Amino Acid Composition of Dried Bovine Dairy Powders from a Range of Product Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Powder Samples Selection
2.2. Total Protein Analysis
2.3. Amino Acid Analysis
2.4. Statistical Analyses
3. Results
3.1. Variability of WPH
3.2. Comparison of WMP and SMP
3.3. Comparison of WPC-C and WPC-L
3.4. Comparison of WPI and WPC-HF
3.5. Comparison of D90 and WPC-C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teodoro, M. The Future of Nutrition: Health and Wellness: 2023 [Industry Report]; Mintel: London, UK, 2003. [Google Scholar]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.; Layman, D. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A comprehensive review on infant formula: Nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S. Quantification of milk fat globule membrane proteins using selected reaction monitoring mass spectrometry. J. Agric. Food Chem. 2009, 57, 6021–6028. [Google Scholar] [CrossRef]
- Elgar, D.F.; Norris, C.S.; Ayers, J.S.; Pritchard, M.; Otter, D.E.; Palmano, K.P. Simultaneous separation and quantitation of the major bovine whey proteins including proteose peptone and caseinomacropeptide by reversed-phase high-performance liquid chromatography on polystyrene–divinylbenzene. J. Chromatog. A 2000, 878, 183–196. [Google Scholar] [CrossRef]
- Pihlanto, A.; Korhonen, H. Bioactive peptides and proteins. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Elsevier: Oxford, UK, 2003; Volume 47, pp. 175–276. [Google Scholar] [CrossRef]
- Tontisirin, K. Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop, Rome, 3–6 December 2002 (Publication No); Food and Agriculture Organisation of the United Nations: Rome, Italy, 2003; ISSN 0254-4725. [Google Scholar]
- Codex Stan 72-1981 (Rev. 2007); Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. Codex Alimentarius Commission: Rome, Italy, 2007; pp. 1–27.
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food. Nutr. Res. 2022, 66, 8242. [Google Scholar] [CrossRef]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- and animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, S.M.; Stein, H.H.; de Vries, S.; Hendriks, W.H.; Moughan, P.J. Determination of true ileal amino acid digestibility in the growing pig for calculation of digestible indispensable amino acid score (DIAAS). J. Nutr. 2020, 150, 2621–2623. [Google Scholar] [CrossRef] [PubMed]
- ISO 21543:2020|IDF 201:2020; Milk Products-Guidelines for the Application of NIR Spectrometry. International Standardization Organization: Geneva, Switzerland, 2001.
