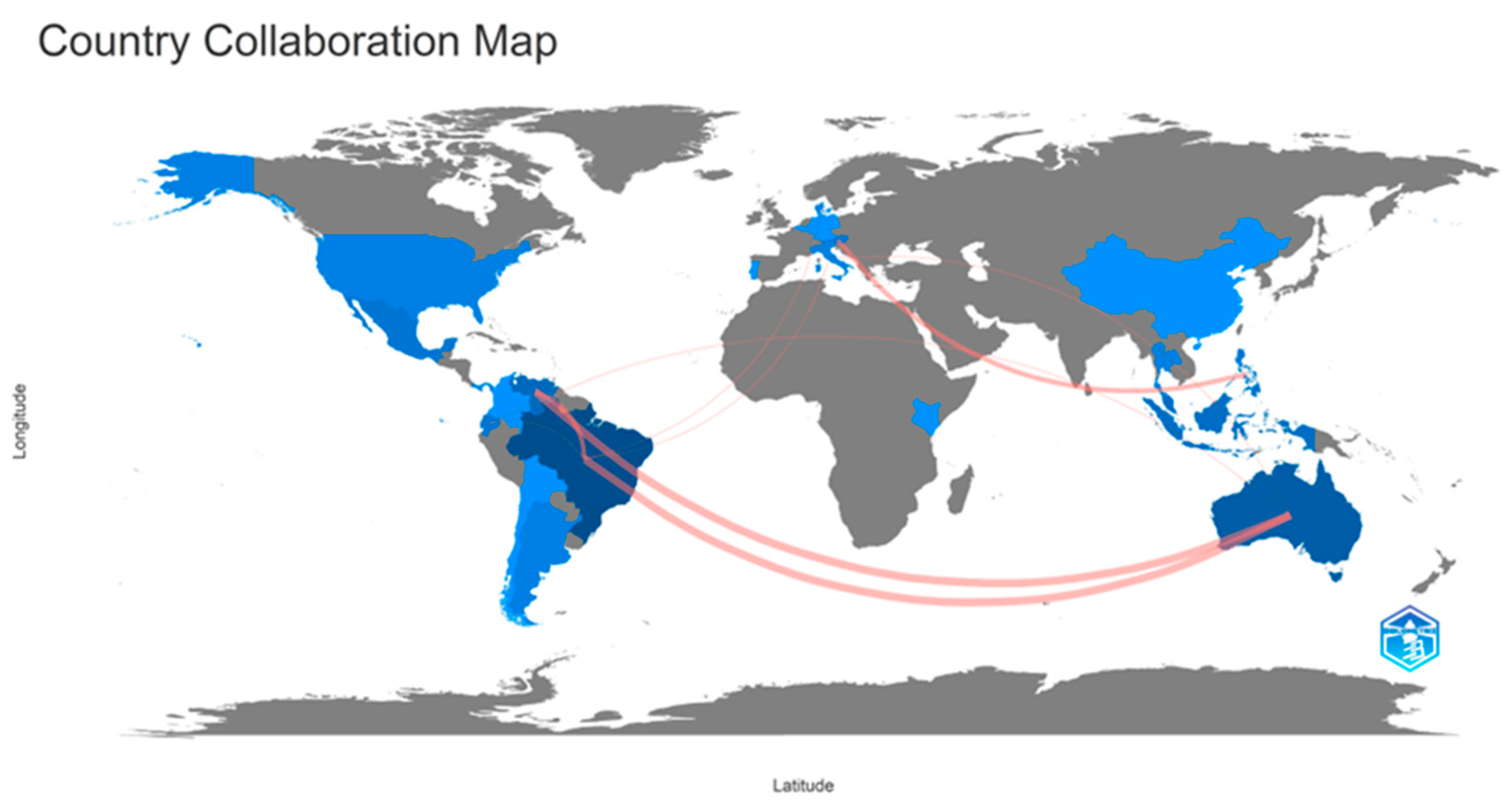
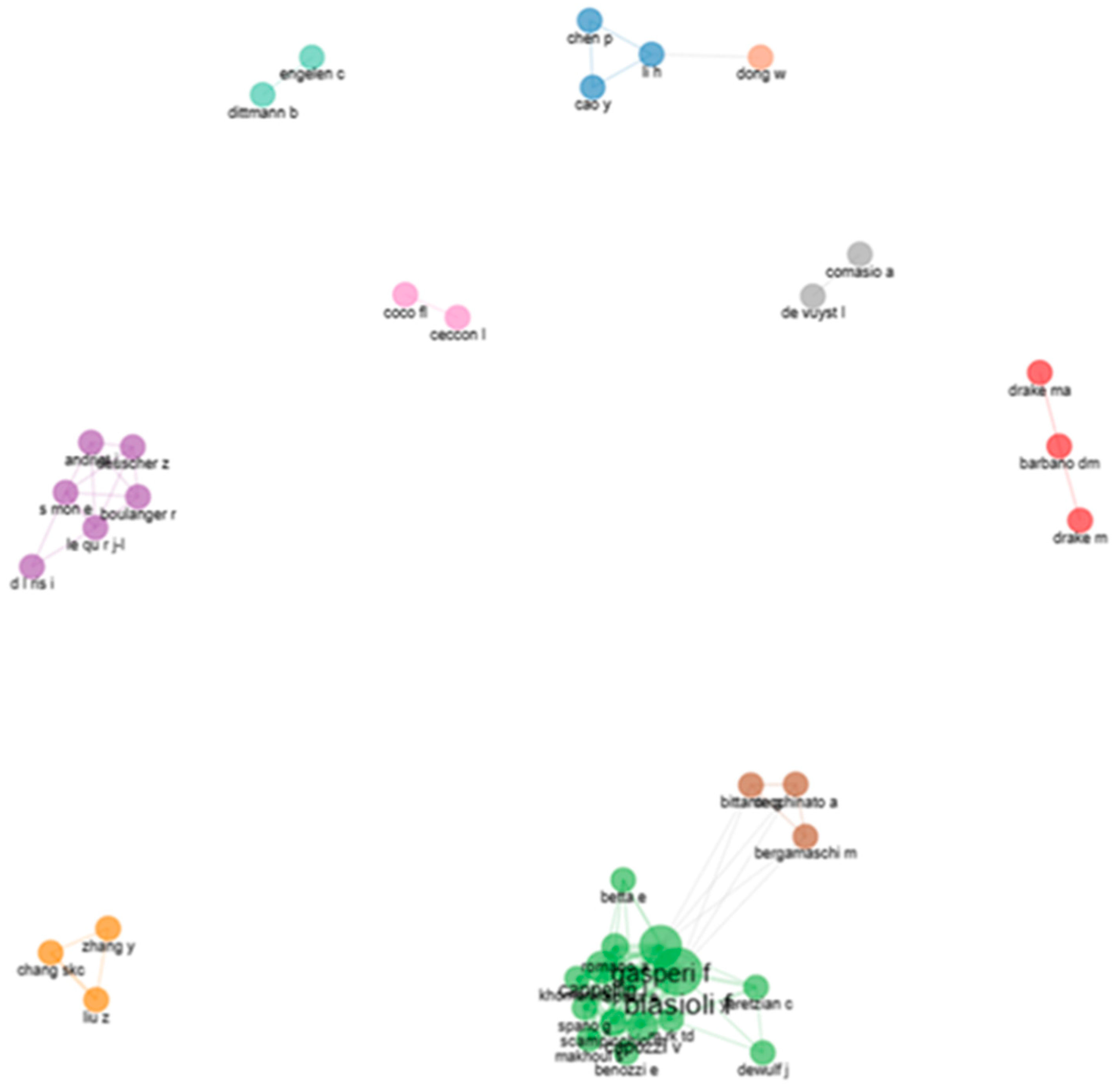
5.1.7. Co-Authors’ Collaborative Networks and Country Collaborative Map in Pot-Pollen Research
5.1.8. Conceptual Structure for Highest Contributions in Pot-Pollen Research
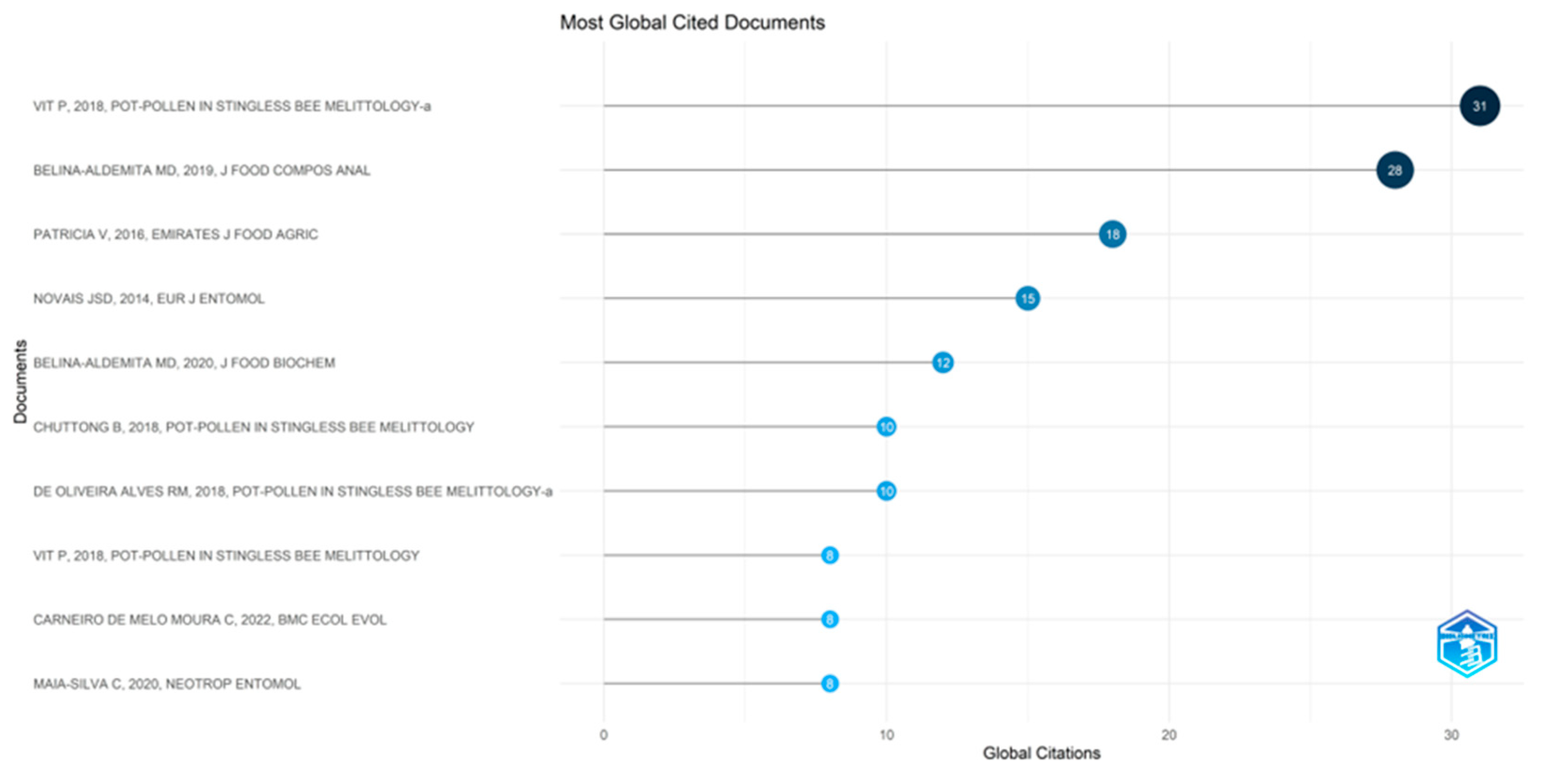
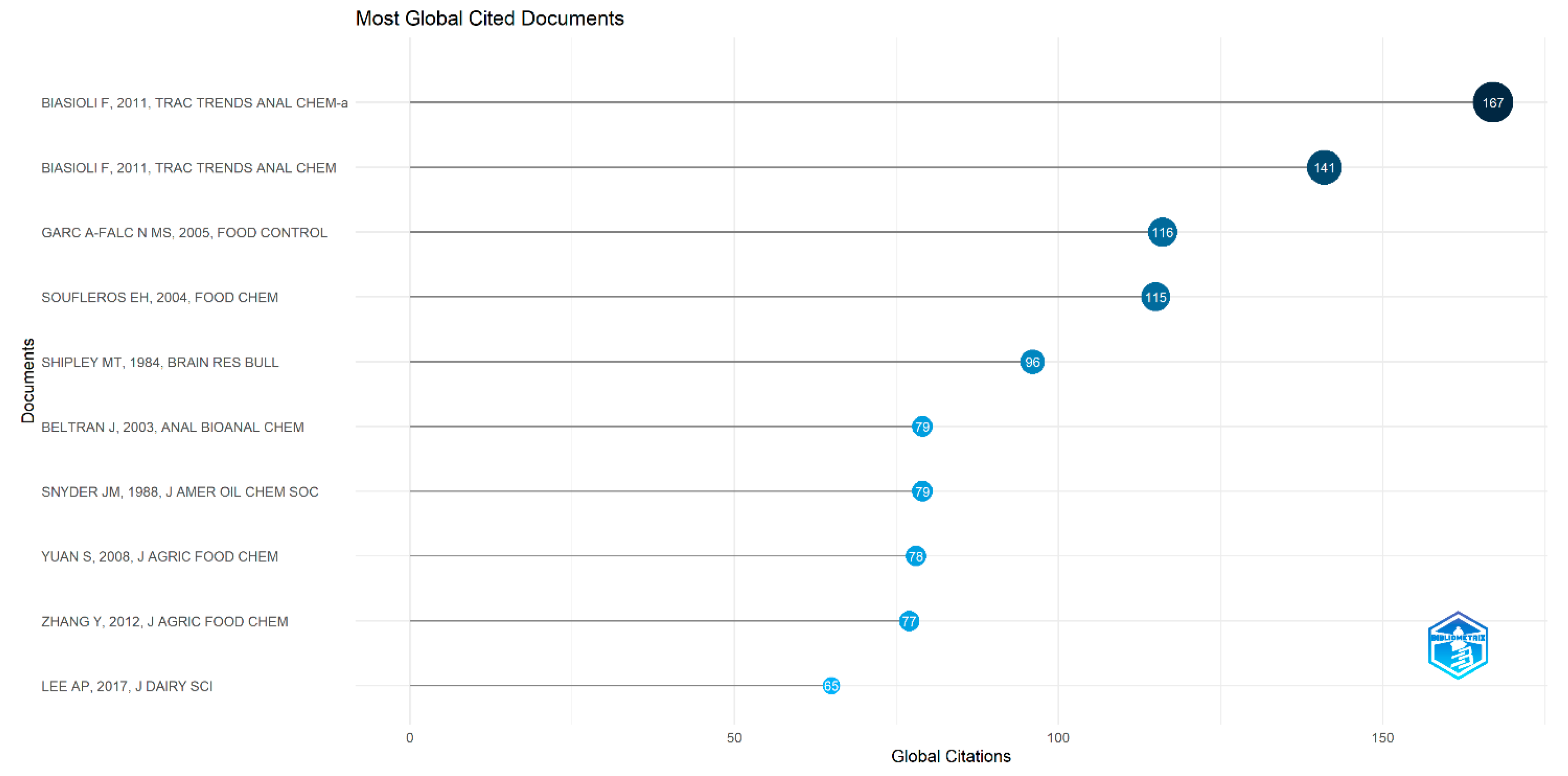
5.1.9. Most Globally Cited Documents in Pot-Pollen Research
5.2. Bibliometrics on Direct Injection in Food Flavor
5.2.1. Most Productive Authors on Direct Injection in Food Flavor
5.2.2. Geographical Distribution of Productive Institutions and Countries on Direct Injection in Food Flavor
5.2.3. Most Frequently Used Sources for Dissemination of Research on Direct Injection in Food Flavor
5.2.4. Main Funding Sources on Direct Injection in Food Flavor
5.2.5. Main Subject Areas of Research on Direct Injection in Food Flavor
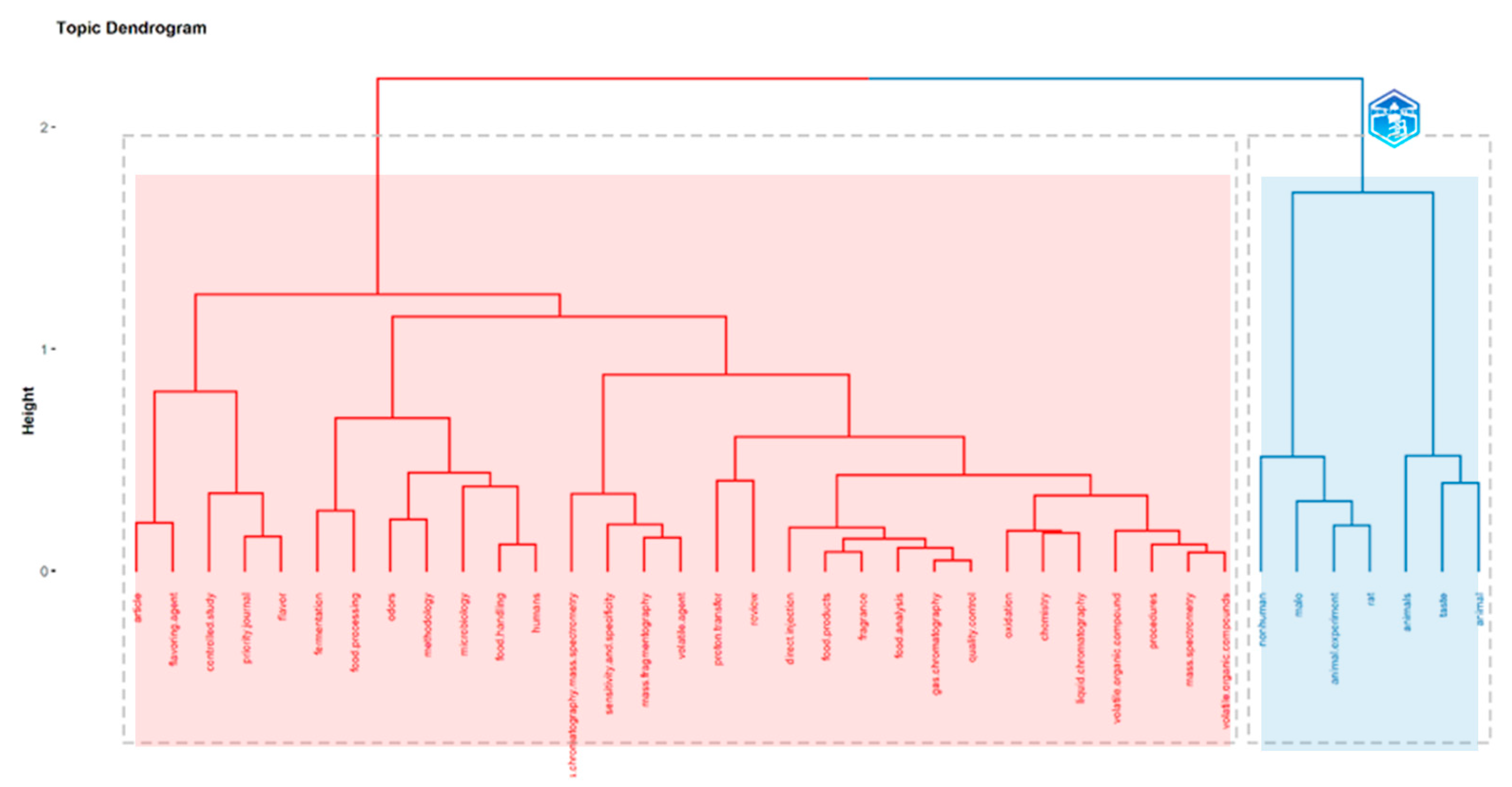
5.2.6. Authors’ Keywords and Keywords Plus: Most Relevant Words and Dendrogram on Direct Injection in Food Flavor
5.2.7. Co-Authors’ Collaborative Networks and Country Collaborative Map on Direct Injection in Food Flavor
5.2.8. Conceptual Structure for Highest Contributions on Direct Injection in Food Flavor
5.2.9. Most Globally Cited Documents on Direct Injection in Food Flavor
5.3. Comparative Metrics Between Pot-Pollen and Direct Injection Food Flavor Publications
6. Pot-Pollen, a Biodiverse Product with a Geographical, Entomological, Botanical, and Less Studied Microbial Origin
