Characterizations of Pectin from Choerospondias axillaris Fruit Pulp: Comparison of Different Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pectin Pretreatment
2.2.1. Hot Water Extraction
2.2.2. Hydrochloric Acid Extraction
2.2.3. Ultrahigh Pressure Extraction
2.2.4. Ultrasonic Extraction
2.3. Physicochemical Properties of Pectin
2.3.1. Determination of Pectin Yield
2.3.2. Determination of Galacturonic Acid Content
2.3.3. Determination of Esterification Degree
2.3.4. Determination of Monosaccharide Composition
2.3.5. Determination of Mw
2.4. Structural Properties
2.4.1. Fourier Transform Infrared (FTIR) Spectra
2.4.2. X-Ray Diffraction
2.4.3. SEM
2.5. Thermogravimetric (TG) and Derivative Thermogravimetric (DTG)
2.6. Properties of Emulsion
2.6.1. Preparation of Pectin Emulsion
2.6.2. Measurement of Emulsifying Activity (EA) and Emulsion Stability (ES)
2.6.3. Droplet Size
2.6.4. Zeta Potential
2.7. Biological Activities
2.7.1. Antioxidant Activities
ABTS Radical Scavenging Activity
Ferric-Reducing Antioxidant Power (FRAP) Assay
2.8. Statistical Analysis
3. Results and Discussion
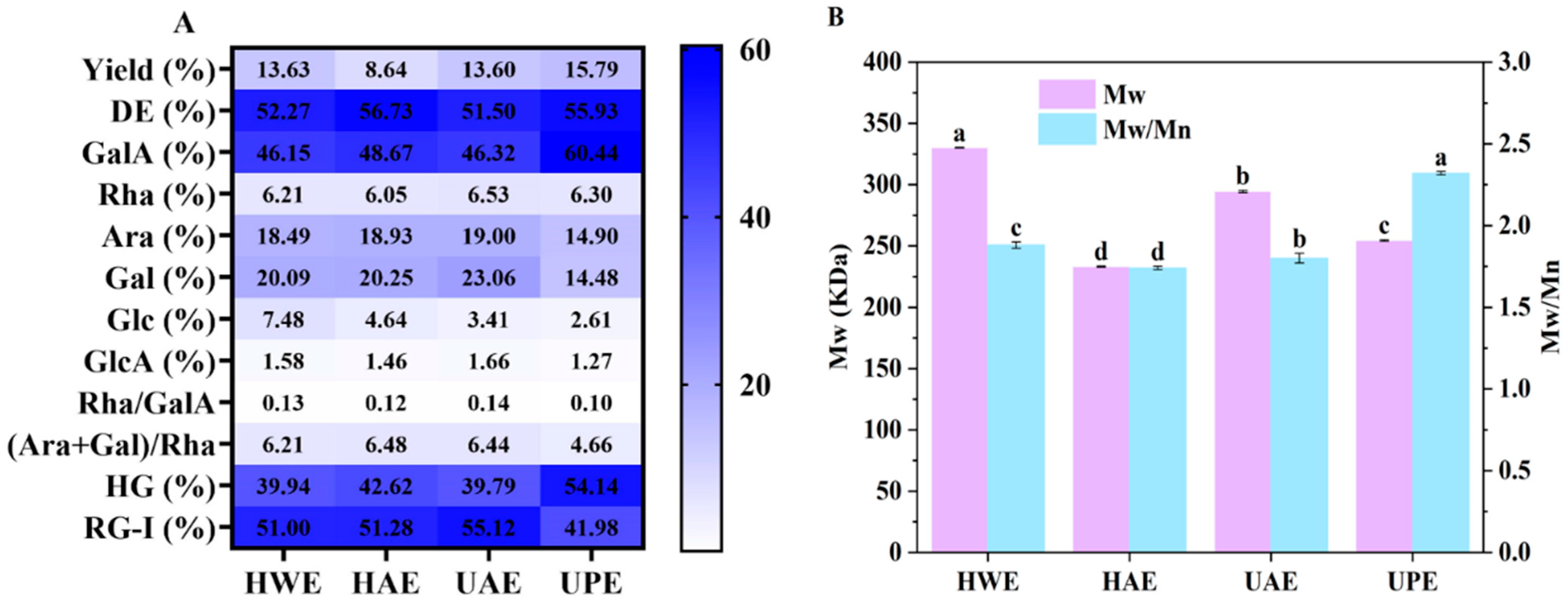
3.1. Physicochemical Characteristics
3.2. Structural Characteristics
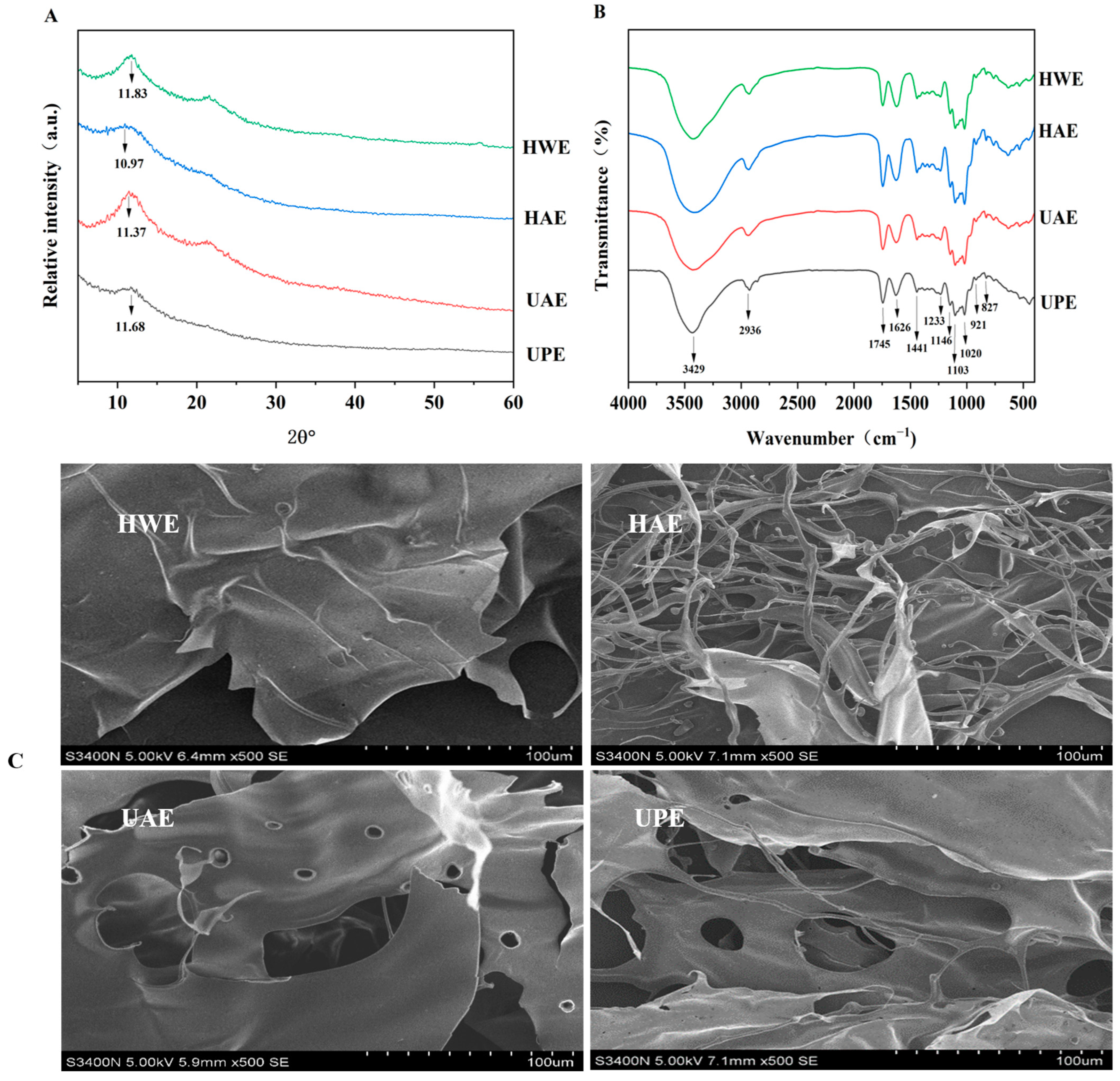
3.2.1. XRD
3.2.2. FTIR
3.2.3. Microstructure
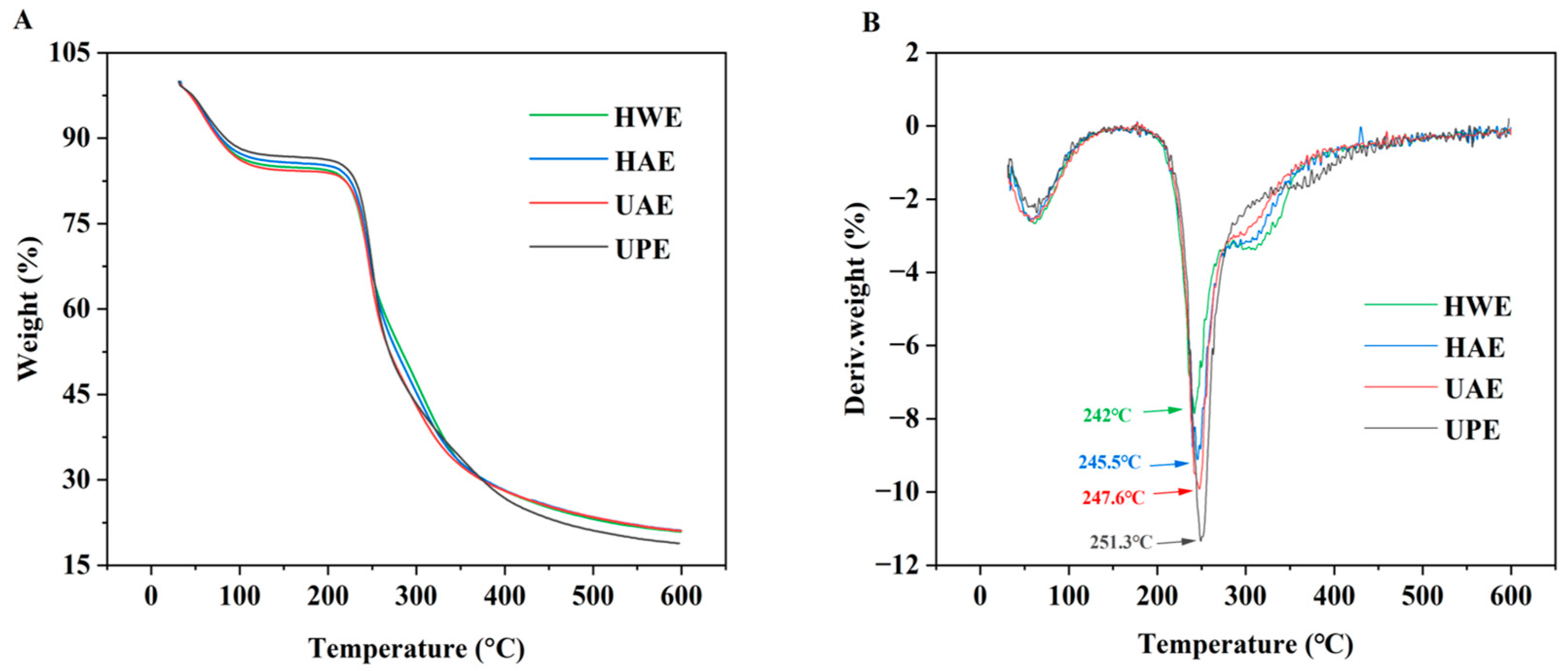
3.3. Thermal Stability
3.4. Emulsifying Properties
3.5. Antioxidant Capacity
3.6. Prebiotic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, S.; Chakraborty, D.; Biswas, S. An alternative perspective of an underutilized fruit tree Choerospondias axillaris in health promotion and disease prevention: A review. Food Biosci. 2022, 47, 101609. [Google Scholar] [CrossRef]
- Dangal, A.; Timsina, P.; Dahal, S. A comprehensive review on study of physical, nutritional, and phytochemical characteristics as well as therapeutic activities of Choerospondias axillaris (Lapsi). Food Biosci. 2023, 53, 102713. [Google Scholar] [CrossRef]
