Advances in Vibrational Spectroscopic Techniques for the Detection of Bio-Active Compounds in Virgin Olive Oils: A Comprehensive Review
Abstract
:1. Introduction
- (1)
- To outline the characteristics, applications, potential, and limitations of these three vibrational spectroscopy techniques for characterizing bio-active components in VOO.
- (2)
- To present examples of how these techniques are applied in both industrial and laboratory settings for the analysis of bio-active components in VOO.
- (3)
- To discuss the principles related to chemometric techniques and nanomaterial signal enhancement, improving the understanding of spectral data analysis and the application of various signal enhancement techniques in the detection of low-concentration bio-active components.
2. Vibrational Spectroscopic Techniques
3. Near-Infrared Spectroscopy (NIRS)
4. Mid-Infrared Spectroscopy (MIRS)

| Bond | Compound/Functional Group | Wavenumber (cm−1) |
|---|---|---|
| O–H stretch | Water, alcohol | 3600–3200 |
| C–H stretch | Alkenes | 3100–3000 |
| C–H stretch | Aromatic ring | 3060–3020 |
| C–H stretch | Methylene group | 2960–2860 |
| C=O stretch | Carboxylic acids | ~1743 |
| C=O stretch | Saturated aldehydes | 1750–1715 |
| C=O stretch (amide I) | Amides | 1700–1600 |
| C=C stretch | Alkenes | 1666–1640 |
| C=C stretch | Aromatic ring | 1625–1590, 1590–1575, 1525–1470, 1409–1425 |
| C–H asymmetric stretch | Methyl and methylene groups | ~1460 |
| O–H deformation | Phenolic compounds | 1390–1330 |
| C–O-H deformation | Phenolic compounds | 1382–1317 |
| C–O vibration | Alkyl-aryl ether | 1310–1210, 1120–1020 |
| C–O stretch | Phenolic compounds | 1260–1180 |
| C–C stretch | Phenyl carbon | 1225–1075 |
| C–O and O-H stretch | Aromatic and alcohol | 1230–1030 |
| –C–H rocking vibration | Methoxy group | 1211–1147 |
| C–O stretching vibration | Phenolic compounds | 1150–1040 |
| C–H out-of-plane deformation | Aromatic ring | 900–700 |
| O–H out-of-plane deformation | Aromatic ring | ~720 |
| Analytes | Units | Range | Sample Size | Spectral Acquisition | Wavelength Range (cm−1) | Spectral Preprocessing | Statistical Methods | Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| TPC | mg/kg | 46–877 | 127 | ATR | 3610–816 | – | PLSR | Rc2 = 0.87 RMSEC = 22.4 | [84] |
| TPC | mg GAE/g | 0.39–1.72 | 449 | ATR | 4000–500 | SNV | PLSR | Rv2 = 0.95 RMSECV = 5.04 | [89] |
| TPC | mg GAE/g | 0.39–1.72 | 449 | ATR | 4000–500 | SNV | PCR | Rv2 = 0.99 RMSECV = 6.99 | [89] |
| TPC | mg/kg | 188.46–491.95 | 64 | ATR | 4000–650 | SD | PLSR | Rc2 = 0.99 RMSEC = 6.06 | [90] |
| TPC | mg/kg | 3.3–13.3 | 104 | ATR | 4000–700 | 2DSG | PLSR | Rv2 = 0.97 RMSEV = 0.59 | [88] |
| TPC | mg/kg | 13.4–946.7 | 93 | ATR | 4000–375 | FD + SNV | PLSR | Rv2 = 0.44 RMSEV = 162.10 RPD = 1.13 | [72] |
| Hydroxytyrosol | mg/kg | 0.3–42.9 | 93 | ATR | 4000–375 | FD + SNV | PLSR | Rv2 = 0.17 RMSEV = 9.96 RPD = 1.35 | [72] |
| Hydroxytyrosol | mg/kg | 0.09–30.72 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.68 RMSECV = 4.66 | [90] |
| Tyrosol | mg/kg | 1.2–32.8 | 93 | ATR | 4000–375 | FD + SNV | PLSR | Rv2 = 0.32 RMSEV = 4.98 RPD = 1.34 | [72] |
| Tyrosol | mg/kg | 0.73–44.19 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.52 RMSECV = 7.97 | [90] |
| Hydroxytyrosol secoiridoids | mg/kg | 40.54–75.20 | 93 | ATR | 4000–375 | FD + SNV | PLSR | Rv2 = 0.19 RMSEV = 106.1 RPD = 0.97 | [72] |
| Tyrosol secoiridoids | mg/kg | 61.10–456.10 | 93 | ATR | 4000–375 | FD + SNV | PLSR | Rv2 = 0.30 RMSEV = 105.7 RPD = 0.98 | [72] |
| Caffeic acid | mg/kg | 0.01–0.60 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.24 RMSECV = 0.09 | [90] |
| p-coumaric acid | mg/kg | 0.02–8.13 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.36 RMSECV = 1.06 | [90] |
| Vanillic acid | mg/kg | 0.01–1.14 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.31 RMSECV = 0.16 | [90] |
| Syringic acid | mg/kg | 0.01–0.38 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.19 RMSECV = 0.06 | [90] |
| Cinnamic acid | mg/kg | 0.01–0.41 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.19 RMSECV = 0.07 | [90] |
| Vanillin | mg/kg | 0.01–1.14 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.31 RMSECV = 0.16 | [90] |
| Apigenin | mg/kg | 0.04–5.29 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.39 RMSECV = 0.92 | [90] |
| Luteolin | mg/kg | 0.02–2.55 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.08 RMSECV = 0.52 | [90] |
| Ortho-diphenols | mg GAE/g | 0.37–0.83 | 449 | ATR | 4000–500 | SNV | PLSR | Rv2 = 0.99 RMSECV = 8.05 | [89] |
| Ortho-diphenols | mg GAE/g | 0.37–0.83 | 449 | ATR | 4000–500 | SNV | PCR | Rv2 = 0.99 RMSECV = 7.69 | [89] |
| Flavonoids | mg GAE/g | 0.78–1.96 | 449 | ATR | 4000–500 | SNV | PLSR | Rv2 = 0.99 RMSECV = 5.28 | [89] |
| Flavonoids | mg GAE/g | 0.78–1.96 | 449 | ATR | 4000–500 | SNV | PCR | Rv2 = 0.98 RMSECV = 3.81 | [89] |