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, M.S.; Rasmussen, C.; Ayala, R.; de Oliveira, F.F. Stingless bee classification and biology (Hymenoptera, Apidae): A review, with an updated key to genera and subgenera. ZooKeys 2023, 1172, 239. [Google Scholar] [CrossRef] [PubMed]
- Vit, P.; Roubik, D. Stingless Bees Process Honey and Pollen in Cerumen Pots; Facultad de Farmacia y Bioanálisis, Universidad de Los Andes: Mérida, Venezuela, 2013; Available online: http://www.saber.ula.ve/handle/123456789/35292 (accessed on 18 October 2024).
- Vit, P.; Pedro, S.R.M.; Roubik, D. (Eds.) Pot-Honey: A Legacy of Stingless Bees; Springer: New York, NY, USA, 2013; p. 654. [Google Scholar]
- Vit, P.; Pedro, S.R.M.; Roubik, D. (Eds.) Pot-Pollen in Stingless Bee Melittology; Springer Nature: Cham, Switzerland, 2018; p. 481. [Google Scholar]
- Bueno, F.G.B.; Kendall, L.; Alves, D.A.; Tamara, M.L.; Heard, T.; Latty, T.; Gloag, R. Stingless bee floral visitation in the global tropics and subtropics. Glob. Ecol. Conserv. 2023, 43, e02454. [Google Scholar] [CrossRef]
- Grüter, C. Stingless Bees: Their Behaviour, Ecology and Evolution; Springer Nature: Cham, Switzerland, 2020; p. 385. [Google Scholar]
- Meléndez, A.; (National University of Singapore, Singapore). After His Conference at Universidad de Los Andes, Institute of Clinical Immunology, Coordinated by Dr. Haydée Urdaneta, Merida, Venezuela. December. Personal Communication, 2004.