- ISO 8968-1:2014|IDF 20-1:2014; Milk and Milk Products–Determination of Nitrogen Content. Part 1: Kjeldahl Principle and Crude Protein Calculation. International Standardization Organization: Geneva, Switzerland, 2014.
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official method 994.12-1997. Amino acids in feeds. Performic acid oxidation. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC International. Official method. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Berry, S.; Sheehy, P.; Williamson, P.; Sharp, J.; Menzies, K.; Lefèvre, C.; Snell, R. Defining the origin and function of bovine milk proteins through genomics: The biological implications of manipulation and modification. In Milk Proteins: From Expression to Food, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: London, UK, 2020; pp. 143–171. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A.; Shah, N.P. Dairy Processing and Quality Assurance; John Wiley & Sons Ltd.: London, UK, 2015; pp. 333–366. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K. Protein and lipid composition of bovine milk-fat-globule membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Kanno, C.; Kim, D.H. A simple procedure for the preparation of bovine milk fat globule membrane and a comparison of its composition, enzymatic activities, and electrophoretic properties with those prepared by other methods. Agric. Biol. Chem. 1990, 54, 2845–2854. [Google Scholar] [CrossRef]
- Mangino, M.E.; Brunner, J.R. Compositional Homology of Membrance-Protein Systems and Membrance-Associated Proteins: Comparaison with Milk Fat Globule Membrance and “Membrance”-Derived Xanthine Oxidease. J. Dairy Sci. 1977, 60, 1208–1216. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Rollema, H.S.; Bot, A.; De Kruif, C.G. Gelation mechanism of milk as influenced by temperature and pH; studied by the use of transglutaminase cross-linked casein micelles. J. Dairy Sci. 2003, 86, 1556–1563. [Google Scholar] [CrossRef]
- Neelima; Sharm, R.; Rajpu, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Tech. 2013, 93, 21–43. [Google Scholar] [CrossRef]
- LaClair, C.E.; Ney, D.M.; MacLeod, E.L.; Etzel, M.R. Purification and use of glycomacropeptide for nutritional management of phenylketonuria. J. Food Sci. 2009, 74, E199–E206. [Google Scholar] [CrossRef]
- Monirujjaman, M.D.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Peyrollier, K.; Hajduch, E.; Blair, A.S.; Hyde, R.; Hundal, H.S. l-Leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: Evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the l-leucine-induced up-regulation of system amino acid transport. Biochem. J. 2000, 350, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.J. Thermal separation of β-lactoglobulin and α-lactalbumin in bovine Cheddar cheese whey. Aus. J. Dairy Tech. 1983, 38, 144–148. [Google Scholar]