| Oleic acid | % | 62.0–80.0 | 86 | ATR | 4000–700 | FD + SD | PLSR | Rv2 = 0.92 | [91] |
| Oleic acid | % | 0.46–1.07 | 47 | ATR | 4000–650 | OSC + WA | PLSR | Rcv2 = 0.93 RMSECV = 0.97 | [92] |
| Oleic acid | mg/kg | 65.66–76.59 | 64 | ATR | 4000–650 | SD | PLSR | Rv2 = 0.94 RMSECV = 0.97 | [90] |
| Oleic acid | mg/kg | 29.9–78.0 | 104 | ATR | 4000–700 | 2DSG | PLSR | Rv2 = 0.99 RMSEV = 1.41 | [88] |
| Linoleic acid | % | 5.3–15.0 | 86 | ATR | 4000–700 | FD + SD | PLSR | Rv2 = 0.94 | [91] |
| Linoleic acid | % | 0.12–0.83 | 47 | ATR | 4000–650 | OSC + WA | PLSR | Rcv2 = 0.93 RMSECV = 0.66 | [92] |
| Linoleic acid | mg/kg | 4.90–15.13 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.91 RMSECV = 0.76 | [90] |
| Linoleic acid | mg/kg | 5.7–41.0 | 104 | ATR | 4000–700 | 2DSG | PLSR | Rv2 = 0.98 RMSEV = 1.40 | [88] |
| Linolenic acid | % | 0.44–0.83 | 47 | ATR | 4000–650 | OSC + WA | PLSR | Rcv2 = 0.64 RMSECV = 0.07 | [92] |
| Linolenic acid | mg/kg | 0.24–0.83 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.00 RMSECV = 0.08 | [90] |
| Linolenic acid | mg/kg | 0.6–1.0 | 104 | ATR | 4000–700 | 2DSG | PLSR | Rv2 = 0.97 RMSEV = 0.02 | [88] |
| Palmitoleic acid | mg/kg | 0.13–1.42 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.52 RMSECV = 0.18 | [90] |
| Chlorophyll a | mg/kg | 0.01–0.26 | 52 | ATR | 4000–650 | 2D | PLSR | Rv2 = 0.18 RMSEV = 0.02 RPD = 0.9 | [83] |
| Chlorophyll b | mg/kg | 0.10–1.70 | 52 | ATR | 4000–650 | 2D | PLSR | Rv2 = 0.24 RMSEV = 0.37 RPD = 1.1 | [83] |
| Lutein | mg/kg | 0.60–6.29 | 52 | ATR | 4000–650 | 2D | PLSR | Rv2 = 0.41 RMSEV = 1.27 RPD = 1.2 | [83] |
| Chlorophylls | mg/kg | 1.075–7.210 | 123 | ATR | 4000–700 | Normalization | PLSR | Rv2 = 0.93 RMSECV = 0.23 RPD = 4.10 | [93] |
| Chlorophylls | mg/kg | 0.51–8.84 | 64 | ATR | 4000–650 | SD | PLSR | Rcv2 = 0.69 RMSECV = 0.95 | [90] |
| Chlorophylls | mg/kg | 0.29–5.64 | 70 | ATR | 4000–700 | SD | PLSR | Rv2 = 0.97 RMSEV = 0.22 | [94] |
| Chlorophylls | mg/kg | 0.29–5.64 | 70 | ATR | 4000–700 | SD | PCR | Rv2 = 0.32 RMSEV = 1.61 | [94] |
| Chlorophylls | mg/kg | 0.29–5.64 | 70 | ATR | 4000–700 | SD | SVM | Rv2 = 0.51 RMSEV = 1.43 | [94] |
| Carotenoids | mg/kg | 0.11–25.63 | 64 | ATR | 4000–700 | SD | PLSR | Rcv2 = 0.46 RMSECV = 3.01 | [90] |
| Squalene | g/kg | 3.25–12.54 | 50 | ATR | 4000–600 | 1D + 2D | PLSR | RMSEC = 0.271 RMSEV = 0.457 | [95] |
5. Raman Spectroscopy
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils; Rev. 13.; International Olive Council: Madrid, Spain, 2019. [Google Scholar]
- Donat-Vargas, C.; Sandoval-Insausti, H.; Peñalvo, J.L.; Iribas, M.C.M.; Amiano, P.; Bes-Rastrollo, M.; Molina-Montes, E.; Moreno-Franco, B.; Agudo, A.; Mayo, C.L.; et al. Olive oil consumption is associated with a lower risk of cardiovascular disease and stroke. Clin. Nutr. 2022, 41, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Lamuela-Raventós, R.M.; Ros, E. The bitter taste of extra virgin olive oil for a sweet long life. J. Am. Coll. Cardiol. 2020, 75, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Sotiroudis, T.G.; Kyrtopoulos, S.A. Anticarcinogenic compounds of olive oil and related biomarkers. Eur. J. Nutr. 2008, 47, 69–72. [Google Scholar] [CrossRef]
- Lee, O.H.; Kim, Y.C.; Kim, K.J.; Kim, Y.C.; Lee, B.Y. The effects of bioactive compounds and fatty acid compositions on the oxidative stability of extra virgin olive oil varieties. Food Sci. Biotechnol. 2007, 16, 415–420. [Google Scholar]
- Gavahian, M.; Khaneghah, A.M.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Sgroi, F.; Sciortino, C.; Giamporcaro, G.; Modica, F. Exploring the impact of beliefs and experiential factors on extra virgin olive oil consumption. J. Agric. Food Res. 2024, 15, 101056. [Google Scholar] [CrossRef]
- Chrysochou, P.; Tiganis, A.; Trigui, I.T.; Grunert, K.G. A cross-cultural study on consumer preferences for olive oil. Food. Qual. Prefer. 2022, 97, 104460. [Google Scholar] [CrossRef]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells-Protection against oxidative stress induced by tert- butylhydroperoxide. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, H.; Soyer, F.; Ozen, B.; Tokatli, F. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J. Agric. Food Chem. 2010, 58, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Fernández-Castillejo, S.; Rubió, L.; Arranz, S.; Catalán, U.; Subirana, I.; Romero, M.P.; Castañer, O.; Pedret, A.; Blanchart, G.; et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects. A randomized, double-blind, cross-over, controlled trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef]
- Khymenets, O.; Fito, M.; Covas, M.I.; Farre, M.; Pujadas, M.A.; Munoz, D.; Konstantinidou, V.; de la Torre, R. Mononuclear cell transcriptome response after sustained virgin olive oil consumption in humans: An exploratory nutrigenomics study. OMICS A J. Integr. Biol. 2009, 13, 7–19. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Mediterranean dietary traditions for the molecular treatment of human cancer: Anti-oncogenic actions of the main olive oil’s monounsaturated fatty acid oleic acid. Curr. Pharm. Biotechnol. 2006, 7, 495–502. [Google Scholar] [CrossRef]