- Rebelo, K.S.; Cazarin, C.B.; Iglesias, A.H.; Stahl, M.A.; Kristiansen, K.; Carvalho-Zilse, G.A.; Grimaldi, R.; Reyes, F.G.; Danneskiold-Samsøe, N.B.; Júnior, M.R. Nutritional composition and bioactive compounds of Melipona seminigra pot-pollen from Amazonas, Brazil. J. Sci. Food Agric. 2021, 101, 4907–4915. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-based medicinal properties of stingless bee products: Recent progress and future directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Rozman, A.S.; Hashim, N.; Maringgal, B.; Abdan, K. A Comprehensive Review of Stingless Bee Products: Phytochemical Composition and Beneficial Properties of Honey, Propolis, and Pollen. Appl. Sci. 2022, 12, 6370. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee pollen as functional food: Insights into its composition and therapeutic properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef]
- Setyawan, A.B.; Rao, U.; Sairazi, N.S.M. Therapeutic potential of stingless bee pollen: A review. Res. J. Pharm. 2023, 16, 2549–2556. [Google Scholar] [CrossRef]
- Vit, P.; Araque, M.; Chuttong, B. A multifaceted bioactive resource of stingless bees: Unlocking the therapeutic anti-antimicrobial-resistance (anti-AMR) potential of pot-pollen. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Fernandes-da-Silva, P.G.; Serrão, J.E. Nutritive value and apparent digestibility of bee-collected and bee-stored pollen in the stingless bee, Scaptotrigona postica Latr. (Hymenoptera, Apidae, Meliponini). Apidologie 2000, 31, 39–45. [Google Scholar] [CrossRef]
- Bárbara, M.S.; Machado, C.S.; Sodré, G.d.S.; Dias, L.G.; Estevinho, L.M.; De Carvalho, C.A.L. Microbiological assessment, nutritional characterization and phenolic compounds of bee pollen from Mellipona mandacaia Smith, 1983. Molecules 2015, 20, 12525–12544. [Google Scholar] [CrossRef]
- Vossler, F.G. Broad protein spectrum in stored pollen of three stingless bees from the chaco dry forest in South America (Hymenoptera, Apidae, Meliponini) and its ecological implications. Psyche 2015, 2015, 659538. [Google Scholar] [CrossRef]
- Vit, P.; Santiago, B.; Silvia, R.; Ruíz, J.; Maza, F.; Pena-Vera, M.; Perez-Perez, E. Chemical and bioactive characterization of pot-pollen produced by Melipona and Scaptotrigona stingless bees from Paria Grande, Amazonas State, Venezuela. Emir. J. Food Agric. 2016, 28, 78–84. [Google Scholar] [CrossRef]
- Contreras-Oliva, A.; Pérez-Sato, J.A.; Gómez-Merino, F.C.; López-Garay, L.A.; Villanueva-Gutiérrez, R.; Crosby-Galván, M.M.; Trejo-Téllez, L.I. Characterization of Scaptotrigona mexicana pot-pollen from Veracruz, Mexico. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 325–337. [Google Scholar]
- Chuttong, B.; Phongphisutthinant, R.; Sringarm, K.; Burgett, M.; Barth, O.M. Nutritional composition of pot-pollen from four species of stingless bees (Meliponini) in Southeast Asia. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 313–324. [Google Scholar]
- De Oliveira Alves, R.M.; da Silva Sodré, G.; Carvalho, C.A.L. Chemical, microbiological, and palynological composition of the “Samburá” Melipona scutellaris pot-pollen. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 349–360. [Google Scholar]
- Vit, P.; Santiago, B.; Peña-Vera, M.; Pérez-Pérez, E. Chemical characterization and bioactivity of Tetragonisca angustula pot-pollen from Mérida, Venezuela. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 339–347. [Google Scholar]
- Belina-Aldemita, M.D.; Opper, C.; Schreiner, M.; D’Amico, S. Nutritional composition of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Compos. Anal. 2019, 82, 103215. [Google Scholar] [CrossRef]
- Oliveira, D.d.J.; Rodrigues dos Santos, D.; Andrade, B.R.; Nascimento, A.S.d.; Oliveira da Silva, M.; da Cruz Mercês, C.; Lucas, C.I.S.; Cavalcante da Silva, S.M.P.; Dib de Carvalho, P.; Silva, F.d.L. Botanical origin, microbiological quality and physicochemical composition of the Melipona scutellaris pot-pollen (“samburá”) from Bahia (Brazil) Region. J. Apic. Res. 2021, 60, 457–469. [Google Scholar] [CrossRef]
- Bobadoye, B.O.; Fombong, A.T.; Kiatoko, N.; Suresh, R.; Teal, P.E.; Salifu, D.; Torto, B. Behavioral responses of the small hive beetle, Aethina tumida, to odors of three meliponine bee species and honey bees, Apis mellifera scutellata. Entomol. Exp. Appl. 2018, 166, 528–534. [Google Scholar] [CrossRef]
- Biasioli, F.; Yeretzian, C.; Märk, T.D.; Dewulf, J.; Van Langenhove, H. Direct-injection mass spectrometry adds the time dimension to (B) VOC analysis. Trends Anal. Chem. 2011, 30, 1003–1017. [Google Scholar] [CrossRef]
- Betta, E.; Contreras, R.; Moreno, E.; Pedro, S.; Khomenko, I.; Vit, P. Venezuelan stingless bee Tetragonisca angustula (Latreille, 1811) pot-pollen and cerumen pollen pot Volatile Organic Compound VOC profiles by HS-SPME/GC-MS. In Proceedings of the DIFFA 2023: 1st International Symposium on Direct Injection Food Flavour Analytics, San Michele all’Adige, TN, Italy, 20–22 September 2023; pp. 183–185. [Google Scholar]
- Capozzi, V.; Yener, S.; Khomenko, I.; Farneti, B.; Cappellin, L.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-ToF-MS Coupled with an Automated Sampling System and Tailored Data Analysis for Food Studies: Bioprocess Monitoring, Screening and Nose-space Analysis. J. Vis. Exp. 2017, 123, e54075. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Barth, O.M. O pólen No Mel Brasileiro; Instituto Oswaldo Cruz Rio de Janeiro: Rio de Janeiro, Brazil, 1989; p. 151. [Google Scholar]
- Roubik, D.W.; Moreno, P.J. Pollen and Spores of Barro Colorado Island [Panama]; Missouri Botanical Garden: St. Louis, MO, USA, 1991; Volume 36, p. 269. [Google Scholar]
- Vit, P. Melissopalynology, Venezuela; APIBA-CDCHT; Universidad de Los Andes: Mérida, Venezuela, 2005; p. 205. [Google Scholar]
- Gilliam, M.; Roubik, D.; Lorenz, B. Microorganisms associated with pollen, honey, and brood provisions in the nest of a stingless bee, Melipona fasciata. Apidologie 1990, 21, 89–97. [Google Scholar] [CrossRef]
- Rosa, C.A.; Lachance, M.-A.; Silva, J.O.; Teixeira, A.C.P.; Marini, M.M.; Antonini, Y.; Martins, R.P. Yeast communities associated with stingless bees. FEMS Yeast Res. 2003, 4, 271–275. [Google Scholar] [CrossRef]
- Silva, M.S.; Arruda, L.M.; Xavier, P.L.; Ramírez, M.X.D.; da Silveira, F.A.; Santana, W.C.; da Silva, P.H.A.; Fietto, L.G.; Eller, M.R. Selection of yeasts from bee products for alcoholic beverage production. Braz. J. Microbiol. 2020, 51, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni, L.; Pavanelli, M.F.; Gregório, A.; Wielewski, P.; Galhardo, D.; da Rosa Santos, P.; Toledo, V.d.A.A.d. Bacterial microbiota in Nannotrigona testaceicornis (Lepeletier, 1836) colonies. J. Apic. Res. 2023, 62, 795–803. [Google Scholar] [CrossRef]
- Atwe, S.U.; Ma, Y.; Gill, H.S. Pollen grains for oral vaccination. J. Control. Release 2014, 194, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Moreno, E.V.P.; Aguilar, I.; Barth, O.M. Melissopalynology of Coffea arabica honey produced by the stingless bee Tetragonisca angustula (Latreille, 1811) from Alajuela, Costa Rica. AIMS Agric. Food 2023, 8, 799–824. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; da Silva Lins, A.C.; Barbosa-Filho, J.M.; da Silva, E.M.S.; Freitas, B.M.; dos Santos, F.d.A.R. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Compos. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Omar, W.A.W.; Yahaya, N.; Ghaffar, Z.A.; Fadzilah, N.H. GC-MS analysis of chemical constituents in ethanolic bee pollen extracts from three species of Malaysian stingless bee. J. Apic. Sci. 2018, 62, 275–284. [Google Scholar] [CrossRef]
- Flavia Massaro, C.; Villa, T.F.; Hauxwell, C. Metabolomics analysis of pot-pollen from three species of Australian stingless bees (Meliponini). In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 401–417. [Google Scholar]
- Vit, P.; Chuttong, B.; Zawawi, N.; Diaz, M.; van der Meulen, J.; Ahmad, H.F.; Tomas-Barberan, F.A.; Meccia, G.; Danmek, K.; Moreno, J.E. A novel integrative methodology for research on pot-honey variations during post-harvest. Sociobiology 2022, 69, e8251. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C.; Di Leo Lira, P.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Vit, P. Metabolites from Microbial Cell Factories in Stingless Bee Nests. In Stingless Bee Nest Cerumen and Propolis; Vit, P., Bankova, V., Popova, M., Roubik, D.W., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 2, pp. 53–114. [Google Scholar]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- FAO and WHO. Standard for honey. Codex Alimentarius Standard, No. CXS 12-1981. Adopted in 1981. Revised in 1987, 2001. Amended in 2019, 2022. Codex Alimentarius Commission: Rome, Italy, 2022; pp. 1–8. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/all-standards/en/ (accessed on 18 October 2024).
- Vit, P.; Pedro, S.R.; Maza, F.; Ramírez, V.M.; Frisone, V. Diversity of stingless bees in Ecuador, pot-pollen standards, and meliponiculture fostering a living museum Meliponini of the world. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 207–227. [Google Scholar]
- Vit, P.; D’Albore, G.R.; Barth, O.M.; Peña-Vera, M.; Pérez-Pérez, E. Characterization of Pot-Pollen from Southern Venezuela. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S.R.M., Roubik, D.W., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 361–375. [Google Scholar]
- Da Silva, G.R.; da Natividade, T.B.; Camara, C.A.; da Silva, E.M.S.; dos Santos, F.d.A.R.; Silva, T.M.S. Identification of sugar, amino acids and minerals from the pollen of Jandaíra stingless bees (Melipona subnitida). Food Nutr. Sci. 2014, 2014, 46901. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.-K.; Zawawi, N. Stingless Bee-Collected Pollen (Bee Bread): Chemical and Microbiology Properties and Health Benefits. Molecules 2021, 26, 957. [Google Scholar] [CrossRef] [PubMed]
- Szczesna, T. Long-chain fatty acids composition of honeybee-collected pollen. J. Apic. Sci. 2006, 50, 65–79. Available online: http://www.jas.org.pl/jas_50_2_2006_8.pdf (accessed on 18 October 2024).