- Ayers, J.S.; Petersen, M.J. Whey protein recovery using a range of novel ion exchangers. NZ J. Dairy Sci. Tech. 1985, 20, 129–142. [Google Scholar]
- Kader, H.A.; Gill, H.; Truong, T. Demineralisation and recovery of whey proteins from commercial full-fat salty Cheddar whey using size-exclusion chromatography. Food Chem. 2023, 405, 134831. [Google Scholar] [CrossRef]
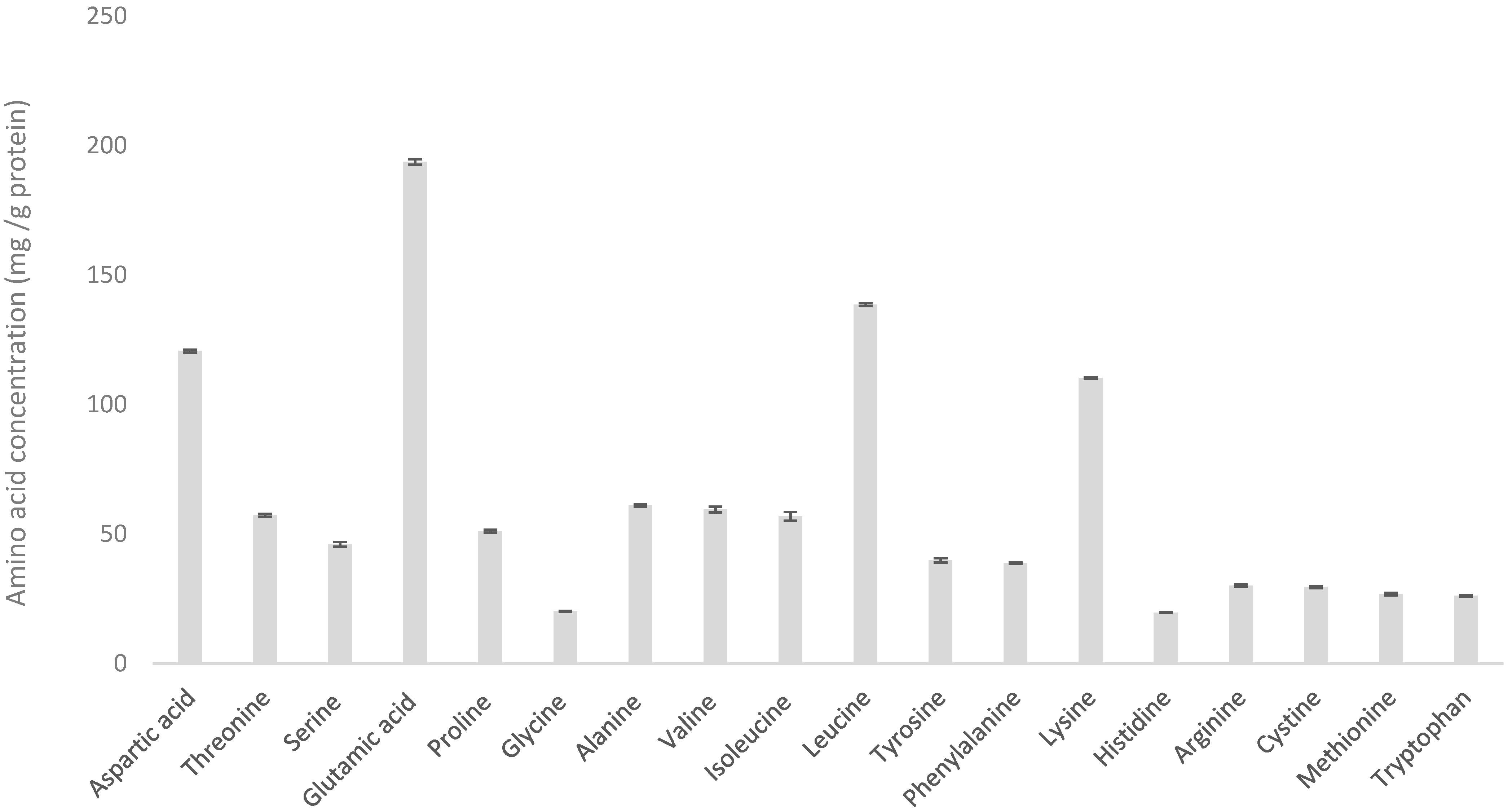
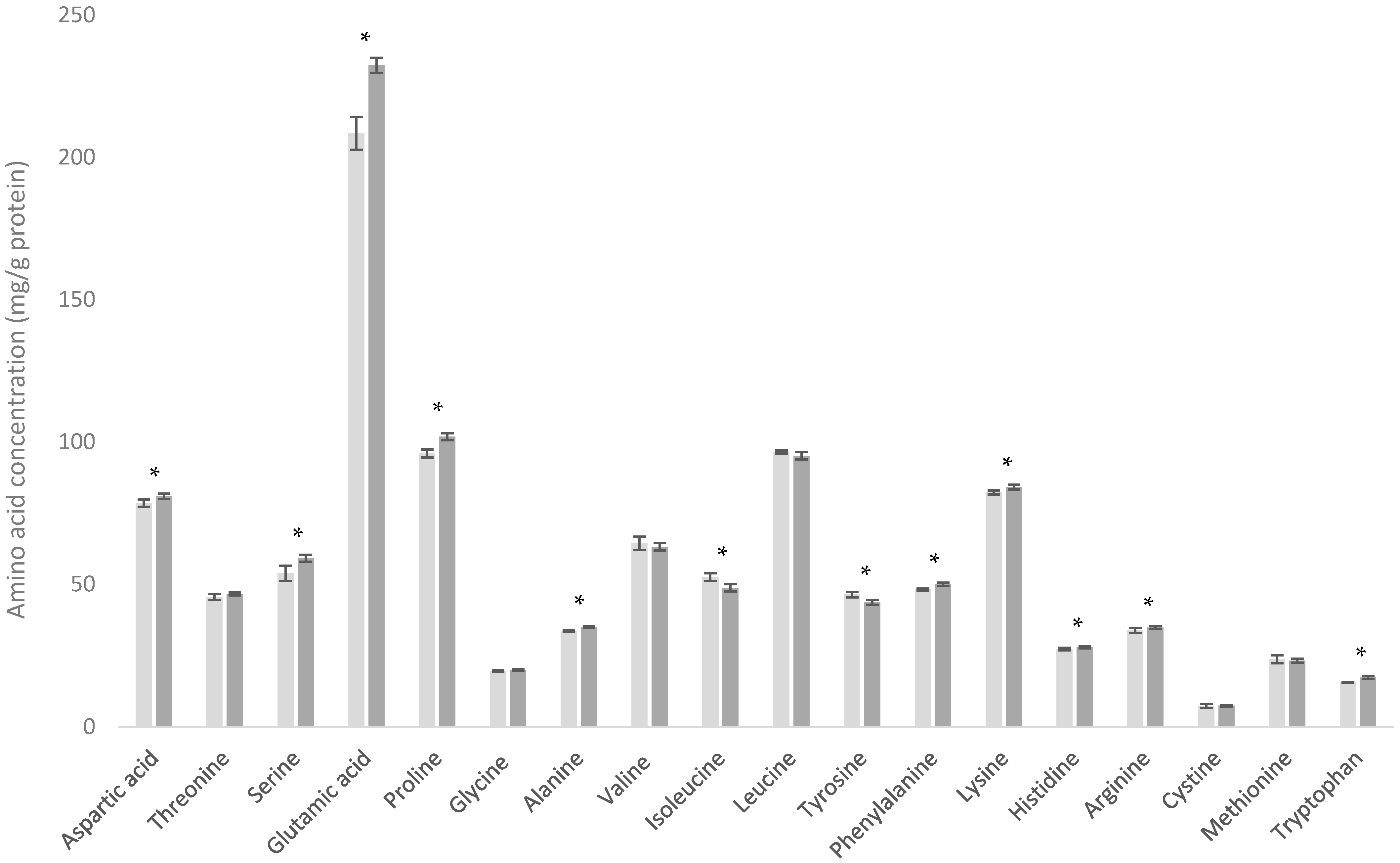

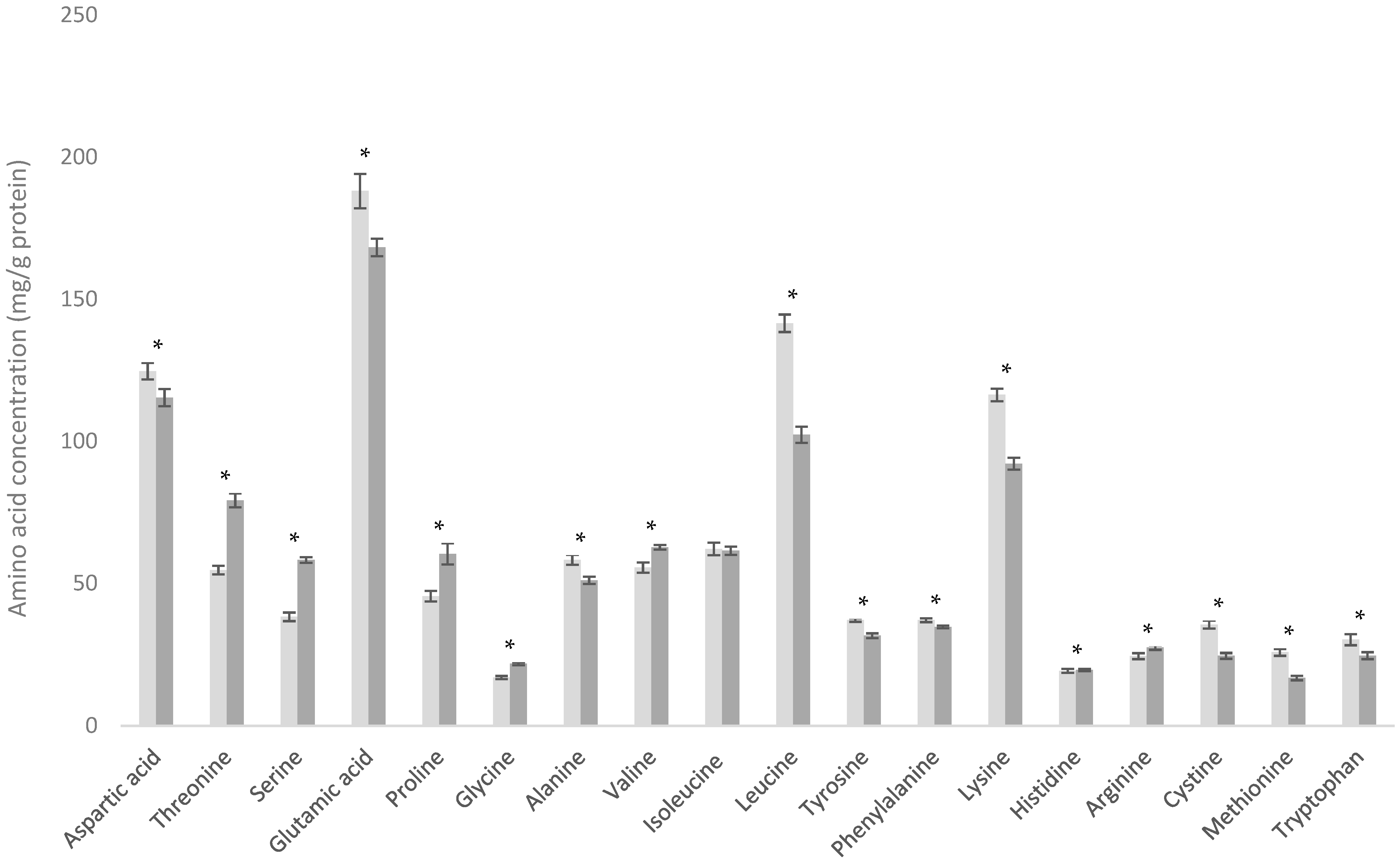

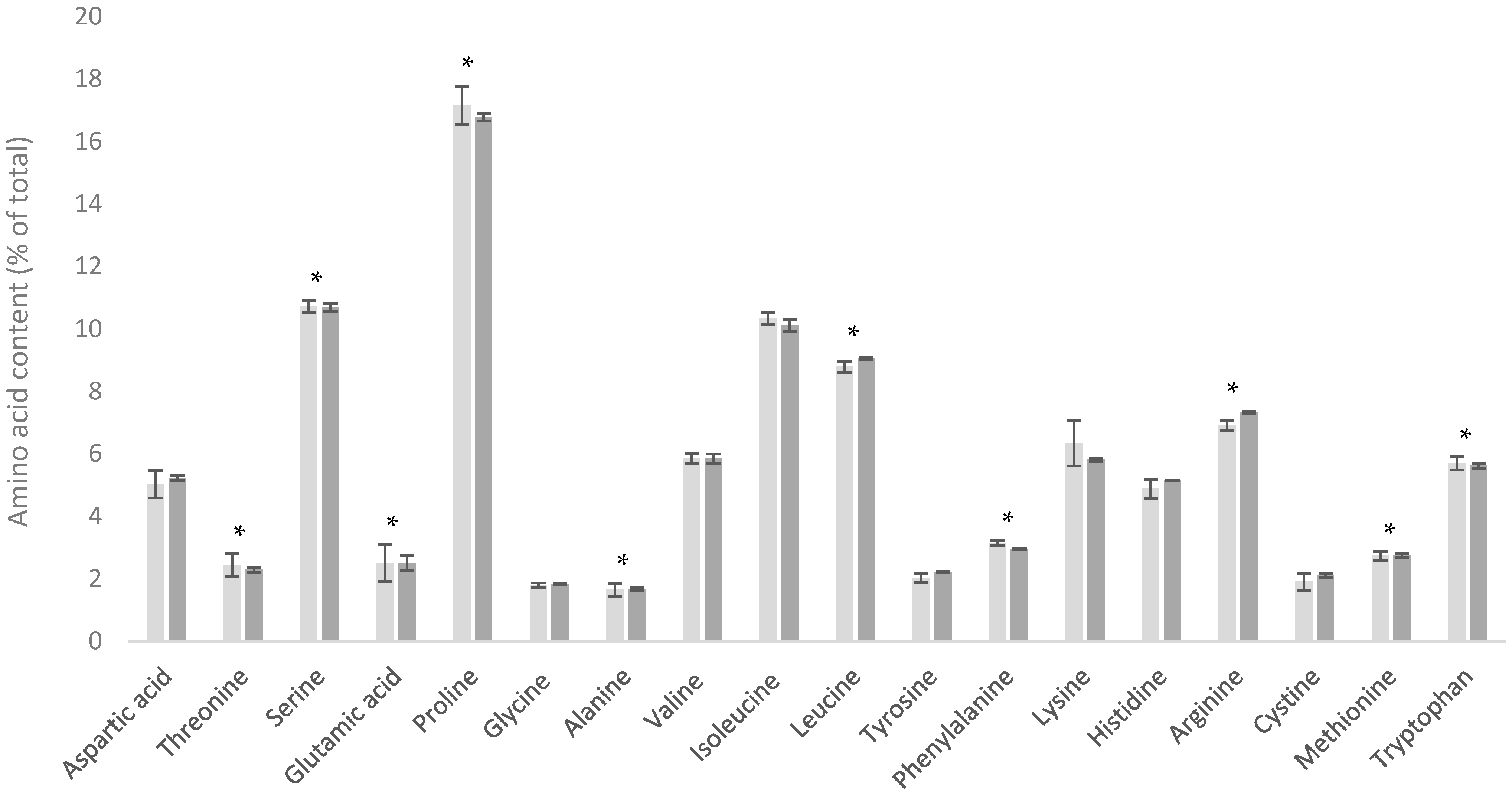
| Amino Acid | Dairy Powder Amino Acid Content (mg/g Protein) | |||||||
|---|---|---|---|---|---|---|---|---|
| WMP | SMP | WPC-C | WPC-L | WPI | WPC-HF | D90 | WPH | |
| Alanine | 33.6 ± 0.3 | 35.0 ± 0.4 | 58.9 ± 0.9 | 57.8 ± 1.1 | 58.3 ± 1.7 | 51.1 ± 1.25 | 50.7 ± 2.7 | 61.1 ± 0.5 |
| Arginine | 33.8 ± 0.9 | 34.8 ± 0.5 | 25.9 ± 0.7 | 28.7 ± 0.8 | 24.5 ± 1.0 | 27.4 ± 0.8 | 23.4 ± 1.3 | 30.1 ± 0.4 |
| Aspartic acid | 78.5 ± 1.3 | 80.9 ± 0.9 | 120.6 ± 1.7 | 121.4 ± 1.7 | 124.7 ± 2.9 | 115.4 ± 3.0 | 104.0 ± 3.4 | 120.7 ± 0.5 |
| Cystine | 7.3 ± 0.7 | 7.4 ± 0.3 | 28.3 ± 0.6 | 28.4 ± 0.7 | 35.5 ± 1.4 | 24.6 ± 1.0 | 23.4 ± 0.5 | 29.5 ± 0.4 |