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-spectrum health protection of extra virgin olive oil compounds. In Studies in Natural Products Chemistry; Rahman, A.U., Ed.; Elsevier Science Bv: Amsterdam, The Netherlands, 2018; Volume 57, pp. 41–77. [Google Scholar]
- Sánchez-Villegas, A.; Sánchez-Tainta, A.; Sanchez-Villegas, A.; SanchezTainta, A. Virgin Olive Oil: A Mediterranean Diet Essential; Academic Press Ltd.; Elsevier Science Ltd.: London, UK, 2018; pp. 59–87. [Google Scholar]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Wurtele, G.; Spiegelhalder, B.; Bartsch, H. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Rao, C.V.; Newmark, H.L.; Reddy, B.S. Chemopreventive effect of squalene on colon cancer. Carcinogenesis 1998, 19, 287–290. [Google Scholar] [CrossRef]
- Smith, T.J.; Yang, G.Y.; Seril, D.N.; Liao, J.; Kim, S. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis by dietary olive oil and squalene. Carcinogenesis 1998, 19, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Erdman, J.W. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, N.; Delort, L.; Le Corre, L.; Satih, S.; Bignon, Y.J.; Bernard-Gallon, D. Gene signature of breast cancer cell lines treated with lycopene. Pharmacogenomics 2006, 7, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Lionetto, L.; Cavallari, M.; Tubaro, A.; Rasio, D.; De Nunzio, C.; Hong, G.M.; Borro, M.; Simmaco, M. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. Int. J. Mol. Sci. 2014, 15, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive oil composition. In Olive Oil; Elsevier: Amsterdam, The Netherlands, 2006; pp. 41–72. [Google Scholar]
- Romero, C.; Brenes, M. Analysis of total contents of hydroxytyrosol and tyrosol in olive oils. J. Agric. Food Chem. 2012, 60, 9017–9022. [Google Scholar] [CrossRef]
- Perri, E.; Raffaelli, A.; Sindona, G. Quantitation of oleuropein in virgin olive oil by ionspray mass spectrometry-Selected reaction monitoring. J. Agric. Food Chem. 1999, 47, 4156–4160. [Google Scholar] [CrossRef]
- Rozanska, A.; Russo, M.; Cacciola, F.; Salafia, F.; Polkowska, Z.; Dugo, P.; Mondello, L. Concentration of potentially bioactive compounds in Italian extra virgin olive oils from various sources by using LC-MS and multivariate data analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef]
- Klen, T.J.; Vodopivec, B.M. Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar] [CrossRef]
- Bouymajane, A.; El Majdoub, Y.O.; Cacciola, F.; Russo, M.; Salafia, F.; Trozzi, A.; Filali, F.R.; Dugo, P.; Mondello, L. Characterization of phenolic compounds, vitamin E and fatty acids from monovarietal virgin olive oils of “Picholine marocaine” cultivar. Molecules 2020, 25, 5428. [Google Scholar] [CrossRef]
- Klikarová, J.; Rotondo, A.; Cacciola, F.; Ceslová, L.; Dugo, P.; Mondello, L.; Rigano, F. The phenolic fraction of Italian extra virgin olive oils: Elucidation through combined liquid chromatography and NMR approaches. Food Anal. Methods 2019, 12, 1759–1770. [Google Scholar] [CrossRef]
- Starec, M.; Calabretti, A.; Berti, F.; Forzato, C. Oleocanthal quantification using 1H NMR spectroscopy and polyphenols HPLC analysis of olive oil from the Bianchera/Belica cultivar. Molecules 2021, 26, 242. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M.Z.; Nenadis, N.; Mastralexi, A.; Servili, M.; Butinar, B.; Vichi, S.; Winkelmann, O.; García-González, D.L.; Toschi, T.G. Toward a harmonized and standardized protocol for the determination of total hydroxytyrosol and tyrosol content in virgin olive oil (VOO). The pros of a fit for the purpose ultra high performance liquid chromatography (UHPLC) procedure. Molecules 2019, 24, 2429. [Google Scholar] [CrossRef] [PubMed]
- Zamljen, T.; Slatnar, A.; Hudina, M.; Veberic, R.; Medic, A. Characterization and quantification of capsaicinoids and phenolic compounds in two types of chili olive oils, Using HPLC/MS. Foods 2022, 11, 2256. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Russo, M.; Cacciola, F.; Mandolfino, F.; Salafia, F.; Vilmercati, A.; Fanali, C.; Casale, M.; De Gara, L.; Dugo, P.; et al. Determination of the phenol and tocopherol content in Italian high-quality extra-virgin olive oils by using LC-MS and multivariate data analysis. Food Anal. Methods 2020, 13, 1027–1041. [Google Scholar] [CrossRef]
- Torres-Cobos, B.; Quintanilla-Casas, B.; Vicario, G.; Guardiola, F.; Tres, A.; Vichi, S. Revealing adulterated olive oils by triacylglycerol screening methods: Beyond the official method. Food Chem. 2023, 409, 135256. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef]