- Belina-Aldemita, M.D.; Fraberger, V.; Schreiner, M.; Domig, K.J.; D’Amico, S. Safety aspects of stingless bee pot-pollen from the Philippines. Die Bodenkultur J. Land Manag. Food Environ. 2020, 71, 87–100. [Google Scholar] [CrossRef]
- Othman, Z.A.; Wan Ghazali, W.S.; Noordin, L.; Yusof, N.A.M.; Mohamed, M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants 2020, 9, 33. [Google Scholar] [CrossRef]
- Fraise, A.P.; Wilkinson, M.; Bradley, C.; Oppenheim, B.; Moiemen, N. The antibacterial activity and stability of acetic acid. J. Hosp. Infect. 2013, 84, 329–331. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef]
- Panico, G.; Fasciolo, G.; Migliaccio, V.; De Matteis, R.; Lionetti, L.; Napolitano, G.; Agnisola, C.; Venditti, P.; Lombardi, A. 1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue. Antioxidants 2023, 12, 1471. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Ma, H.; Yang, W. Butanediol induces brown blotch (Rhizoctonia solani) resistance in creeping bentgrass by enhancing the anti-oxidation of reactive oxygen species and sucrose metabolism. Australas. Plant Pathol. 2022, 51, 281–294. [Google Scholar] [CrossRef]
- Ishimwe, J.A.; Garrett, M.R.; Sasser, J.M. 1,3-Butanediol attenuates hypertension and suppresses kidney injury in female rats. Am. J. Physiol. Renal Physiol. 2020, 319, F106–F114. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.B.; Wasli, H.; Bourgou, S.; Khamessi, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Insights on Juniperus phoenicea essential oil as potential anti-proliferative, anti-tyrosinase, and antioxidant candidate. Molecules 2023, 28, 7547. [Google Scholar] [CrossRef]
- Efdi, M.; Okselni, T.; Itam, A.; Arifin, B.; Novela, M.; Hidayat, T.; Fadli. Essential Oil Extraction of Piper betle, Piper ramipilum, and Piper aduncum and their Antibacterial Activity against Food borne Pathogens. J. Essent. Oil-Bear. Plants. 2023, 26, 446–458. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Bhat, K.; Sogi, S.H.P. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.M.; Bloomfield, S.F. Effect of ethanol, propylene glycol and glycerol on the interaction of methyl and propyl p-hydroxybenzoate with Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Pharm. 1997, 147, 51–60. [Google Scholar] [CrossRef]
- Lv, B.; Bian, M.; Huang, X.; Sun, F.; Gao, Y.; Wang, Y.; Fu, Y.; Yang, B.; Fu, X. n-Butanol potentiates subinhibitory aminoglycosides against bacterial persisters and multidrug-resistant MRSA by rapidly enhancing antibiotic uptake. ACS Infect. Dis. 2022, 8, 373–386. [Google Scholar] [CrossRef]
- Li, S.; Jia, X.; Wen, J. Improved 2-methyl-1-propanol production in an engineered Bacillus subtilis by constructing inducible pathways. Biotechnol. Lett. 2012, 34, 2253–2258. [Google Scholar] [CrossRef]
- Chai, W.-M.; Liu, X.; Hu, Y.-H.; Feng, H.-L.; Jia, Y.-L.; Guo, Y.-J.; Zhou, H.-T.; Chen, Q.-X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef]
- Kowalski, S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013, 141, 1378–1382. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Relationship of Thermal Treatment and Antioxidant Capacity in Cooked Foods. Antioxidants 2022, 11, 2324. [Google Scholar] [CrossRef]
- Utami, L.A.; Putri, D.H. The Effect of Ethanol Solvent Concentration on Antimicrobial Activities the Extract of Andalas Endophytic Bacteria (Morus macroura Miq.) Fermentation Product. Eksakta Berkala Ilmiah Bidang MIPA 2020, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lens, C.; Malet, G.; Cupferman, S. Antimicrobial activity of Butyl acetate, Ethyl acetate and Isopropyl alcohol on undesirable microorganisms in cosmetic products. Int. J. Cosmet. Sci. 2016, 38, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, B.; Ma, P.; Liu, M.; Wang, Z. Synthesis and properties of macrocyclic butanoic acid conjugates as a promising delivery formulation for the nutrition of colon. Sci. World J. 2013, 2013, 914234. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. ROS stress and cell membrane disruption are the main antifungal mechanisms of 2-phenylethanol against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef] [PubMed]
- Choub, V.; Won, S.-J.; Ajuna, H.B.; Moon, J.-H.; Choi, S.-I.; Lim, H.-I.; Ahn, Y.S. Antifungal activity of volatile organic compounds from Bacillus velezensis CE 100 against Colletotrichum gloeosporioides. Horticulturae 2022, 8, 557. [Google Scholar] [CrossRef]
- Luo, W.; Wang, K.; Luo, J.; Liu, Y.; Tong, J.; Qi, M.; Jiang, Y.; Wang, Y.; Ma, Z.; Feng, J. Limonene anti-TMV activity and its mode of action. Pestic. Biochem. Physiol. 2023, 194, 105512. [Google Scholar] [CrossRef]
- Tareq, A.M.; Hossain, M.M.; Uddin, M.; Islam, F.; Khan, Z.; Karim, M.M.; Lyzu, C.; Ağagündüz, D.; Reza, A.S.M.A.; Emran, T.B.; et al. Chemical profiles and pharmacological attributes of Apis cerana indica beehives using combined experimental and computer-aided studies. Heliyon 2023, 9, e15016. [Google Scholar] [CrossRef]
- Bonsakhteh, B.; Rustaiyan, A. Nano Drug Delivery Study of Anticancer Properties on Jackfruit using QM/MM Methods. Orient. J. Chem. 2014, 30, 1703. [Google Scholar] [CrossRef]
- Jo, H.; Cha, B.; Kim, H.; Brito, S.; Kwak, B.M.; Kim, S.T.; Bin, B.-H.; Lee, M.-G. α-Pinene enhances the anticancer activity of natural killer cells via ERK/AKT pathway. Int. J. Mol. Sci. 2021, 22, 656. [Google Scholar] [CrossRef]
- Adewunmi, Y.; Namjilsuren, S.; Walker, W.D.; Amato, D.N.; Amato, D.V.; Mavrodi, O.V.; Patton, D.L.; Mavrodi, D.V. Antimicrobial activity of, and cellular pathways targeted by, p-anisaldehyde and epigallocatechin gallate in the opportunistic human pathogen Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2020, 86, e02482-19. [Google Scholar] [CrossRef]
- Gogacz, M.; Peszke, J.; Natorska-Chomicka, D.; Ruszała, M.; Dos Santos Szewczyk, K. Anticancer Effects of Propolis Extracts Obtained Using the Cold Separation Method on Breast Cancer Cell Lines. Plants 2023, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.C.M.; Sviripa, V.M.; Huang, M.; Kril, L.; Watt, D.S.; Liu, C.; Lin, H.-S. Analysis of trans-2, 6-difluoro-4′-(N, N-dimethylamino) stilbene (DFS) in biological samples by liquid chromatography-tandem mass spectrometry: Metabolite identification and pharmacokinetics. Anal. Bioanal. Chem. 2015, 407, 7319–7332. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Jamil, S.; Sadeq, T.W.; Mohammed Ameen, M.S.; Kohli, K. Development and Evaluation of Nanoformulations Containing Timur Oil and Rosemary Oil for Treatment of Topical Fungal Infections. Gels 2023, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An updated comprehensive review of its pharmacological activities, ethnomedicinal, phytopharmaceutical formulation, clinical, phytochemical, and toxicological aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Qadirifard, M.S.; Arabpour, Z.; Afsahi, S.; Sorkheh, F.; Hamidpour, S.N.; Ahangarzadeh, N.; Dabestani, B.; Ansari, A.; Deravi, N. Sclareol and cancer prevention: A mini-review. Oncol. Rev. 2021, 11, 112–119. [Google Scholar] [CrossRef]
- Sulbarán-Mora, M.; Pérez-Pérez, E.; Vit, P. Antibacterial activity of ethanolic extracts of pot-pollen produced by eight meliponine species from Venezuela. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 391–399. [Google Scholar]
- Carneiro, A.L.B.; Gomes, A.A.; Alves da Silva, L.; Alves, L.B.; Cardoso da Silva, E.; da Silva Pinto, A.C.; Tadei, W.P.; Pohlit, A.M.; Simas Teixeira, M.F.; Gomes, C.C. Antimicrobial and Larvicidal activities of stingless bee pollen from Maues, Amazonas, Brazil. Bee World 2019, 96, 98–103. [Google Scholar] [CrossRef]
- Pérez-Pérez, E.; Sulbarán-Mora, M.; Barth, O.M.; Flavia Massaro, C.; Vit, P. Bioactivity and botanical origin of Austroplebeia and Tetragonula Australian pot-pollen. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 377–390. [Google Scholar]
- Lopes, A.J.O.; Vasconcelos, C.C.; Garcia, J.B.S.; Pinheiro, M.S.D.; Pereira, F.A.N.; Camelo, D.d.S.; Morais, S.V.d.; Freitas, J.R.B.; Rocha, C.Q.d.; Ribeiro, M.N.d.S. Anti-Inflammatory and Antioxidant Activity of Pollen Extract Collected by Scaptotrigona affinis postica: In silico, in vitro, and in vivo Studies. Antioxidants 2020, 9, 103. [Google Scholar] [CrossRef]
- María, S.V.; De Carvalho Katia, G.; Florencia, A.; María, M.L.; Gerardo, G.; Nancy, V.; Mariana, R.C. Biopolymer production by bacteria isolated from native stingless bee honey, Scaptotrigona jujuyensis. Food Biosci. 2021, 42, 101077. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Puthong, S.; Arung, E.T.; Chanchao, C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014, 4, 549–556. [Google Scholar] [CrossRef]
- Omar, W.A.W.; Azhar, N.A.; Fadzilah, N.H.; Kamal, N.N.S.N.M. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 265–269. [Google Scholar] [CrossRef]
- Rebelo, K.S.; Nunez, C.E.C.; Cazarin, C.B.B.; Júnior, M.R.M.; Kristiansen, K.; Danneskiold-Samsøe, N.B. Pot-pollen supplementation reduces fasting glucose and modulates the gut microbiota in high-fat/high-sucrose fed C57BL/6 mice. Food Funct. 2022, 13, 3982–3992. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Rocha, C.Q.D.; Lima, S.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.E.; El-Magd, M.A.; El-Said, K.S.; El-Sharnouby, M.; Tousson, E.M.; Salama, A.F. Potential therapeutic effect of thymoquinone and/or bee pollen on fluvastatin-induced hepatitis in rats. Sci. Rep. 2021, 11, 15688. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; p. 71. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 18 October 2024).
- WHO. WHO Bacterial Priority Pathogens List, 2024 Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; p. 56. Available online: https://www.who.int/antimicrobial-resistance/en/ (accessed on 18 October 2024).