| Glutamic acid | 208.5± 5.8 | 232.4 ± 2.7 | 189.2 ± 2.4 | 177.5 ± 3.3 | 188.1 ± 6.1 | 168.2 ± 3.1 | 166.6 ± 5.7 | 193.7 ± 1.1 |
| Glycine | 19.7 ± 0.4 | 20.0 ± 0.3 | 20.6 ± 0.3 | 20.7 ± 0.4 | 17.0 ± 0.6 | 21.8 ± 0.5 | 18.1 ± 0.8 | 20.2 ± 0.2 |
| Histidine | 27.2 ± 0.5 | 27.9 ± 0.4 | 19.0 ± 0.4 | 20.6 ± 0.4 | 19.3 ± 0.7 | 19.7 ± 0.4 | 18.0 ± 0.9 | 19.6 ± 0.1 |
| Isoleucine | 52.6 ± 1.3 | 48.8 ± 1.3 | 66.0 ± 2.7 | 51.3 ± 2.5 | 62.1 ± 2.2 | 61.5 ± 1.4 | 58.5 ± 2.5 | 56.9 ± 1.7 |
| Leucine | 96.5 ± 0.6 | 95.1 ± 1.3 | 114.1 ± 1.5 | 126.7 ± 2.4 | 141.5 ± 3.1 | 102.3 ± 2.9 | 103.0 ± 3.7 | 138.5 ± 0.6 |
| Lysine | 82.3 ± 0.7 | 84.2 ± 0.8 | 102.1 ± 1.5 | 106.4 ± 1.7 | 116.4 ± 2.2 | 92.1 ± 2.1 | 90.8 ± 3.4 | 110.3 ± 0.3 |
| Methionine | 23.8 ± 1.4 | 23.3 ± 0.7 | 25.0 ± 0.6 | 23.2 ± 0.5 | 25.8 ± 1.1 | 16.8 ± 0.8 | 20.7 ± 0.6 | 26.8 ± 0.4 |
| Phenylalanine | 48.1 ± 0.4 | 50.0 ± 0.6 | 33.5 ± 0.5 | 37.2 ± 0.9 | 37.1 ± 0.7 | 34.7 ± 0.5 | 31.4 ± 1.3 | 38.8 ± 0.2 |
| Proline | 96.0 ± 1.5 | 101.9 ± 1.2 | 65.5 ± 1.5 | 47.2 ± 1.1 | 45.5 ± 1.8 | 60.4 ± 3.7 | 59.5 ± 2.9 | 51.1 ± 0.6 |
| Serine | 53.9 ± 2.6 | 59.1 ± 1.2 | 58.1 ± 1.5 | 48.7 ± 0.9 | 38.3 ± 1.5 | 58.2 ± 1.0 | 48.4 ± 2.4 | 46.0 ± 0.9 |
| Threonine | 45.5 ± 1.0 | 46.6 ± 0.5 | 82.7 ± 1.5 | 59.8 ± 1.1 | 54.7 ± 1.5 | 79.2 ± 2.4 | 70.3 ± 2.8 | 57.3 ± 0.6 |
| Tryptophan | 15.6 ± 0.2 | 17.4 ± 0.5 | 23.8 ± 0.9 | 28.6 ± 0.6 | 30.2 ± 1.9 | 24.6 ± 1.1 | 20.1 ± 0.9 | 26.2 ± 0.3 |