- Ammar, S.; Kelebek, H.; Zribi, A.; Abichou, M.; Selli, S.; Bouaziz, M. LC-DAD/ESI-MS/MS characterization of phenolic constituents in Tunisian extra-virgin olive oils: Effect of olive leaves addition on chemical composition. Food Res. Int. 2017, 100, 477–485. [Google Scholar] [CrossRef]
- Kritikou, E.; Kalogiouri, N.P.; Kostakis, M.; Kanakis, D.C.; Martakos, I.; Lazarou, C.; Pentogennis, M.; Thomaidis, N.S. Geographical characterization of olive oils from the North Aegean region based on the analysis of biophenols with UHPLC-QTOF-MS. Foods 2021, 10, 2102. [Google Scholar] [CrossRef]
- Meier, R.J. Vibrational spectroscopy: A ‘vanishing’ discipline? Chem. Soc. Rev. 2005, 34, 743–752. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Sruthi, N.U.; Kumar, A.; Kothakota, A.; Thirumdas, R.; Ramesh, S.V.; Cozzolino, D. Recent applications of vibrational spectroscopic techniques in the grain industry. Food Rev. Int. 2023, 39, 209–239. [Google Scholar] [CrossRef]
- Chen, Z.P.; Lovett, D.; Morris, J. Process analytical technologies and real time process control a review of some spectroscopic issues and challenges. J. Process Control 2011, 21, 1467–1482. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- García Martín, J.F. Potential of near-infrared spectroscopy for the determination of olive oil quality. Sensors 2022, 22, 2831. [Google Scholar] [CrossRef] [PubMed]
- Swinehart, D.F. The beer-lambert law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- García-Martín, J.F. Optical path length and wavelength selection using Vis/NIR spectroscopy for olive oil’s free acidity determination. Int. J. Food Sci. Technol. 2015, 50, 1461–1467. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I.; Chree, A.; Scaife, J.R. Multivariate determination of free fatty acids and moisture in fish oils by partial least-squares regression and near-infrared spectroscopy. LWT-Food Sci. Technol. 2005, 38, 821–828. [Google Scholar] [CrossRef]
- Christy, A.A.; Kasemsumran, S.; Du, Y.P.; Ozaki, Y. The detection and quantification of adulteration in olive oil by near-infrared spectroscopy and chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Sato, T.; Kawano, S.; Iwamoto, M. Near infrared spectral patterns of fatty acid analysis from fats and oils. J. Am. Oil Chem. Soc. 1991, 68, 827–833. [Google Scholar] [CrossRef]
- García-González, D.L.; Baeten, V.; Pierna, J.A.F.; Tena, N. Infrared, Raman, and fluorescence spectroscopies: Methodologies and applications. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 335–393. [Google Scholar]
- Murray, I. The NIR spectra of homologous series of organic compounds. In Proceedings of the international NIR/NIT Conference, Budapest, Hungary, 1 January 1986; pp. 13–28. [Google Scholar]
- Cayuela, J.A.; García-Martín, J.F. Sorting olive oil based on alpha-tocopherol and total tocopherol content using near-infra-red spectroscopy (NIRS) analysis. J. Food Eng. 2017, 202, 79–88. [Google Scholar] [CrossRef]
- Holman, R.T.; Edmondson, P.R. Near-infrared spectra of fatty acids and some related substance. Anal. Chem. 1956, 28, 1533–1538. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D. Evaluation of minor components, sensory characteristics and quality of virgin olive oil by near infrared (NIR) spectroscopy. Food Res. Int. 2013, 50, 250–258. [Google Scholar] [CrossRef]
- Sinelli, N.; Casale, M.; Di Egidio, V.; Oliveri, P.; Bassi, D.; Tura, D.; Casiraghi, E. Varietal discrimination of extra virgin olive oils by near and mid infrared spectroscopy. Food Res. Int. 2010, 43, 2126–2131. [Google Scholar] [CrossRef]
- Sánchez, J.A.C.; Moreda, W.; García, J.M. Rapid determination of olive oil oxidative stability and its major quality parameters using Vis/NIR transmittance spectroscopy. J. Agric. Food Chem. 2013, 61, 8056–8062. [Google Scholar] [CrossRef]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. Trac Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Manley, M.; Eberle, K. Comparison of Fourier transform near infrared spectroscopy partial least square regression models for South African extra virgin olive oil using spectra collected on two spectrophotometers at different resolutions and path lengths. J. Near Infrared Spectrosc. 2006, 14, 111–126. [Google Scholar] [CrossRef]
- Johnson, J.B.; Walsh, K.B.; Naiker, M.; Ameer, K. The use of infrared spectroscopy for the quantification of bioactive compounds in food: A review. Molecules 2023, 28, 3215. [Google Scholar] [CrossRef]
- Abu-Khalaf, N.; Hmidat, M. Visible/Near Infrared (VIS/NIR) spectroscopy as an optical sensor for evaluating olive oil quality. Comput. Electron. Agric. 2020, 173, 105445. [Google Scholar] [CrossRef]
- Vanstone, N.; Moore, A.; Martos, P.; Neethirajan, S. Detection of the adulteration of extra virgin olive oil by near-infrared spectroscopy and chemometric techniques. Food Qual. Saf. 2018, 2, 189–198. [Google Scholar] [CrossRef]