- Vit, P.; Chuttong, B.; de Oliveira Santos, A.D.; Contreras, R.R. Nutraceutical properties and bibliometrics on chemical research of stingless bee pollen. JNH 2024, 2, 17–24. [Google Scholar] [CrossRef]
- Araque, M.; Vit, P. Evaluation of the potential synergistic effect of Tetragonisca angustula pot-pollen with amikacin and meropenem against extensively drug-resistant bacteria of clinical origin. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Vit, P.; Meccia, G. (Eds.) Memorias del 2024 Taller Internacional de Meliponicultura Mustafa, Puerto Ayacucho, Venezuela, 2–5 July; APIBA-ULA: Merida, Venezuela, 2024; pp. 1–72. [Google Scholar]
- Kacemi, R.; Campos, M.G. Translational research on bee pollen as a source of nutrients: A scoping review from bench to real world. Nutrients 2023, 15, 2413. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, web of science, and Google scholar: Strengths and weaknesses. Fed. Am. Soc. Exp. Biol. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Vossler, F.G.; Tellería, M.C.; Cunningham, M. Floral resources foraged by Geotrigona argentina (Apidae, Meliponini) in the Argentine Dry Chaco forest. Grana 2010, 49, 142–153. [Google Scholar] [CrossRef]
- Abdul Halim, L.; Basrawi, F.; Md Yudin, A.S.; Abdul Razak, A.; Johari, N.A.; Muhamad, A.; Mamat, M.R. Drying of stingless bees pot-pollen using swirling fluidized bed dryer. Dry. Technol. 2022, 40, 197–204. [Google Scholar] [CrossRef]
- Cerbulo-Vazquez, A.; Garcia-Espinosa, M.; Briones-Garduno, J.C.; Arriaga-Pizano, L.; Ferat-Osorio, E.; Zavala-Barrios, B.; Cabrera-Rivera, G.L.; Miranda-Cruz, P.; Garcia de la Rosa, M.T.; Prieto-Chavez, J.L.; et al. The percentage of CD39+ monocytes is higher in pregnant COVID-19+ patients than in nonpregnant COVID-19+ patients. PLoS ONE 2022, 17, e0264566. [Google Scholar] [CrossRef]
- Nogueira, D.S. Overview of Stingless Bees in Brazil (Hymenoptera: Apidae: Meliponini). EntomoBrasilis 2023, 16, e1041. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; Vasconcelos, M.R.D.S.; Oda-Souza, M.; Oliveira, F.F.D.; López, A.M.Q. Honey and bee pollen produced by Meliponini (Apidae) in Alagoas, Brazil: Multivariate analysis of physicochemical and antioxidant profiles. Food Sci. Technol. 2018, 38, 493–503. [Google Scholar] [CrossRef]
- Barth, O.M. Melissopalynology in Brazil: A review of pollen analysis of honeys, propolis and pollen loads of bees. Sci. Agric. 2004, 61, 342–350. [Google Scholar] [CrossRef]
- González, G.; Hinojo, M.; Mateo, R.; Medina, A.; Jiménez, M. Occurrence of mycotoxin producing fungi in bee pollen. Int. J. Food Microbiol. 2005, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, M.; Nogueira-Neto, P.; Lavie, P. Étude de quelques charactéristiques des miels récoltés par certains Méliponines brésiliens. C. R. Acad. Sci. 1964, 258, 3107–3109. [Google Scholar]
- Menezes, C.; Vollet-Neto, A.; Contrera, F.A.F.L.; Venturieri, G.C.; Imperatriz-Fonseca, V.L. The role of useful microorganisms to stingless bees and stingless beekeeping. In Pot-Honey: A Legacy of Stingless Bees; Springer: New York, NY, USA, 2013; pp. 153–171. [Google Scholar]
- De Paula, G.T.; Menezes, C.; Pupo, M.T.; Rosa, C.A. Stingless bees and microbial interactions. Curr. Opin. Insect Sci. 2021, 44, 41–47. [Google Scholar] [CrossRef]
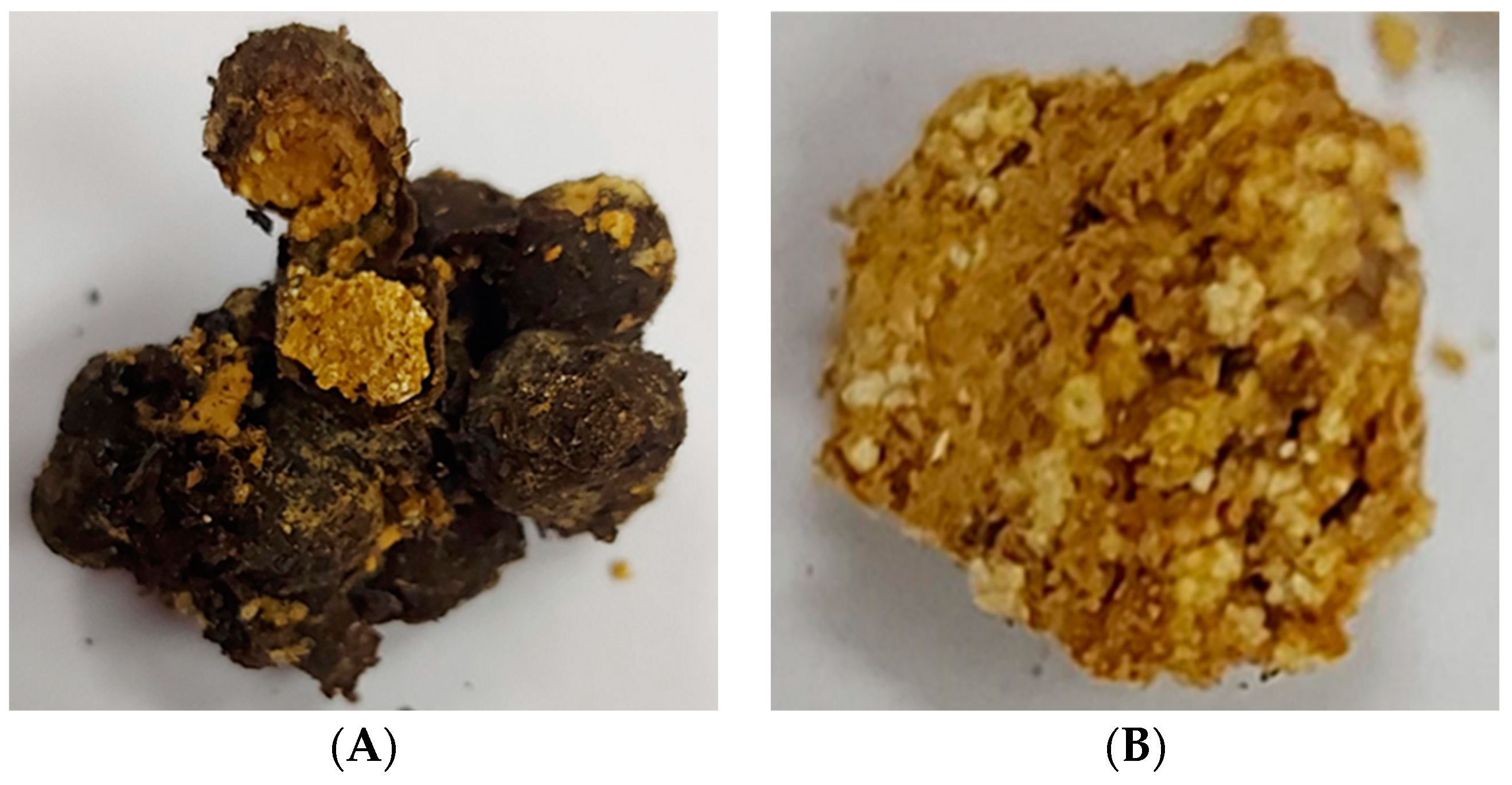

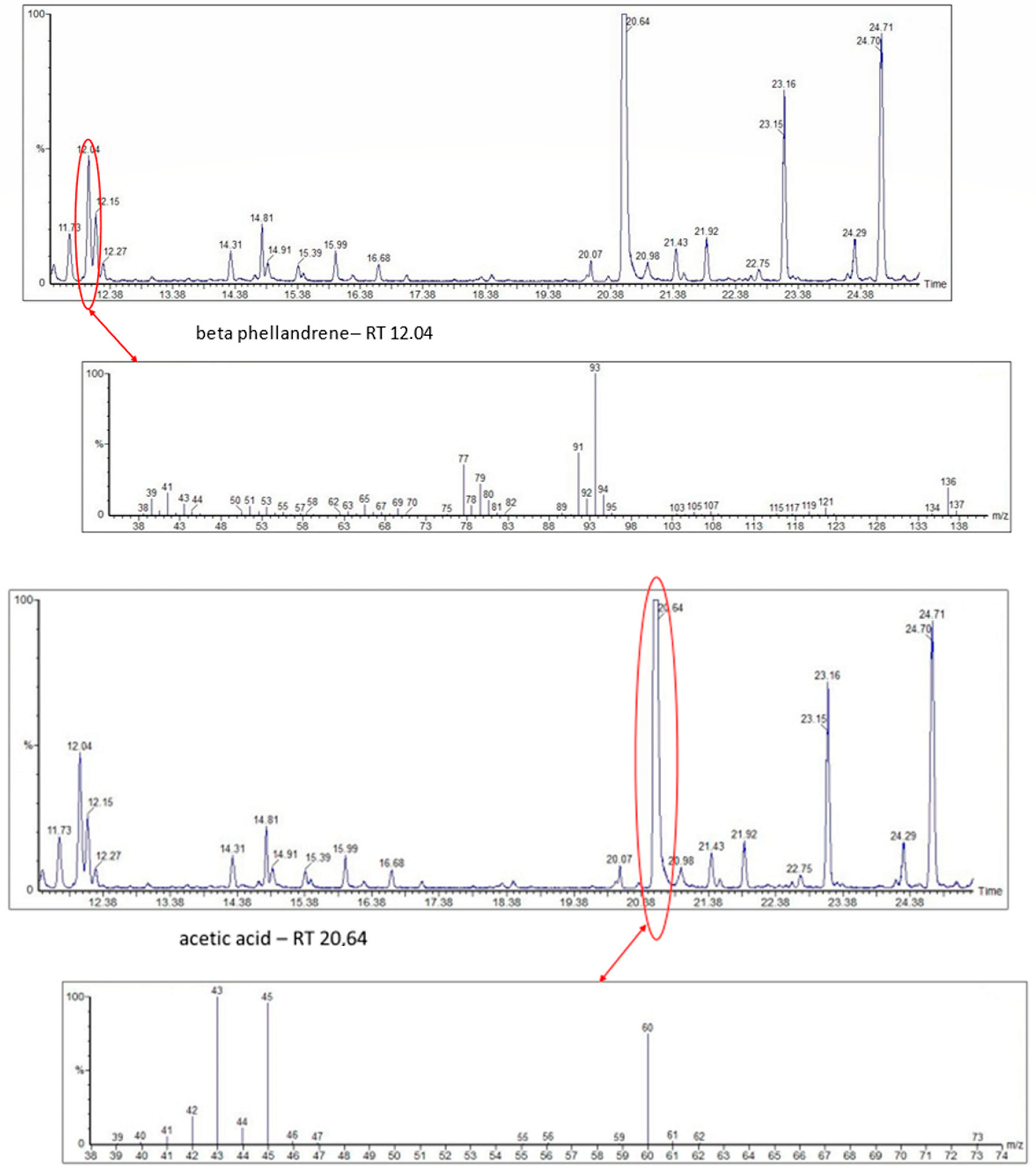
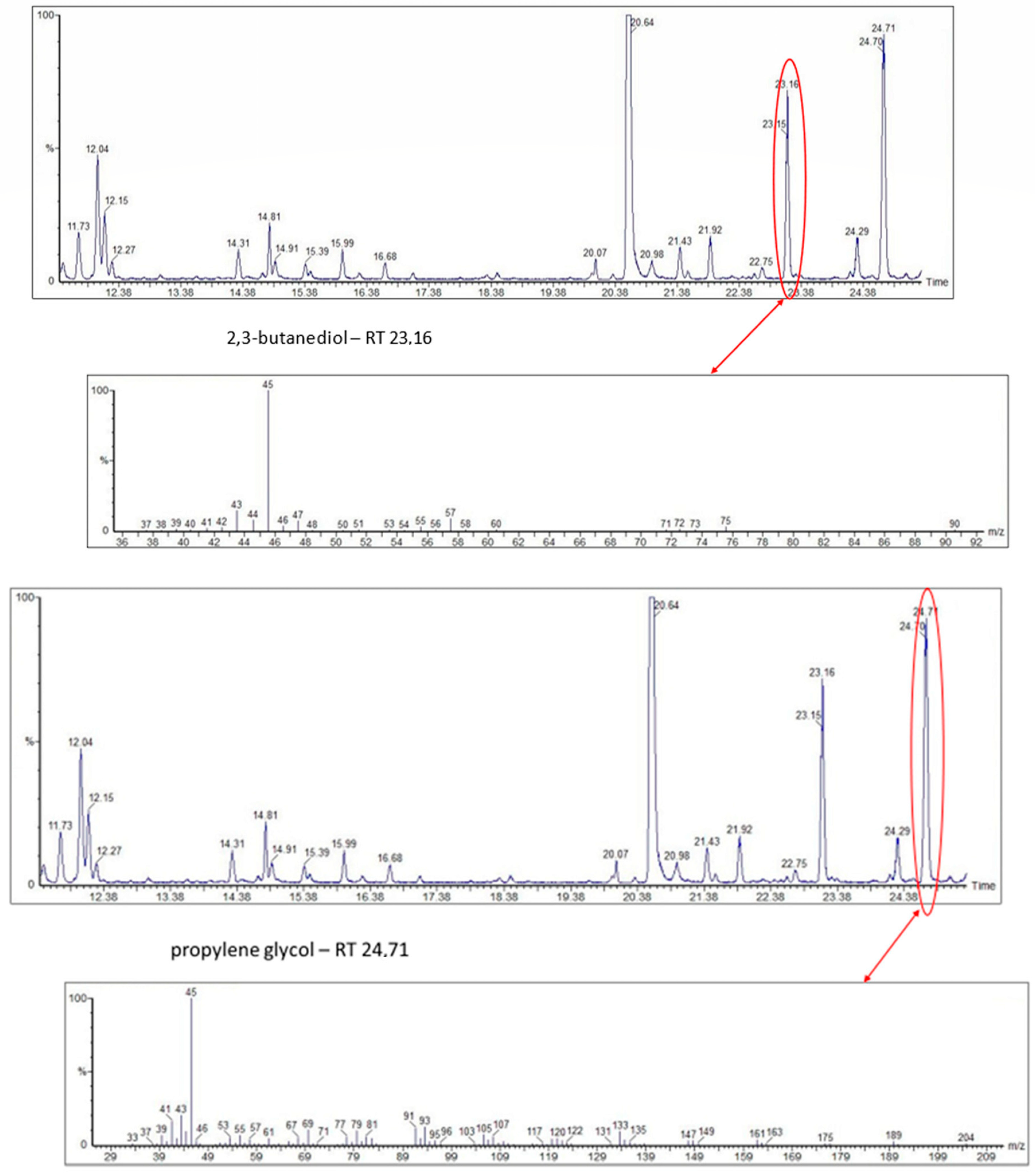




| No. Australia | 1 No. Venezuela | Chemical Classes of Volatile Organic Compounds (VOCs) | Presence of VOCs in Stingless Bee Species | |||
|---|---|---|---|---|---|---|
| Australia | Venezuela | |||||
| Austroplebeia australis | Tetragonula carbonaria | Tetragonula hogkingsi | Tetragonisca angustula | |||
| 1. Acids (11) | ||||||
| 1 | 1 | acetic acid | - | + | + | + |
| 2 | propanoic acid | - | - | - | + | |
| 3 | 2-methyl propanoic acid | - | - | - | + | |
| 4 | butanoic acid | - | - | - | + | |
| 5 | 3-methyl butanoic acid | - | - | - | + | |
| 6 | 2-methyl butanoic acid | - | - | - | + | |
| 7 | 3-methyl pentanoic acid | - | - | - | + | |
| 8 | pentanoic acid | - | - | - | + | |
| 9 | tiglic acid | - | - | - | + | |
| 10 | hexanoic acid | - | - | - | + | |
| 11 | octanoic acid | - | - | - | + | |
| 2. Alcohols (16) | ||||||
| 12 | 2-propanol | - | - | - | + | |
| 13 | ethanol | - | - | - | + | |
| 14 | 2-methyl-3-buten-2-ol | - | - | - | + | |
| 15 | 2-methyl-1-propanol | - | - | - | + | |
| 16 | 2-pentanol | - | - | - | + | |
| 17 | 1-butanol | - | - | - | + | |
| 18 | 2-methyl butanol + 3-methyl butanol3 | - | - | - | + | |
| 19 | 1-pentanol | - | - | - | + | |
| 20 | 2-heptanol | - | - | - | + | |
| 21 | hexanol | - | - | - | + | |
| 22 | 3-octanol | - | - | - | + | |
| 23 | 1-heptanol | - | - | - | + | |
| 24 | 6-methyl-5-hepten-2-ol | - | - | - | + | |
| 25 | 2-ethyl-1-hexanol | - | - | - | + | |
| 26 | benzyl alcohol | - | - | - | + | |
| 27 | 2-phenylethanol | - | - | - | + | |
| 2 | cis-geraniol | + | - | - | - | |
| 3 | geraniol | + | + | + | - | |
| 4 | benzemethanol | + | + | + | - | |
| 5 | epiglobulol | - | + | + | - | |
| 6 | (−)spathulenol | + | + | + | - | |
| 3. Aldehydes (7) | ||||||
| 7 | 28 | hexanal | + | + | + | + |
| 29 | (Z)o(E)-2-heptenal | - | - | - | + | |
| 8 | 30 | nonanal | + | + | - | + |
| 31 | (E)-2-octenal | - | - | - | + | |
| 32 | furfural | - | - | - | + | |
| 33 | benzaldehyde | - | - | - | + | |
| 34 | benzeneacetaldehyde | - | - | - | + | |
| 9 | p-anisaldehyde | + | + | + | - | |
| 10 | α-citral | + | - | - | - | |
| 11 | methanone | + | + | + | - | |
| 4. Esters (16) | ||||||
| 35 | methyl acetate | - | - | - | + | |
| 36 | ethyl acetate | - | - | - | + | |
| 37 | isopropyl acetate | - | - | - | + | |
| 38 | butanoic acid ethyl ester | - | - | - | + | |
| 39 | butanoic acid-2-methyl ethyl ester | - | - | - | + | |
| 40 | ethyl isovalerate | - | - | - | + | |
| 41 | 3-methyl butyl acetate | - | - | - | + | |
| 42 | hexanoic acid ethyl ester | - | - | - | + | |
| 43 | heptanoic acid ethyl ester | - | - | - | + | |
| 44 | ethyl (L)-(-)-lactate | - | - | - | + | |
| 45 | ethyl 3-hydroxybutanoate | - | - | - | + | |
| 46 | ethyl succinate | - | - | - | + | |
| 47 | ethyl benzene acetate | - | - | - | + | |
| 48 | 2-phenethyl acetate | - | - | - | + | |
| 49 | delta octalactone | - | - | - | + | |
| 50 | 5,6-dihydro-6-propyl-2H-pyran-2-one | - | - | - | + | |
| 5. Ketones (8) | ||||||
| 51 | acetone | - | - | - | + | |
| 52 | 2-butanone | - | - | - | + | |
| 53 | 3-pentanone | - | - | - | + | |
| 54 | 2-methyl-3-pentanone | - | - | - | + | |
| 55 | 2-heptanone | - | - | - | + | |
| 56 | 3-octanone | - | - | - | + | |
| 57 | 6-methyl-5-hepten-2-one | - | - | - | + | |
| 58 | 2-nonanone | - | - | - | + | |
| 12 | 4-ketoisophorone | + | - | - | - | |
| 13 | methanone | + | + | + | - | |
| 6. Monoterpenes (17) | ||||||
| 59 | 1R-α-pinene | - | - | - | + | |
| 60 | α-thujene | - | - | - | + | |
| 61 | camphene | - | - | - | + | |
| 62 | β-pinene | - | - | - | + | |
| 63 | β-thujene | - | - | - | + | |
| 64 | 3-carene | - | - | - | + | |
| 65 | α-phellandrene | - | - | - | + | |
| 66 | β-myrcene | - | - | - | + | |
| 67 | α-terpinene | - | - | - | + | |
| 68 | limonene | - | - | - | + | |
| 69 | eucalyptol | - | - | - | + | |
| 70 | β-phellandrene | - | - | - | + | |
| 71 | γ-terpinene | - | - | - | + | |
| 72 | linalool | - | - | - | + | |
| 73 | terpinen-4-ol | - | - | - | + | |
| 74 | α-terpineol | - | - | - | + | |
| 75 | borneol | - | - | - | + | |
| 14 | α-pinene | + | + | + | - | |
| 7. Oxides (5) | ||||||
| 76 | cis-linalool oxide | - | - | - | + | |
| 77 | trans-linalool oxide | - | - | - | + | |
| 78 | trans-pyranoid linalool oxide | - | - | - | + | |