- Rong, W.; Shi, Q.; Yang, Y.; Su, W.; Li, M.; Qin, M.; Bai, S.; Zhu, Q.; Wang, A. Fructus choerospondiatis: A comprehensive review of its traditional Uses, chemical composition, pharmacological activities, and clinical studies. J. Ethnopharmacol. 2024, 323, 117696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, J.; Zhao, S.; Liu, D.; Zhao, C.; Zheng, J. The structure-function relationships of pectins separated from three citrus parts: Flavedo, albedo, and pomace. Food Hydrocoll. 2023, 136, 108308. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, K.; Descallar, F.B.A.; Li, A.; Yang, X.; Yang, H. Gelation Behaviors of some special plant-sourced pectins: A review inspired by examples from traditional gel foods in China. Trends Food Sci. Technol. 2022, 126, 26–40. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Li, Z.; Xi, J.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Zhang, M. Effect of glycosylation with apple pectin, citrus pectin, mango pectin and sugar beet pectin on the physicochemical, interfacial and emulsifying properties of coconut protein Isolate. Food Res. Int. 2022, 156, 111363. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.A.; Fabra, M.J.; Martínez-Abad, A.; Μartínez-Sanz, Μ.; Gorria, M.; López-Rubio, A. Understanding the different emulsification mechanisms of pectin: Comparison between watermelon rind and two commercial pectin sources. Food Hydrocoll. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the behavior of three different sources of pectin at the oil-water interface and the stability of the emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; Julian McClements, D.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Lu, J.; Bu, Z.; Yu, Y.; Wu, J.; Yang, W.; Xu, Y.; Peng, J. Influence of citric acid and hydrochloric acid with High-pressure processing on characteristics of pectic polysaccharide from Choerospondias axillaris Fruit Peel. Food Bioprocess. Technol. 2023, 16, 1235–1245. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Liu, W.; Liu, J. Comparison of bioactivities and phenolic composition of Choerospondias axillaris peels and fleshes. J. Sci. Food Agric. 2016, 96, 2462–2471. [Google Scholar] [CrossRef]