- Vieira, L.S.; Assis, C.; de Queiroz, M.; Neves, A.A.; de Oliveira, A.F. Building robust models for identification of adulteration in olive oil using FT-NIR, PLS-DA and variable selection. Food Chem. 2021, 345, 128866. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Yousfi, K.; Martínez, M.C.; García, J.M. Rapid determination of olive oil chlorophylls and carotenoids by using visible spectroscopy. J. Am. Oil Chem. Soc. 2014, 91, 1677–1684. [Google Scholar] [CrossRef]
- Márquez, A.J. Monitoring carotenoid and chlorophyll pigments in virgin olive oil by visible-near infrared transmittance spectroscopy.: On-line application. J. Near Infrared Spectrosc. 2003, 11, 219–226. [Google Scholar] [CrossRef]
- Arroyo-Cerezo, A.; Yang, X.P.; Jiménez-Carvelo, A.M.; Pellegrino, M.; Savino, A.F.; Berzaghi, P. Assessment of extra virgin olive oil quality by miniaturized near infrared instruments in a rapid and non-destructive procedure. Food Chem. 2024, 430, 137043. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, I.S.; Dag, Ç.; Özinanç, G.; Suçsoran, Ö.; Ertas, E.; Bekiroglu, S. Quantification of sterols and fatty acids of extra virgin olive oils by FT-NIR spectroscopy and multivariate statistical analyses. LWT Food Sci. Technol. 2018, 91, 125–132. [Google Scholar] [CrossRef]
- Milinovic, J.; Garcia, R.; Rato, A.E.; Cabrita, M.J. Rapid assessment of monovarietal portuguese extra virgin olive oil’s (EVOO’s) fatty acids by Fourier-transform near-infrared spectroscopy (FT-NIRS). Eur. J. Lipid Sci. Technol. 2019, 121, 1800392. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.E.; Reboredo-Rodríguez, P.; Salvador, M.D.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J.; Fregapane, G. Assessment of polar phenolic compounds of virgin olive oil by NIR and mid-IR spectroscopy and their impact on quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1600099. [Google Scholar] [CrossRef]
- Cayuela, J.A.; García-Martín, J.F. Nondestructive measurement of squalene in olive oil by near infrared spectroscopy. LWT Food Sci. Technol. 2018, 88, 103–108. [Google Scholar] [CrossRef]
- Ding, F.C.; Zuo, C.Z.; García-Martín, J.F.; Ge, Y.; Tu, K.; Peng, J.; Xiao, H.M.; Lan, W.J.; Pan, L.Q. Non-invasive prediction of mango quality using near-infrared spectroscopy: Assessment on spectral interferences of different packaging materials. J. Food Eng. 2023, 357, 111653. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Shurvell, H. Spectra-structure correlations in the mid-and far-infrared. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared spectroscopy coupled with chemometrics: A tool for the analysis of intact food systems and the exploration of their molecular structure-quality relationships—A review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. In Annual Review of Food Science and Technology; Doyle, M.P., Klaenhammer, T.R., Eds.; Annual Review of Food Science and Technology; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 2, pp. 467–483. [Google Scholar]
- Jaggi, N.; Vij, D.R. Fourier transform infrared spectroscopy. In Handbook of Applied Solid State Spectroscopy; Vij, D.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 411–450. [Google Scholar]
- Ramer, G.; Lendl, B. Attenuated total reflection Fourier transform infrared spectroscopy. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Tahir, H.E.; Zou, X.B.; Xiao, J.B.; Mahunu, G.K.; Shi, J.Y.; Xu, J.L.; Sun, D.W. Recent progress in rapid analyses of vitamins, phenolic, and volatile compounds in foods using vibrational spectroscopy combined with chemometrics: A review. Food Anal. Meth. 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B.; Tokatli, F. Use of FTIR and UV-visible spectroscopy in determination of chemical characteristics of olive oils. Talanta 2019, 201, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caravaca, A.M.; Maggio, R.M.; Verardo, V.; Cichelli, A.; Cerretani, L. Fourier transform infrared spectroscopy-Partial Least Squares (FTIR-PLS) coupled procedure application for the evaluation of fly attack on olive oil quality. LWT Food Sci. Technol. 2013, 50, 153–159. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Relationships between the composition of edible oils and lard and the ratio of the absorbance of specific bands of their Fourier transform infrared spectra. Role of some bands of the fingerprint region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Zhu, Q.Q. Latent variable regression for supervised modeling and monitoring. IEEE CAA J. Autom. Sin. 2020, 7, 800–811. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Karimi, S. Construction of stable multivariate calibration models using unsupervised segmented principal component regression. J. Chemometr. 2011, 25, 139–150. [Google Scholar] [CrossRef]
- Aykas, D.P.; Karaman, A.D.; Keser, B.; Rodriguez-Saona, L. Non-targeted authentication approach for extra virgin olive oil. Foods 2020, 9, 221. [Google Scholar] [CrossRef]