| 79 | cis-pyranoid linalool oxide | - | - | - | + | |
| 15 | 80 | caryophyllene oxide | + | + | + | + |
| 8. Sesquiterpenes (11) | ||||||
| 81 | α-cubebene | - | - | - | + | |
| 16 | 82 | α-copaene | + | + | + | + |
| 83 | β-bourbonene | - | - | - | + | |
| 84 | β-copaene | - | - | - | + | |
| 17 | 85 | caryophyllene | - | + | + | + |
| 86 | aromandendrene | - | - | - | + | |
| 87 | humulene | - | - | - | + | |
| 88 | γ-muurolene | - | - | - | + | |
| 89 | β-selinene | - | - | - | + | |
| 90 | γ-cadinene | - | - | - | + | |
| 91 | cedrol | - | - | - | + | |
| 18 | sesquiterpene 1 | + | + | + | - | |
| 19 | sesquiterpene 2 | + | + | + | - | |
| 20 | sesquiterpene 3 | + | + | + | - | |
| 21 | sesquiterpene 4 | + | + | + | - | |
| 22 | alloaromadendrene | + | + | + | - | |
| 23 | (+)-ledene | - | + | + | - | |
| 24 | sesquiterpene 5 | - | + | + | - | |
| 25 | sesquiterpene 6 | + | + | + | - | |
| 26 | sesquiterpene 7 | + | + | - | - | |
| 27 | cis-α-bisabolene | + | + | - | - | |
| 9. Others (4) | ||||||
| 92 | 2,3-butanediol (polyol) | - | - | - | + | |
| 93 | propylen glycol (polyol) | - | - | - | + | |
| 94 | estragole (phenylpropene) | - | - | - | + | |
| 95 | glycerin (polyol) | - | - | - | + | |
| 28 | labd-14-ene | - | + | + | - | |
| 10. Hydrocarbons | ||||||
| 29 | 1H-cycloprop[e]azulene | + | + | + | - | |
| 30 | hydrocarbon 1 | + | + | + | - | |
| 31 | hydrocarbon 2 | + | + | + | - | |
| 32 | hydrocarbon 3 | - | + | + | - | |
| 33 | hydrocarbon 4 | + | + | + | - | |
| 34 | hydrocarbon 5 | + | + | + | - | |
| 35 | hydrocarbon 6 | + | + | + | - | |
| Total | 28 | 32 | 29 | 95 | ||
| Metabolites Alphabetical Order | Chemical Formula | Chemical Structures | Origin Food, Microorganisms, Plants | Olfactory Attributes |
|---|---|---|---|---|
| Acetic acid | CH3COOH | 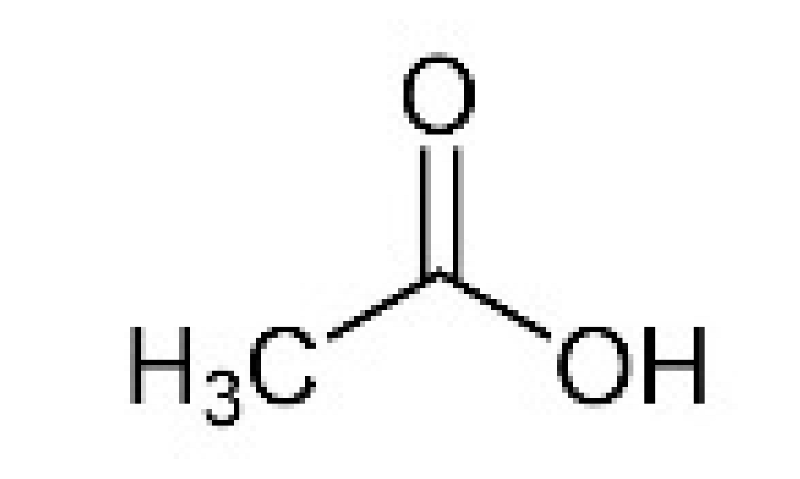 | Acetic acid bacteria | Pungent smell, vinegar |
| p-Anisaldehyde | C8H8O8 | 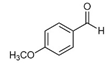 | Vanilla pompona, Solidago odora, Nigella sativa | Anise-like |
| 2,3-Butanediol | C4H10O2 |  | Bacillus spp. | Neutral, wine |
| Butanoic acid | C4H8O2 | 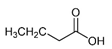 | Butter ghee milk | Rancid butter |
| Ethanol | CH3CH2OH | 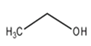 | Yeasts | Distilled-like but not any particular rum, tequila, vodka, wine |
| Ethyl acetate | C4H8O2 | 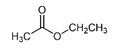 | Vitis rotundifolia, Cinnamomum sieboldii | Pleasant, sweet, fruity |
| Furfural | C4H3OCHO |  | Chemical transformation | Almond-like |
| Glycerin | C3H8O3 |  | Beer honey vinegar wine | Odorless |
| Limonene | C10H16 |  | Oils of grapefruit, lemon, and orange | Lemon-like, citrusy |
| 3-Methyl butanoic acid | C5H10O2 | 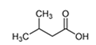 | Valeriana officinalis | Rancid, cheesy, sweaty |
| 3-Methyl butyl acetate | C7H14O2 | 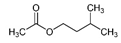 | Vitis rotundifolia, Nicotiana bonariensis | Artificial banana |
| 2-Methyl-1-propanol | C4H10O | 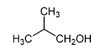 | Fresh tea leaves | Sweet, musty |
| β-Phellandrene | C10H16 | 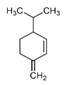 | Chemical transformation, Canada balsam oil | Peppery, minty, citrusy |
| 2-Phenylethanol | C8H10O | 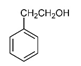 | Vitis rotundifolia, Lonicera japonica, Moringa oleifera | Floral |
| α-Pinene | C10H16 | 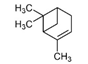 | Eucalyptus oil, pine trees, rosemary | (+)-α-Pinene minty, (−)-α-Pinene pine, turpentine |
| Propylene glycol | C3H8O2 | 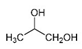 | Soybean and canola oils | Odorless |
| Metabolites Descending Order of Abundance | Chemical Structures | Metabolite Abundance | Origin M, Microbial P, Plant C, Chemical Transformation | Biological Activity (Reference) |
|---|---|---|---|---|
| Acetic acid | 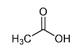 | 50.41 | M | Antibacterial activity in diluting solutions against P. aeruginosa [55,56] |
| 2,3-Butanediol | 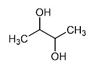 | 23.88 | M | Reduction in oxidative stress, anti-inflammatory, and antihypertensive properties [57,58,59] |
| β-Phellandrene | 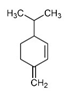 | 9.73 | P | Anticancer and antibacterial activity [60,61] |
| Propylene glycol |  | 7.08 | M | Antibacterial activity against S. aureus and P. aeruginosa [62,63] |
| 2-Methyl-1-propanol |  | 4.47 | M | Antibacterial activity against S. aureus [64,65] |
| Furfural | 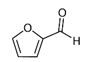 | 3.70 | C | Antibacterial, antioxidant, and anti-inflammatory activity [66,67,68] |
| Ethanol |  | 3.32 | M | Effect of ethanol concentrations in antimicrobial activity of extracts [69]; extraction solvent used to prepare herbal remedies, a cutaneous penetration enhancer, and disinfecting and sterilizing agent |
| Ethyl acetate |  | 2.44 | M, P | More than 5% ethyl acetate is antimicrobial [70]; extraction solvent of analytes in complex biological matrices |
| Glycerin | 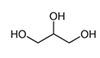 | 1.82 | M, P | Antibacterial activity against Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, and Escherichia coli [62]; sweetener in syrups and excipient in eyewash solutions |
| Butanoic acid |  | 2.52 | M | Mucosal health and energy source for colon cells [71]; additive to increase fruit fragrance |
| 2-Phenylethanol | 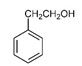 | 2.85 | M, P | Broad-spectrum antifungal activity by suppressing mycelium growth, structural damage to mycelia, ROS stress, and cell membrane disruption [72]; antimicrobial, antiseptic, and disinfectant that is used also as aromatic essence and preservative |
| 3-Methyl butanoic acid | 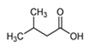 | 4.74 | M, P | Antifungal activity against spore germination and mycelial growth of Colletotrichum gloeosporioides emitted by Bacillus velezensis CE 100 [73]; antifibrotic agent to treat scleroderma as an antirheumatic drug; flavoring cheese |
| Limonene | 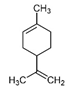 | 2.48 | P | Broad-spectrum and long-lasting resistance to pathogen infection—insecticide, antifungal, anti-viral, and plant immunity activator [74]; promotes weight loss, prevents cancer, treats cancer, and treats bronchitis; cutaneous penetration enhancer for medicinal ointments and creams |
| 5,6-Dihydro-6-propyl-2H-pyran-2-one | 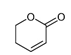 | 1.59 | P | Anxiolytic, antidepressant, anti-inflammatory, thrombolytic, and cytotoxic activities [75]; treating benign prostatic hypertrophy or hyperplasia, prostatic cancer, alopecia, hirsutism, and acne vulgaris |
| 3-Methyl butyl acetate | 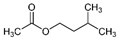 | 1.67 | P | 3-methyl butyl acetate has main anticancer role [76]; masking agent, perfuming agent, and solvent in cosmetics industry |
| Metabolites Ascending Linear Retention Indices | Chemical Structures | Metabolite Abundance | Origin M, Microbial P, Plant | Biological Activity (Reference) | ||
|---|---|---|---|---|---|---|
| Stingless Bees 1 | ||||||
| Aa | Tc | Th | ||||
| α-Pinene |  | 1.6 | 4.4 | 1.5 | P | The anticancer effect of α-pinene was mediated by natural killer (NK) cell activation and cytotoxicity via extracellular signal-regulated kinase/protein kinase B (ERK/AKT) signaling pathways [77], Anti-inflammatory properties, antimicrobial properties, antiulcerogenic and gastroprotective properties, and the ability to aid memory are |
| p-Anisaldehyde | 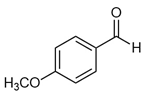 | 6.7 | 0.2 | 0.2 | P | intermediate in the synthesis of other compounds important in pharmaceuticals and perfumery. Antimicrobial activity against Pseudomonas aeruginosa is via membrane transport, lipid biosynthesis, and stress response, as well as synergism with epigallocatechin gallate [78]. |