- Zhong, L.; Li, X.; Duan, M.; Song, Y.; He, N.; Che, L. Impacts of high hydrostatic pressure processing on the structure and properties of pectin. LWT 2021, 148, 111793. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Dorado, C.; Bai, J.; Chau, H.K.; Hotchkiss, A.T.; Yadav, M.P.; Cameron, R.G. Modification of pectin with high-pressure processing treatment of fresh orange peel before pectin extraction: Part II. The effects on gelling capacity and emulsifying properties of pectin. Food Hydrocoll. 2024, 149, 109536. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Physicochemical and macromolecule properties of RG-I enriched pectin from citrus wastes by manosonication extraction. Int. J. Biol. Macromol. 2021, 176, 332–341. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Ultrasound-assisted extraction of pectin from Citrus Limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Biosci. 2023, 51, 102231. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Pinheiro, E.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Li, T.; Liu, Y.; Zhang, Z.; Zhang, Y. Purification, Characterization and in vitro antioxidant activity of a polysaccharide AAP–3–1 from Auricularia auricula. Int. J. Biol. Macromol. 2020, 162, 1453–1464. [Google Scholar] [CrossRef]
- Deng, Z.; Pan, Y.; Chen, W.; Chen, W.; Yun, Y.; Zhong, Q.; Zhang, W.; Chen, H. Effects of cultivar and growth region on the structural, emulsifying and rheological characteristic of mango peel pectin. Food Hydrocoll. 2020, 103, 105707. [Google Scholar] [CrossRef]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef]
- Peng, J.; Bu, Z.; Ren, H.; He, Q.; Yu, Y.; Xu, Y.; Wu, J.; Cheng, L.; Li, L. Physicochemical, structural, and functional properties of wampee (Clausena lansium (Lour.) Skeels) fruit peel pectin extracted with different organic acids. Food Chem. 2022, 386, 132834. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, X.; Yang, B.; Yu, Q.; Wei, X.; Ding, Y.; Kan, J. New insight into bamboo shoot (Chimonobambusa quadrangularis) polysaccharides: Impact of extraction processes on Its prebiotic activity. Food Hydrocoll. 