- Machado, M.; Machado, N.; Gouvinhas, I.; Cunha, M.; De Almeida, J.; Barros, A. Quantification of chemical characteristics of olive fruit and oil of cv Cobrançosa in two ripening stages using MIR spectroscopy and chemometrics. Food Anal. Meth. 2015, 8, 1490–1498. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. Prediction of various chemical parameters of olive oils with Fourier transform infrared spectroscopy. LWT Food Sci. Technol. 2015, 63, 978–984. [Google Scholar] [CrossRef]
- Maggio, R.M.; Kaufman, T.S.; Del Carlo, M.; Cerretani, L.; Bendini, A.; Cichelli, A.; Compagnone, D. Monitoring of fatty acid composition in virgin olive oil by Fourier transformed infrared spectroscopy coupled with partial least squares. Food Chem. 2009, 114, 1549–1554. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Ozen, B.; Tokatli, F. Comparison of fatty acid profiles and mid-infrared spectral data for classification of olive oils. Eur. J. Lipid Sci. Technol. 2010, 112, 218–226. [Google Scholar] [CrossRef]
- Zaroual, H.; El Hadrami, E.; Karoui, R. Preliminary study on the potential application of Fourier-transform mid-infrared for the evaluation of overall quality and authenticity of Moroccan virgin olive oil. J. Sci. Food Agric. 2021, 101, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Zaroual, H.; El Hadrami, E.; Chénè, C.; Karoui, R. Fourier transform infrared spectroscopy coupled with chemometrics for the monitoring of virgin olive oil quality during storage up to 18 months. Eur. Food Res. Technol. 2024, 250, 1969–1986. [Google Scholar] [CrossRef]
- Tarhan, I. A comparative study of ATR-FTIR, UV-visible and fluorescence spectroscopy combined with chemometrics for quantification of squalene in extra virgin olive oils. Spectroc. Acta Pt. A Mol. Biomol. Spectr. 2020, 241, 118714. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, C.; Harwood, J. Fatty acid and lipid structure. In The Lipid Handbook with CD-ROM; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–50. [Google Scholar]
- Abbas, O.; Compere, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Keresztury, G. Raman spectroscopy: Theory. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; Volume 1, pp. 71–87. [Google Scholar]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Wood, S.; Hollis, J.R.; Kim, J.S. Raman spectroscopy as an advanced structural nanoprobe for conjugated molecular semiconductors. J. Phys. D Appl. Phys. 2017, 50, 073001. [Google Scholar] [CrossRef]
- Stanton, S.G.; Pecora, R.; Hudson, B.S. Resonance enhanced dynamic rayleigh-scattering. J. Chem. Phys. 1981, 75, 5615–5626. [Google Scholar] [CrossRef]
- Windarsih, A.; Lestari, L.A.; Erwanto, Y.; Putri, A.R.; Irnawati; Fadzillah, N.A.; Rahnnawati, N.; Rohman, A. Application of Raman spectroscopy and chemometrics for quality controls of fats and oils: A review. Food Rev. Int. 2023, 39, 3906–3925. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.W.; Wolverson, D.; Valev, V.K. Raman techniques: Fundamentals and frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Kagan, M.R.; McCreery, R.L. Reduction of fluorescence interference in raman-spectroscopy via analyte adsorption on graphitic carbon. Anal. Chem. 1994, 66, 4159–4165. [Google Scholar] [CrossRef]
- Madonini, F.; Villa, F. Single photon avalanche diode arrays for time-resolved Raman spectroscopy. Sensors 2021, 21, 4287. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hu, L.M.; Ding, F.C.; Liu, J.; Su, J.; Tu, K.; Peng, J.; Lan, W.J.; Pan, L.Q. Introducing high-performance star-shaped bimetallic nanotags into SERS aptasensor: An ultrasensitive and interference-free method for chlorpyrifos detection. Biosens. Bioelectron. 2024, 263, 116577. [Google Scholar] [CrossRef] [PubMed]
- Efremov, E.V.; Ariese, F.; Gooijer, C. Achievements in resonance Raman spectroscopy review of a technique with a distinct analytical chemistry potential. Anal. Chim. Acta 2008, 606, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Rodrigues, V.; Calheiros, R.; Marques, M.P.M. Characterization of antioxidant olive oil biophenols by spectroscopic methods. J. Sci. Food Agric. 2011, 91, 309–314. [Google Scholar] [CrossRef]
- Portarena, S.; Anselmi, C.; Leonardi, L.; Proietti, S.; Bizzarri, A.R.; Brugnoli, E.; Baldacchini, C. Lutein/β-carotene ratio in extra virgin olive oil: An easy and rapid quantification method by Raman spectroscopy. Food Chem. 2023, 404, 134748. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Portaccio, M.; Delfino, I.; Lepore, M. Surface-enhanced Raman spectroscopy for monitoring extravirgin olive oil bioactive components. J. Chem. 2019, 2019, 9537419. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Rapid Determination of free fatty acid in extra virgin olive oil by Raman spectroscopy and multivariate analysis. J. Am. Oil Chem. Soc. 2009, 86, 507–511. [Google Scholar] [CrossRef]