| Tyrosine | 46.4 ± 1.0 | 43.7 ± 0.8 | 31.1 ± 0.6 | 34.8 ± 0.9 | 37.1 ± 0.7 | 31.6 ± 0.8 | 24.0 ± 1.3 | 39.9 ± 0.8 |
| Valine | 64.4 ± 2.4 | 63.2 ± 1.4 | 63.4 ± 2.3 | 51.9 ± 2.7 | 55.5 ± 1.8 | 62.7 ± 0.9 | 57.1 ± 2.1 | 59.5 ± 1.2 |
| Total | 1033.6 | 1071.8 | 1127.5 | 1070.6 | 1111.4 | 1052.3 | 988.0 | 1126.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilmour, S.R.; Holroyd, S.E.; Fuad, M.D.; Elgar, D.; Fanning, A.C. Amino Acid Composition of Dried Bovine Dairy Powders from a Range of Product Streams. Foods 2024, 13, 3901. https://doi.org/10.3390/foods13233901
Gilmour SR, Holroyd SE, Fuad MD, Elgar D, Fanning AC. Amino Acid Composition of Dried Bovine Dairy Powders from a Range of Product Streams. Foods. 2024; 13(23):3901. https://doi.org/10.3390/foods13233901
Chicago/Turabian StyleGilmour, Simon R., Stephen E. Holroyd, Maher D. Fuad, Dave Elgar, and Aaron C. Fanning. 2024. "Amino Acid Composition of Dried Bovine Dairy Powders from a Range of Product Streams" Foods 13, no. 23: 3901. https://doi.org/10.3390/foods13233901
APA StyleGilmour, S. R., Holroyd, S. E., Fuad, M. D., Elgar, D., & Fanning, A. C. (2024). Amino Acid Composition of Dried Bovine Dairy Powders from a Range of Product Streams. Foods, 13(23), 3901. https://doi.org/10.3390/foods13233901