2019, 95, 367–377. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y.; Lian, Y.; Zheng, J. The Structure–property relationships of acid- and alkali-extracted grapefruit peel pectins. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, L.; Li, S.; Meng, T.; Wang, L.; Zhang, W. The effect of sonication-synergistic natural deep eutectic solvents on extraction yield, structural and physicochemical properties of pectins extracted from mango peels. Ultrason. Sonochem. 2022, 86, 106045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cai, J. Preparation of branched RG-I-Rich pectin from red dragon fruit peel and the characterization of its probiotic properties. Carbohydr. Polym. 2023, 299, 120144. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Zhu, C.; Wei, M. Characterization of edible film fabricated with HG-type hawthorn pectin gained using different extraction methods. Carbohydr. Polym. 2022, 285, 119270. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, J.; Chi, J.; Zhang, M.; Zhang, R.; Jia, X.; Mei, D.; Dong, L.; Yi, Y.; Huang, F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int. J. Biol. Macromol. 2022, 223, 663–672. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Guo, X.; Guo, X.; Meng, H.; Chen, X.; Zeng, Q.; Yu, S. Influences of different pectins on the emulsifying performance of conjugates formed between pectin and whey protein isolate. Int. J. Biol. Macromol. 2019, 123, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cheng, H.; Wu, D.; Chen, J.; Ye, X.; Chen, S. Enhanced extraction assisted by pressure and ultrasound for targeting RG-I enriched pectin from citrus peel wastes: A mechanistic study. Food Hydrocoll. 2022, 133, 107778. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Pan, M.-K.; Zhou, F.-F.; Liu, Y.; Wang, J.-H. Na+-induced gelation of a low-methoxyl pectin extracted from Premna microphylla Turcz. Food Hydrocoll. 2021, 110, 106153. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Chen, J.; Du, X.; Lu, Z.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; et al. Systematic evaluation of a series of pectic polysaccharides extracted from apple pomace by regulation of subcritical water conditions. Food Chem. 2022, 368, 130833. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, W.; Wang, Y.; Xu, Y.; Zhang, W.; Lao, F.; Liao, X.; Wu, J. Physicochemical and Structural Properties of Three Pectin Fractions from Muskmelon (Cucumis melo) and Their Correlation with Juice Cloud Stability. Food Hydrocoll. 2022, 124, 107313. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, Y.; Wang, C.; Tian, Z. Pyrolysis behavior of pectin under the conditions that simulate cigarette smoking. J. Anal. Appl. Pyrolysis 2011, 91, 232–240. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.-M.; Cheng, X.-C.; Wang, X.-D. Effect of drying pretreatment methods on structure and properties of pectins extracted from Chinese quince fruit. Int. J. Biol. Macromol. 2019, 137, 801–808. [Google Scholar] [CrossRef]
- Huang, Z.; Zong, M.-H.; Lou, W.-Y. Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa Champ. Food Hydrocoll. 2022, 124, 107217. [Google Scholar] [CrossRef]
- Han, H.; Luo, Y.; Bai, J.; Tao, Z.; Wang, S.; Lei, X.; Feng, Y.; Ren, Y. Electron beam irradiation pretreatment for efficient extraction of pectin from spaghetti squash peel: Structural, functional, and biological properties. Food Hydrocoll. 2024, 148, 109451. [Google Scholar] [CrossRef]
- Lin, J.; Lei, H.; Han, Z.; Zeng, X. Conformational flexibility and molecular weight influence the emulsification performance of pectin: A comparative study of sugar beet pectin and citrus pectin. Food Hydrocoll. 2024, 146, 109272. [Google Scholar] [CrossRef]
- Kavya, M.; Ranjit Jacob, A.; Nisha, P. Pectin emulsions and emulgels: Bridging the correlation between rheology and microstructure. Food Hydrocoll. 2023, 143, 108868. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Hernández-Ortega, C.; Welti-Chanes, J.; Putnik, P.; Barba, F.J.; Mallikarjunan, K.; Escobedo-Avellaneda, Z.; Roohinejad, S. High pressure processing of food-grade emulsion systems: Antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll. 2019, 87, 307–320. [Google Scholar] [CrossRef]