- Berezin, K.V.; Dvoretskii, K.N.; Chernavina, M.L.; Novoselova, A.V.; Nechaev, V.V.; Antonova, E.M.; Shagautdinova, I.T.; Likhter, A.M. The use of Raman spectroscopy and methods of quantum chemistry for assessing the relative concentration of triglycerides of oleic and linoleic acids in a mixture of olive oil and sunflower seed oil. Opt. Spectrosc. 2018, 125, 311–316. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Cañada, M.J. Direct, reagent-free determination of free fatty acid content in olive oil and olives by Fourier transform Raman spectrometry. Anal. Chim. Acta 2003, 487, 211–220. [Google Scholar] [CrossRef]
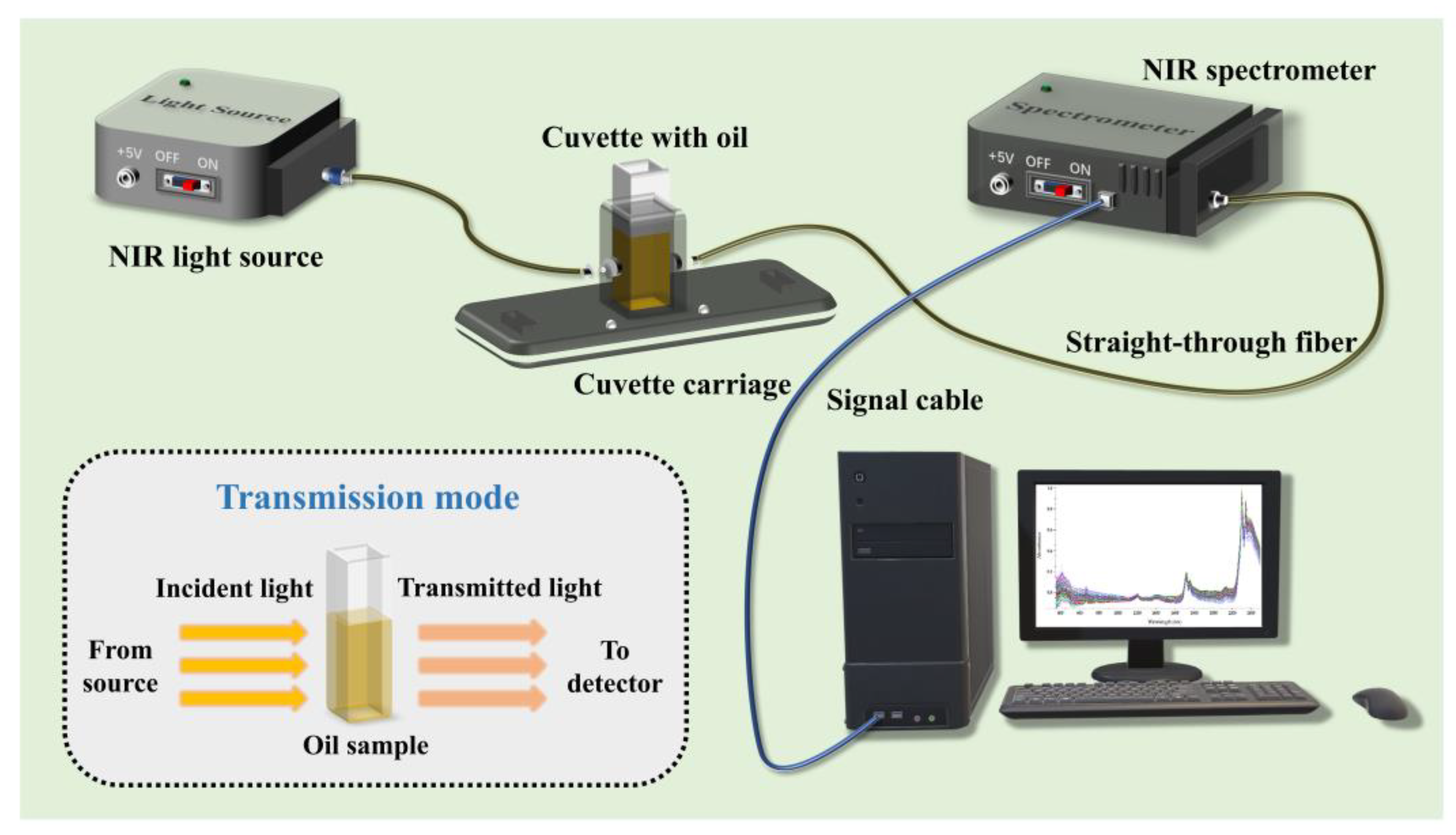
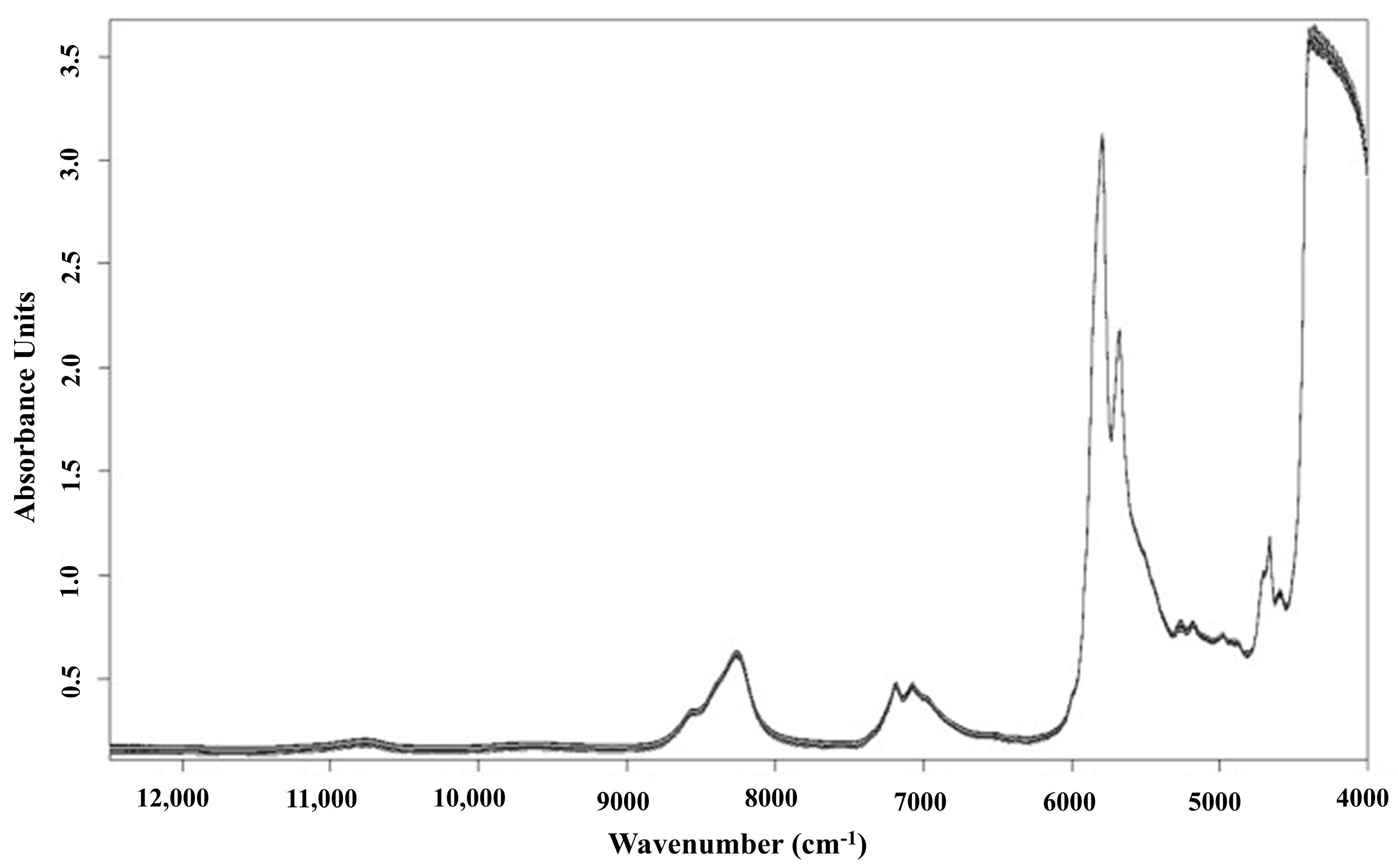
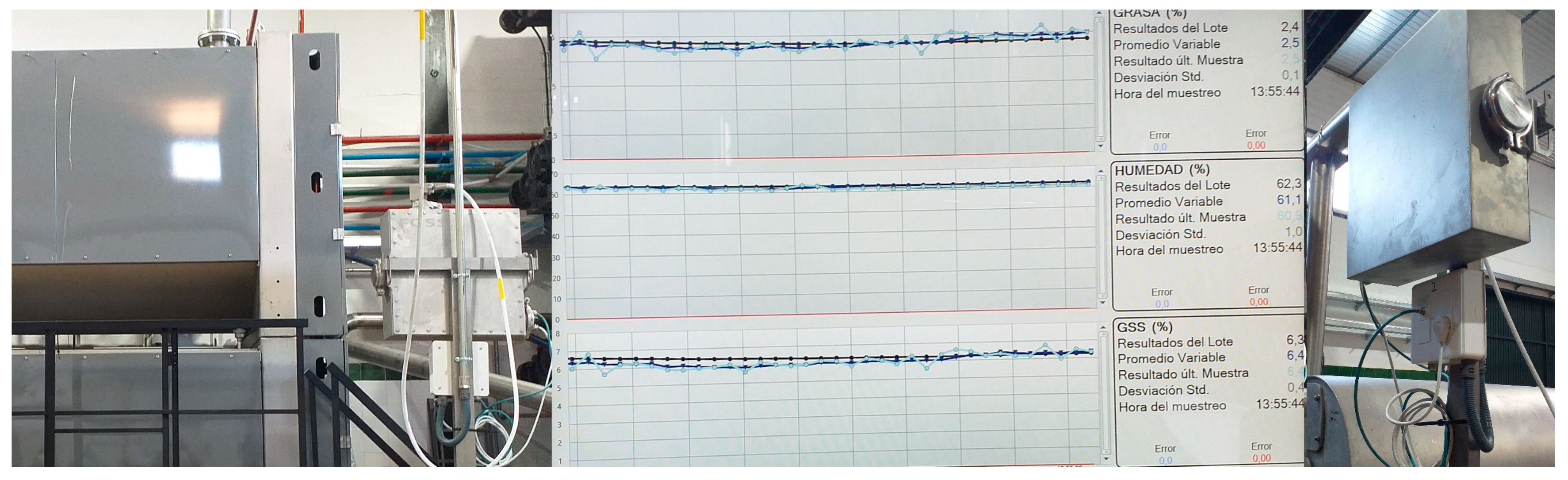
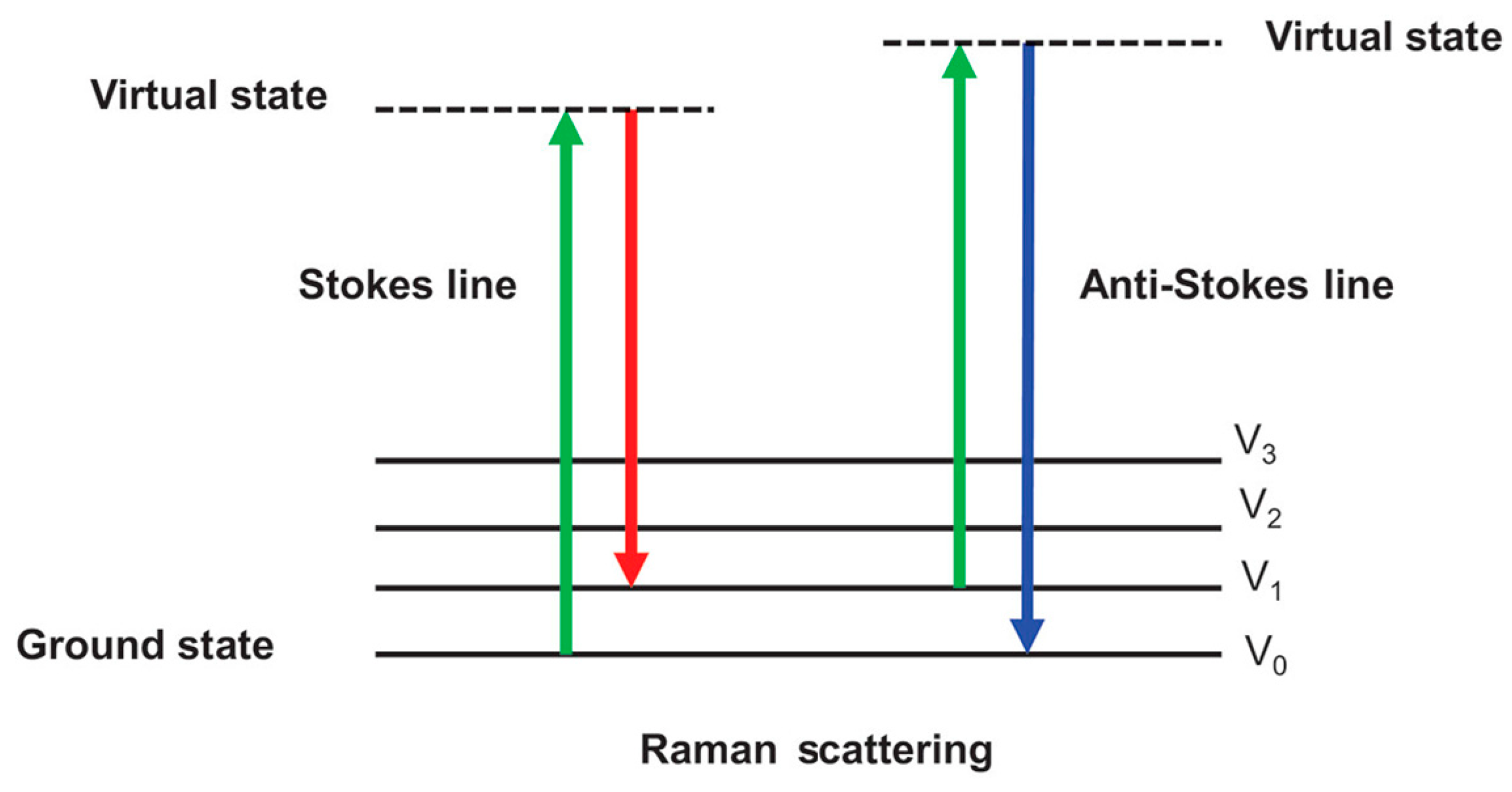
| Selected Wavelength (nm) | Wavenumber (cm−1) | Functional Groups | Assignment | Reference |
|---|---|---|---|---|
| 1167 | 8569 | –CH3 | C–H stretch second overtone | [58] |
| 1208 | 8278 | –CH2 | C–H stretch second overtone | [50] |
| 1220 | 8197 | HC=CH– | C–H stretch second overtone | [59] |
| 1373 | 7283 | –CH3 | 2C–H stretch + C–H deformation | [58] |
| 1400 | 7143 | –OH | O–H stretch | [55] |
| 1462 | 6840 | –CH2 | 2C–H stretch + C–H deformation | [58] |
| 1720 | 5814 | –CH2, –CH3, =CH2 | C–H first overtone | [52] |
| 1760 | 5682 | –CH2, –CH3, =CH2 | C–H first overtone | [51] |
| 1832 | 5459 | –COOR | C–H first overtone | [54] |
| 1844 | 5423 | –CH2 | C–H first overtone | [58] |
| 1950 | 5128 | –OH | O–H stretch first overtone | [55] |
| 2022 | 4946 | –COOR | C–H str. + C=O str. | [58] |
| 2049 | 4880 | –COOR | C–H str. + C=O str. | [58] |
| 2144 | 4664 | HC=CH– | C–H str. + C=C str. | [54] |
Analytes | Units | Range | Samples Number | Wavelength Range (nm) | Path Length (mm) | Spectra Preprocessing | Statistical Methods | Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| TPC | mg/kg | 110.73–593.95 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.79 RMSEV = 44.50 RPD = 1.71 | [57] |
| TPC | mg/kg | 44.49–738.76 | 98 | 978–2500 | 0.5 | MSC | PLSR | Rv2 = 0.34 RMSEV = 82.10 RPD = 1.24 | [62] |
| TPC | mg/kg | 44.49–738.76 | 98 | 1100–2500 | 0.2 | MSC | PLSR | Rv2 = 0.21 RMSEV = 89.66 RPD = 1.13 | [62] |
| TPPC | mg/kg | 13.4–946.7 | 93 | 800–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.82 RMSEV = 76.70 RPD = 2.36 | [72] |
| Hydroxytyrosol | mg/kg | 0.3–42.9 | 93 | 800–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.55 RMSEV = 4.84 RPD = 1.25 | [72] |
| Hydroxytyrosol | mg/kg | 1.07–36.12 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.20 RMSEV = 4.06 RPD = 1.03 | [57] |
| Tyrosol | mg/kg | 1.2–32.8 | 93 | 800–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.55 RMSEV = 5.27 RPD = 1.43 | [72] |
| Tyrosol | mg/kg | 1.57–64.39 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.34 RMSEV = 3.20 RPD = 1.06 | [57] |
| Hydroxytyrosol derivatives | mg/kg | 21.41–380.37 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.85 RMSEV = 25.50 RPD = 1.99 | [57] |
| Hydroxytyrosol derivatives | mg/kg | 40.54–75.20 | 93 | 800–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.82 RMSEV = 43.1 RPD = 2.39 | [72] |
| Tyrosol derivatives | mg/kg | 68.33–315.92 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.57 RMSEV = 23.80 RPD = 1.23 | [57] |
| Tyrosol derivatives | mg/kg | 61.1–456.1 | 93 | 800–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.84 RMSEV = 41.5 RPD = 2.31 | [72] |
| Oleuropein | mg/kg | – | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.88 RMSEV = 179.00 RPD = 2.13 | [57] |
| α-tocopherol | mg/kg | 90.96–249.33 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.60 RMSEV = 15.20 RPD = 1.30 | [57] |
| α-tocopherol | mg/kg | 54.50–755.90 | 206 | 1100–2300 | 10 | 1DSG + 2DSG | PLSR | Rcv = 0.91 SEC = 36.14 | [55] |
| β-tocopherol | mg/kg | 9.11–17.20 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.14 RMSEV = 1.53 RPD = 1.04 | [57] |
| β-tocopherol | mg/kg | 0.7–14.1 | 211 | 1100–2300 | 10 | 1DSG + 2DSG | PLSR | Rcv = 0.52 SEC = 0.58 | [55] |
| γ-tocopherol | mg/kg | 10.73–36.56 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.40 RMSEV = 2.23 RPD = 1.17 | [57] |
| γ-tocopherol | mg/kg | 2.5–103.8 | 211 | 1100–2300 | 10 | 1DSG + 2DSG | PLSR | Rcv = 0.88 SEC = 5.34 | [55] |
| Total tocopherols | mg/kg | 110.8–278.8 | 97 | 800–2500 | 8 | FD + SD + MSC | PLSR | Rv2 = 0.44 RMSEV = 19.30 RPD = 1.17 | [57] |
| Total tocopherols | mg/kg | 64.2–1078.0 | 213 | 1100–2300 | 10 | 1DSG + 2DSG | PLSR | Rcv2 = 0.88 SEC = 57.15 | [55] |
| Linoleic acid | % | 0.00–15.68 | 104 | 978–2500 | 0.5 | MSC | PLSR | Rv2 = 0.88 RMSEV = 0.83 RPD = 2.81 | [62] |
| Linoleic acid | % | 0.00–15.68 | 104 | 1100–2500 | 0.2 | MSC | PLSR | Rv2 = 0.90 RMSEV = 0.83 RPD = 2.81 | [62] |
| Linoleic acid | % | 4.39–24.83 | 73 | 833–2500 | 8 | SD | PLSR | Rv2 = 0.99 RMSEV = 0.23 RPD = 16.00 | [70] |
| Linoleic acid | % | 3.00–22.00 | 82 | 772–2222 | 8 | SNV | PLSR | Rv2 = 0.99 RMSEV = 0.46 RPD = 8.80 | [71] |