| 1H-Cycloprop[e]azulene | 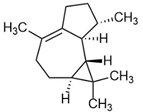 | 1.4 | 7.7 | 6.4 | P | Biological activity in breast cancer cell lines towards estrogen receptors was found using the MTT test [79]. Azulene and its derivatives are antiallergic, antibacterial, and anti-inflammatory therapies. |
| cis-α-Bisabolene | 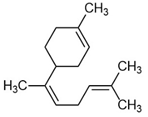 | 0.6 | 0.1 | - | P | An antifungal agent for the plant has anticancer properties but β-bisabolene is more active against human and murine breast cancer cells [80]. It is used as an effective ligand with pregnane X receptor alternative to vitamin E and can penetrate the blood–brain barrier [81]. |
| Epiglobulol | 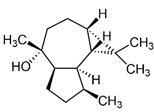 | - | 0.3 | 0.1 | P | An anti-inflammatory sesquiterpene alcohol isolated from the bark of Moringa oleifera and Eucalyptus leaves [82]. |
| Labd-14-ene-8 | 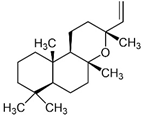 | - | 0.8 | 0.4 | M, P | Presented in a review on sclareol human or murine cancer (cervical, breast, erythroleukemia, colon, gastric, lung epithelial) prevention and treatment [83]. A plant metabolite or a fungal metabolite, as well as an antibacterial agent. |
| Bibliometric Descriptor | Counts |
|---|---|
| All Documents | |
| Time span | 2014–2023 |
| Scopus database | |
| Number of documents | 40 |
| Number of articles | 18 |
| Number of books | 1 |
| Number of book chapters | 15 |
| Number of conference papers | 4 |
| Number of conference reviews | 1 |
| Number of reviews | 1 |
| Number of languages | 3 |
| Bibliometrix | |
| Annual growth rate (%) | 8.01 |
| Sources (no. journals, books) | 20 |
| Author’s keywords DE (no.) | 73 |
| Keywords Plus ID (no.) | 170 |
| Average citations per document | 6.08 |
| Document average age (years) | 3.62 |
| Authors (no.) | 153 |
| Single-authored documents (no.) | 3 |
| Multi-authored documents (no.) | 38 |
| International co-authorship (%) | 45 |
| Average co-authors per document (no.) | 4.88 |
| References (total no.) | 1976 |
| Ranking | NP 1 | Pot-Pollen Research | ||
|---|---|---|---|---|
| Author | Affiliation, City | Country | ||
| 1 | 6 | Vit, P. | Food Science Departament, Faculty of Pharmacy and Bioanalysis, Universidad de Los Andes, Mérida | Venezuela |
| 2 | 4 | Barth, O.M. | Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro | Brazil |
| 3 | 4 | Pérez-Pérez, E. | Laboratory of Biotechnological and Molecular Analysis, Faculty of Pharmacy and Bioanalysis, Universidad de Los Andes, Mérida | Venezuela |
| 4 | 3 | Agussalim | Department of Animal Nutrition and Feed Science, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna No. 3, Bulaksumur, Yogyakarta | Indonesia |
| 5 | 3 | Belina-Aldemita, M.D. | Institute of Chemistry, College of Arts and Sciences, University of the Philippines Los Banos, College, Laguna | Philippines |
| 6 | 3 | D’Amico, S. | Institute for Animal Nutrition and Feed, AGES—Austrian Agency for Health and Food Safety, Spargelfeldstrase 191, Vienna, 1220 | Austria |
| 7 | 3 | Pedro, S.R.M. | Biology Department, Faculty of Philosophy, Science and Letters, Universidade de São Paulo, Ribeirão Preto, SP | Brazil |
| 8 | 3 | Schreiner, M. | Department of Food Science and Technology, BOKU—University of Natural Resources and Life Sciences Vienna, Muthgasse 18, Vienna | Austria |
| 9 | 3 | Yudin, A.S.M. | Meliponini Engineering Laboratory (MePEL), Faculty of Mechanical and Automotive Engineering Technology, Universiti Malaysia Pahang, Pekan, Pahang | Malaysia |
| 10 | 2 | Basrawi, F. | Meliponini Engineering Laboratory (MePEL), Faculty of Mechanical and Automotive Engineering Technology, Universiti Malaysia Pahang, Pekan, Pahang | Malaysia |
| Ranking | NP 1 | Pot-Pollen Research | |
|---|---|---|---|
| Institution | Country | ||
| 1 | 7 | Universidad de Los Andes, Merida | Venezuela |
| 2 | 7 | The University of Sydney | Australia |
| 3 | 5 | Universidade de São Paulo | Brazil |
| 4 | 4 | Fundacao Oswaldo Cruz | Brazil |
| 5 | 3 | Austrian Agency for Health and Food Safety | Austria |
| 6 | 3 | Universidade Federal do Reconcavo da Bahia | Brazil |
| 7 | 3 | Universitat fur Bodenkultur Wien | Austria |
| 8 | 3 | Universitas Gadjah Mada | Indonesia |
| 9 | 3 | University of the Philippines Los Banos | Philippines |
| 10 | 3 | Universiti Malaysia Pahang Al-Sultan Abdullah | Malaysia |
| Ranking | NP 1 | Pot-Pollen Research |
|---|---|---|
| Country | ||
| 1 | 15 | Brazil |
| 2 | 8 | Australia |
| 3 | 7 | Venezuela |
| 4 | 4 | Indonesia |
| 5 | 4 | Malaysia |
| 6 | 3 | Austria |
| 7 | 3 | Ecuador |
| 8 | 3 | Mexico |
| 9 | 3 | Philippines |
| 10 | 2 | Argentina |
| Ranking | NP 1 | Pot-Pollen Research |
|---|---|---|
| Sources (h Index, Quartile, Impact Score) Publisher, Country 2 | ||
| 1 | 16 | Pot Pollen In Stingless Bee Melittology Springer, Switzerland |
| 2 | 3 | Iop Conference Series Materials Science and Engineering (h 54, discontinued, 0.50) IOP Publishing Ltd., United Kingdom |
| 3 | 2 | Journal of Apicultural Research (h 66, Q2, 2.08) Taylor and Francis Ltd., United Kingdom |
| 4 | 2 | Livestock Research for Rural Development (h 35, Q3, 0.56) Centro para la Investigacion en Sistemas Sostenibles de Produccion Agropecuaria, Colombia |
| 5 | 2 | Neotropical Entomology (h 54, Q2, 1.89) Springer US, United States |
| 6 | 1 | Biodiversitas (h 22, Q3, 1.50) Biology department, Sebelas Maret University Surakarta, Indonesia |
| 7 | 1 | BMC Ecology and Evolution (h 138, Q1, 2.69) BioMed Central Ltd., United Kingdom |
| 8 | 1 | Bodenkultur (h 20, Q4, 0.26) De Gruyter Open Ltd., Germany |
| 9 | 1 | Drying Technology (h 102, Q1, 4.56) Taylor and Francis Ltd., United States |
| 10 | 1 | Emirates Journal of Food and Agriculture (h 37, Q3, 1.31) United Arab Emirates University, UAE |
| Ranking | NP 1 | Pot-Pollen Research | |
|---|---|---|---|
| Funding Sponsor | Country | ||
| 1 | 24 | Conselho Nacional de Desenvolvimento Científico e Tecnológico | Brazil |
| 2 | 5 | Coordenação de Aperfeiçoamento de Pessoal de Nível Superior | Brazil |
| 3 | 4 | Universiti Malaysia Pahang | Malaysia |
| 4 | 3 | Bundesministerium für Wissenschaft, Forschung und Wirtschaft | Austria |
| 5 | 3 | Ministry of Higher Education, Malaysia | Malaysia |
| 6 | 3 | Österreichische Agentur für Internationale Mobilität und Kooperation in Bildung, Wissenschaft und Forschung | Austria |
| 7 | 2 | Fundação de Amparo à Pesquisa do Estado do Amazonas | Brazil |
| 8 | 2 | Fundação de Amparo à Pesquisa do Estado de São Paulo | Brazil |
| 9 | 1 | Carlsbergfondet | Denmark |
| 10 | 1 | Consejo Nacional de Investigaciones Científicas y Técnicas | Argentina |
| Ranking | NP 1 | Pot-Pollen Research |
|---|---|---|
| Scopus Subject Area | ||
| 1 | 33 | Agricultural and Biological Sciences |
| 2 | 20 | Engineering |
| 3 | 19 | Biochemistry, Genetics, and Molecular Biology |
| 4 | 17 | Environmental Science |
| 5 | 4 | Materials Science |
| 6 | 2 | Chemistry |
| 7 | 1 | Immunology and Microbiology |
| 8 | 1 | Multidisciplinary |
| 9 | 1 | Nursing |
| 10 | 1 | Pharmacology, Toxicology, and Pharmaceutics |
| Bibliometric Descriptor | Counts |
|---|---|
| All Documents | |
| Time span | 1976:2023 |
| Scopus database | |
| Number of documents | 48 |
| Number of articles | 38 |
| Number of book chapters | 1 |
| Number of conference papers | 2 |
| Number of reviews | 7 |
| Number of languages | 3 |
| Bibliometrix | |
| Annual growth rate (%) | 3.89 |
| Sources (no. journals, books) | 36 |
| Author’s keywords DE (no.) | 207 |
| Keywords Plus ID (no.) | 740 |
| Average citations per document | 36.73 |
| Document average age (years) | 14 |
| Authors (no.) | 190 |