- Jia, Y.; Du, J.; Li, K.; Li, C. Emulsification mechanism of persimmon pectin with promising emulsification capability and stability. Food Hydrocoll. 2022, 131, 107727. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Deng, R.; Zheng, Y.; Liu, D.; Liu, J.; Zhang, M.; Xi, G.; Song, L.; Liu, P. The effect of ultrasonic power on the physicochemical properties and antioxidant activities of frosted figs pectin. Ultrason. Sonochem. 2024, 106, 106883. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Cahú, T.B.; Isay Saad, S.M.; Blennow, A.; Jespersen, L. The Effect of Pectins on Survival of Probiotic Lactobacillus Spp. in Gastrointestinal Juices Is Related to Their Structure and Physical Properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Lee, H.-B.; Son, S.-U.; Lee, J.-E.; Lee, S.-H.; Kang, C.-H.; Kim, Y.-S.; Shin, K.-S.; Park, H.-Y. Characterization, Prebiotic and Immune-Enhancing Activities of Rhamnogalacturonan-I-Rich Polysaccharide Fraction from Molokhia Leaves. Int. J. Biol. Macromol. 2021, 175, 443–450. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luyang, Z.; Bu, Z.; Wu, J.; Yu, Y.; Cheng, L.; Peng, J.; Xu, Y. Characterizations of Pectin from Choerospondias axillaris Fruit Pulp: Comparison of Different Extraction Methods. Foods 2024, 13, 3920. https://doi.org/10.3390/foods13233920
Luyang Z, Bu Z, Wu J, Yu Y, Cheng L, Peng J, Xu Y. Characterizations of Pectin from Choerospondias axillaris Fruit Pulp: Comparison of Different Extraction Methods. Foods. 2024; 13(23):3920. https://doi.org/10.3390/foods13233920
Chicago/Turabian StyleLuyang, Zian, Zhibin Bu, Jijun Wu, Yuanshan Yu, Lina Cheng, Jian Peng, and Yujuan Xu. 2024. "Characterizations of Pectin from Choerospondias axillaris Fruit Pulp: Comparison of Different Extraction Methods" Foods 13, no. 23: 3920. https://doi.org/10.3390/foods13233920
APA StyleLuyang, Z., Bu, Z., Wu, J., Yu, Y., Cheng, L., Peng, J., & Xu, Y. (2024). Characterizations of Pectin from Choerospondias axillaris Fruit Pulp: Comparison of Different Extraction Methods. Foods, 13(23), 3920. https://doi.org/10.3390/foods13233920