| Linoleic acid | % | 3.31–41.90 | 25 | 900–1700 | 8 | SNV + SG | PLSR | Rv2 = 0.92 RMSEV = 0.57 | [69] |
| Linoleic acid | % | 3.31–41.90 | 25 | 1350–2150 | 8 | SNV + SG | PLSR | Rv2 = 0.72 RMSEV = 0.76 | [69] |
| Linolenic acid | % | 0.44–1.79 | 73 | 833–2500 | 8 | FD + SNV | PLSR | Rv2 = 0.85 RMSEV = 0.08 RPD = 2.80 | [70] |
| Oleic acid | % | 45.07–80.57 | 25 | 900–1700 | 8 | SNV + SG | PLSR | Rv2 = 0.86 RMSEV = 1.46 | [69] |
| Oleic acid | % | 45.07–80.57 | 25 | 1350–2150 | 8 | SNV + SG | PLSR | Rv2 = 0.58 RMSEV = 2.35 | [69] |
| Oleic acid | % | 58.90–77.90 | 104 | 978–2500 | 0.5 | MSC | PLSR | Rv2 = 0.56 RMSEV = 1.47 RPD = 1.50 | [62] |
| Oleic acid | % | 58.90–77.90 | 104 | 1100–2500 | 0.2 | MSC | PLSR | Rv2 = 0.53 RMSEV = 1.53 RPD = 1.44 | [62] |
| Oleic acid | % | 49.14–79.69 | 73 | 833–2500 | 8 | FD + SLS | PLSR | Rv2 = 0.99 RMSEV = 0.28 RPD = 17.60 | [70] |
| Oleic acid | % | 56.00–80.00 | 82 | 772–2222 | 8 | SNV | PLSR | Rv2 = 0.96 RMSEV = 1.03 RPD = 4.70 | [71] |
| Cholesterol | % | 0.00–1.35 | 73 | 833–2500 | 8 | COE | PLSR | Rv2 = 0.42 RMSEV = 0.14 RPD = 1.32 | [70] |
| Campesterol | % | 13.2–3.99 | 73 | 833–2500 | 8 | MSC | PLSR | Rv2 = 0.17 RMSEV = 0.57 RPD = 1.14 | [70] |
| Stigmasterol | % | 0.17–1.88 | 73 | 833–2500 | 8 | SLS | PLSR | Rv2 = 0.22 RMSEV = 0.35 RPD = 1.14 | [70] |
| β-sitosterol | % | 45.94–89.66 | 73 | 833–2500 | 8 | FD + MSC | PLSR | Rv2 = 0.40 RMSEV = 6.41 RPD = 1.30 | [70] |
| Δ5-avenasterol | % | 3.28–17.98 | 73 | 833–2500 | 8 | FD | PLSR | Rv2 = 0.27 RMSEV = 2.77 RPD = 1.17 | [70] |
| Total sterol | mg/kg | 687.9–3087.4 | 73 | 833–2500 | 8 | FD + MSC | PLSR | Rv2 = 0.84 RMSEV = 192.00 RPD = 2.64 | [70] |
| Chlorophylls | mg/kg | 0.082–25.23 | 97 | 978–2500 | 0.5 | MSC | PLSR | Rv2 = 0.31 RMSEV = 4.42 RPD = 1.20 | [62] |
| Chlorophylls | mg/kg | 0.082–25.23 | 97 | 1100–2500 | 0.2 | MSC | PLSR | Rv2 = 0.56 RMSEV = 3.58 RPD = 1.49 | [62] |
| Chlorophylls | mg/kg | 0.70–27.50 | 183 | 450–2500 | 1 | DT | PLSR | Rc2 = 0.99 RMSEV = 0.66 RPD = 7.70 | [68] |
| Chlorophylls | mg/kg | 1.40–88.10 | 255 | 1100–2500 | 5 | SG | PLSR | Rc2 = 0.56 | [67] |
| Chlorophylls | mg/kg | 1.40–88.10 | 255 | 350–2500 | 5 | SG | PLSR | Rc2 = 0.96 RMSEV = 3.50 RPD = 4.10 | [67] |
| Carotenoids | mg/kg | 0.12–13.13 | 96 | 978–2500 | 0.5 | MSC | PLSR | Rv2 = 0.52 RMSEV = 1.35 RPD = 1.44 | [62] |
| Carotenoids | mg/kg | 0.12–13.13 | 96 | 1100–2500 | 0.2 | MSC | PLSR | Rv2 = 0.66 RMSEV = 1.14 RPD = 1.71 | [62] |
| Carotenoids | mg/kg | 1.60–18.10 | 183 | 450–2500 | 1 | DT | PLSR | Rc2 = 0.99 RMSEV = 0.96 RPD = 5.20 | [68] |
| Carotenoids | mg/kg | 2.10–38.50 | 255 | 1100–2500 | 5 | SG | PLSR | Rc2 = 0.62 | [67] |
| Carotenoids | mg/kg | 2.10–38.50 | 255 | 350–2500 | 5 | SG | PLSR | Rc2 = 0.95 RMSEV = 1.80 RPD = 3.90 | [67] |
| Squalene | g/kg | 1.00–10.10 | 177 | 1100–2300 | – | MN + SNV + 1DSG + 2DSG | PLSR | Rc2 = 0.86 RMSEV = 1.20 RPD = 2.30 | [73] |
| Squalene | g/kg | 1.00–10.10 | 177 | 350–2500 | 10 | MN + SNV + 1DSG + 2DSG | PLSR | Rc2 = 0.76 RMSEV = 1.00 RPD = 1.90 | [73] |
| Analytes | Raman Shift (cm−1) | Associated Chemical Bond/Structure | Reference |
|---|---|---|---|
| Hydroxytyrosol | 780 | O–H bending | [108] |
| Carotenoids | 1004 | C–CH3 bending | [109] |
| Carotenoids | 1150, 1525 | C=C stretching, C–H bending | [110] |
| Carotenoids | 1156 | C–C stretching | [111] |
| TPC | 1237 | C–O stretching | [110] |
| Oleic and linoleic acid | 1270 | In-phase C–H bending | [112] |
| Oleic and linoleic acid | 1306 | –CH2 torsional bending | [112] |
| Oleic acid | 1350 | C–H bending | [110] |
| Oleic acid | 1442, 1655 | C=C stretching | [113] |
| Carotenoids | 1523 | C=C stretching | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Sánchez-Villasclaras, S.; Pan, L.; Lan, W.; García-Martín, J.F. Advances in Vibrational Spectroscopic Techniques for the Detection of Bio-Active Compounds in Virgin Olive Oils: A Comprehensive Review. Foods 2024, 13, 3894. https://doi.org/10.3390/foods13233894
Ding F, Sánchez-Villasclaras S, Pan L, Lan W, García-Martín JF. Advances in Vibrational Spectroscopic Techniques for the Detection of Bio-Active Compounds in Virgin Olive Oils: A Comprehensive Review. Foods. 2024; 13(23):3894. https://doi.org/10.3390/foods13233894
Chicago/Turabian StyleDing, Fangchen, Sebastián Sánchez-Villasclaras, Leiqing Pan, Weijie Lan, and Juan Francisco García-Martín. 2024. "Advances in Vibrational Spectroscopic Techniques for the Detection of Bio-Active Compounds in Virgin Olive Oils: A Comprehensive Review" Foods 13, no. 23: 3894. https://doi.org/10.3390/foods13233894
APA StyleDing, F., Sánchez-Villasclaras, S., Pan, L., Lan, W., & García-Martín, J. F. (2024). Advances in Vibrational Spectroscopic Techniques for the Detection of Bio-Active Compounds in Virgin Olive Oils: A Comprehensive Review. Foods, 13(23), 3894. https://doi.org/10.3390/foods13233894