| Single-authored documents (no.) | 1 |
| Multi-authored documents (no.) | 189 |
| International co-authorship (%) | 22.92 |
| Average co-authors per document (no.) | 4.81 |
| References (total no.) | 2.169 |
| Ranking | NP 1 | Direct Injection in Food Flavor Research | ||
|---|---|---|---|---|
| Author | Affiliation, City | Country | ||
| 1 | 8 | Biasioli, F. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), Italy | Italy |
| 2 | 6 | Gasperi, F. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), via E. Mach 1, San Michele all’Adige, 38010, Italy | Italy |
| 3 | 4 | Cappellin, L. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), via E. Mach, 1, S. Michele, 38010, Italy | Italy |
| 4 | 4 | Capozzi, V. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), via E. Mach 1, San Michele all’Adige, 38010, Italy | Italy |
| 5 | 3 | Aprea, E. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), via E. Mach 1, San Michele all’Adige, 38010, Italy | Italy |
| 6 | 3 | Khomenko, I. | Department of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), via E. Mach 1, San Michele all’Adige, 38010, Italy | Italy |
| 7 | 3 | Le Quere, J.L | Centre des Sciences du Goût et de l’Alimentation (CSGA), CNRS, INRAE, Institut Agro, Université de Bourgogne, Dijon, F-21000, France | France |
| 8 | 3 | Mark, T.D. | Institut für Ionenphysik und Angewandte Physik, Leopold-Franzens Universität Innsbruck, Technikerstr. 25, Innsbruck, 6020, Austria | Austria |
| 9 | 3 | Romano, A. | Faculty of Science and Technology, Free University of Bolzano, Bolzano, 39100, Italy | Italy |
| 10 | 3 | Scampicchio, M. | Faculty of Science and Technology, Free University of Bolzano, Bolzano, 39100, Italy | Italy |
| Ranking | NP 1 | Direct Injection in Food Flavor Research | |
|---|---|---|---|
| Institution | Country | ||
| 1 | 8 | Fondazione Edmund Mach | Italy |
| 2 | 4 | Universita degli Studi di Foggia | Italy |
| 3 | 4 | Universitat Innsbruck | Austria |
| 4 | 3 | Centre des Sciences du Godt et de Alimentation | France |
| 5 | 3 | Free University of Bozen-Bolzano | Italy |
| 6 | 3 | CNRS Centre National de la Recherche Scientifique | France |
| 7 | 2 | AgroParisTech | France |
| 8 | 2 | Centre INRAE Bourgogne-Franche-Comte | France |
| 9 | 2 | Cornell University | United States |
| 10 | 2 | L’lnstitut Agro Dijon | France |
| Ranking | NP 1 | Direct Injection in Food Flavor Research |
|---|---|---|
| Country | ||
| 1 | 10 | United States |
| 2 | 9 | Italy |
| 3 | 8 | France |
| 4 | 7 | China |
| 5 | 5 | Spain |
| 6 | 4 | Austria |
| 7 | 3 | The Netherlands |
| 8 | 2 | Belgium |
| 9 | 2 | Canada |
| 10 | 2 | India |
| Ranking | NP 1 | Direct Injection in Food Flavor Research |
|---|---|---|
| Sources (h Index, Quartile, Impact Score) Publisher, Country 2 | ||
| 1 | 4 | Journal of Agricultural and Food Chemistry (h 328, Q1, 3.21) American Chemical Society, United States |
| 2 | 3 | Food Research International (h 195, Q1, 8.96) Elsevier Ltd., United Kingdom |
| 3 | 3 | Journal of Mass Spectrometry (h123, Q3, 2.13) Wiley-Blackwell, United States |
| 4 | 2 | Analytical and Bioanalytical Chemistry (h 182, Q2, 4.04) Springer Verlag, Germany |
| 5 | 2 | Food and Fermentation Industries (h 43, Q2, 3.85) Multidisciplinary Digital Publishing Institute (MDPI), Switzerland |
| 6 | 2 | Journal of Dairy Science (h 216, Q1, 3.70) Elsevier Ltd., United States |
| 7 | 2 | Molecules (h 199, Q1, 4.71) Multidisciplinary Digital Publishing Institute (MDPI), Switzerland |
| 8 | 2 | Trac Trends in Analytical Chemistry (h 198, Q1, 13.53) Elsevier, the Netherlands |
| 9 | 1 | ACS Symposium Series (h 71, Q4, 0.66) American Chemical Society, United States |
| 10 | 1 | American Journal of Physiology Regulatory Integrative and Comparative Physiology (h 189, Q2, 2.58) American Physiological Society, United States |
| Ranking | NP 1 | Direct Injection in Food Flavor Research | |
|---|---|---|---|
| Funding Sponsor | Country | ||
| 1 | 2 | European Regional Development Fund | European Union |
| 2 | 2 | National Institute of Food and Agriculture | United States |
| 3 | 1 | Agropolis Fondation | France |
| 4 | 1 | Chinese Academy of Sciences | China |
| 5 | 1 | Cornell University | United States |
| 6 | 1 | Department of Science and Technology, Ministry of Science and Technology, India | India |
| 7 | 1 | European Commission | European Union |
| 8 | 1 | Foundation for lchthyosis and Related Skin Types | United States |
| 9 | 1 | Horizon 2020 | European Union |
| 10 | 1 | Horizon 2020 Framework Programme | European Union |
| Ranking | NP 1 | Direct Injection in Food Flavor Research |
|---|---|---|
| Scopus Subject Area | ||
| 1 | 22 | Agricultural and Biological Sciences |
| 2 | 22 | Chemistry |
| 3 | 18 | Biochemistry, Genetics, and Molecular Biology |
| 4 | 5 | Immunology and Microbiology |
| 5 | 4 | Chemical Engineering |
| 6 | 4 | Pharmacology, Toxicology, and Pharmaceutics |
| 7 | 3 | Neuroscience |
| 8 | 1 | Engineering |
| 9 | 1 | Environmental Science |
| 10 | 1 | Materials Science |
| Bibliometric Descriptor | Counts of all Documents | |
|---|---|---|
| Pot-Pollen | Direct Injection Food Flavor | |
| Time span | 2014:2023 | 1976:2023 |
| Scopus database | ||
| Number of documents | 40 | 48 |
| Number of articles | 18 | 38 |
| Number of books | 1 | - |
| Number of book chapters | 15 | 1 |
| Number of conference papers | 4 | 2 |
| Number of conference reviews | 1 | - |
| Number of reviews | 1 | 7 |
| Subject areas | ||
| Agricultural and Biological Sciences | 33 | 22 |
| Engineering | 20 | 1 |
| Biochemistry, Genetics, and Molecular Biology | 19 | 18 |
| Environmental Science | 17 | 1 |
| Materials Science | 4 | 1 |
| Chemistry | 2 | 22 |
| Immunology and Microbiology | 1 | 5 |
| Pharmacology, Toxicology, and Pharmaceutics | 1 | 4 |
| Chemical Engineering | - | 4 |
| Neuroscience | - | 3 |
| Bibliometrix | ||
| Annual growth rate (%) | 8.01 | 3.89 |
| Sources (No. journals, books) | 20 | 36 |
| Author’s keywords DE (no.) | 73 | 207 |
| Keywords Plus ID (no.) | 170 | 740 |
| Average citations per document | 6.08 | 36.73 |
| Document average age (years) | 3.62 | 14 |
| Authors (no.) | 153 | 190 |
| Single-authored documents (no.) | 3 | 1 |
| Multi-authored documents (no.) | 37 | 47 |
| International co-authorship (%) | 45 | 22.92 |
| Average co-authors per document (no.) | 4.88 | 4.81 |
| References (total no.) | 1976 | 2.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vit, P.; Araque, M.; Chuttong, B.; Moreno, E.; Contreras, R.R.; Wang, Q.; Wang, Z.; Betta, E.; Bankova, V. Pot-Pollen Volatiles, Bioactivity, Synergism with Antibiotics, and Bibliometrics Overview, Including Direct Injection in Food Flavor. Foods 2024, 13, 3879. https://doi.org/10.3390/foods13233879
Vit P, Araque M, Chuttong B, Moreno E, Contreras RR, Wang Q, Wang Z, Betta E, Bankova V. Pot-Pollen Volatiles, Bioactivity, Synergism with Antibiotics, and Bibliometrics Overview, Including Direct Injection in Food Flavor. Foods. 2024; 13(23):3879. https://doi.org/10.3390/foods13233879
Chicago/Turabian StyleVit, Patricia, Maria Araque, Bajaree Chuttong, Enrique Moreno, Ricardo R. Contreras, Qibi Wang, Zhengwei Wang, Emanuela Betta, and Vassya Bankova. 2024. "Pot-Pollen Volatiles, Bioactivity, Synergism with Antibiotics, and Bibliometrics Overview, Including Direct Injection in Food Flavor" Foods 13, no. 23: 3879. https://doi.org/10.3390/foods13233879
APA StyleVit, P., Araque, M., Chuttong, B., Moreno, E., Contreras, R. R., Wang, Q., Wang, Z., Betta, E., & Bankova, V. (2024). Pot-Pollen Volatiles, Bioactivity, Synergism with Antibiotics, and Bibliometrics Overview, Including Direct Injection in Food Flavor. Foods, 13(23), 3879. https://doi.org/10.3390/foods13233879










