Phenolic Characterization and Quality Evaluation of Herbal Coffee from Roasted Juniper Berry Fruits (Juniperus drupacea L.): Elucidating the Impact of Roasting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Juniper Berry Fruits
2.2. Chemicals
2.3. Roasting and Preparation of Herbal Coffee

2.4. Color and Dry Matter Analyses
2.5. Antioxidant Activity (AA) Analysis
2.6. Total Phenolic Content (TPC) Analysis
2.7. Hydroxymethylfurfural (HMF) Analysis
2.8. Sugar Content Analysis
2.9. Phenolic Profile Analysis
2.10. Sensory Analysis
2.11. Statistical Data Analysis
3. Results
3.1. Dry Matter (DM) and Color Analyses Results
| 120 °C | 160 °C | 200 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyses | Unroasted Fruits | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | T | Tp | T × Tp |
| DM % | 83.67 ± 0.18 a | 84.95 ± 0.24 b | 88.30 ± 0.14 de | 88.27 ± 0.04 de | 87.46 ± 0.38 c | 87.78 ± 0.04 cd | 88.96 ± 0.82 e | 91.65 ± 0.31 f | 94.32 ± 0.34 g | 88.40 ± 0.18 de | 91.77 ± 0.32 f | 94.87 ± 0.06 gh | 95.14 ± 0.29 h | ** | ** | ** |
| L* | 38.72 ± 0.02 e | 38.93 ± 0.16 e | 44.44 ± 0.33 i | 40.34 ± 0.02 fg | 38.09 ± 0.40 e | 40.98 ± 0.08 gh | 40.04 ± 0.37 f | 39.95 ± 0.33 f | 33.79 ± 0.03 c | 41.47 ± 0.09 h | 35.96 ± 1.18 d | 32.10 ± 0.05 b | 30.97 ± 0.05 a | ** | ** | ** |
| a* | 13.58 ± 0.01 f | 13.96 ± 0.55 fgh | 14.25 ± 0.32 ghi | 13.54 ± 0.02 f | 12.52 ± 0.13 e | 14.40 ± 0.02 hi | 14.15 ± 0.13 ghi | 13.84 ± 0.20 fg | 7.68 ± 0.04 c | 14.59 ± 0.03 i | 10.44 ± 0.14 d | 6.08 ± 0.02 b | 5.11 ± 0.14 a | ** | ** | ** |
| b* | 15.71 ± 0.01 f | 16.81 ± 1.61 gh | 18.84 ± 0.04 j | 15.49 ± 0.02 f | 14.12 ± 0.55 e | 18.09 ± 0.04 ij | 17.20 ± 0.09 hi | 16.07 ± 0.34 fg | 8.23 ± 0.01 c | 18.51 ± 0.08 j | 12.23 ± 0.35 d | 6.56 ± 0.07 b | 5.31 ± 0.22 a | ** | ** | ** |
| C* | 20.76 ± 0.01 fg | 21.88 ± 1.51 gh | 23.58 ± 0.11 i | 20.11 ± 0.67 f | 18.87 ± 0.50 e | 23.54 ± 0.66 i | 22.27 ± 0.15 h | 21.33 ± 0.40 gh | 11.26 ± 0.04 c | 23.57 ± 0.04 i | 16.08 ± 0.17 d | 8.94 ± 0.06 b | 7.39 ± 0.23 a | ** | ** | ** |
| h | 49.20 ± 0.06 cd | 49.09 ± 0.93 cd | 53.03 ± 0.49 f | 49.69 ± 1.19 cd | 48.43 ± 0.81 bc | 51.77 ± 0.33 ef | 50.57 ± 0.12 de | 48.27 ± 0.63 bc | 46.98 ± 0.10 ab | 51.75 ± 0.16 ef | 49.51 ± 1.21 cd | 47.19 ± 0.22 ab | 46.78 ± 0.56 a | ** | ** | ** |
| DPPH | 11.87 ± 0.05 d | 15.13 ± 0.38 f | 17.99 ± 0.03 h | 16.39 ± 0.02 g | 12.26 ± 0.07 d | 14.87 ± 0.06 f | 14.61 ± 0.14 f | 13.47 ± 1.13 e | 10.88 ± 0.05 c | 12.28 ± 0.19 d | 9.65 ± 0.07 b | 6.99 ± 0.02 a | 6.78 ± 0.04 a | ** | ** | ** |
| ABTS | 15.99 ± 0.03 cd | 20.89 ± 0.03 g | 29.36 ± 0.07 i | 21.19 ± 0.02 g | 17.76 ± 0.34 ef | 23.36 ± 0.07 h | 21.63 ± 0.20 g | 18.53 ± 1.54 f | 15.12 ± 0.04 c | 16.72 ± 0.23 de | 13.72 ± 0.41 b | 11.44 ± 0.02 a | 11.04 ± 0.29 a | ** | ** | ** |
| TPC | 1851.40 ± 1.41 d | 2351.70 ± 14.42 g | 2887.25 ± 1.06 i | 2494.00 ± 5.66 h | 1897.75 ± 46.03 de | 2139.50 ± 1.41 f | 2082.30 ± 6.08 f | 2004.40 ± 40.02 e | 1840.40 ± 2.40 cd | 1836.00 ± 25.03 cd | 1766.42 ± 8.37 bc | 1708.15 ± 7.71 b | 1543.05 ± 8.27 a | ** | ** | ** |
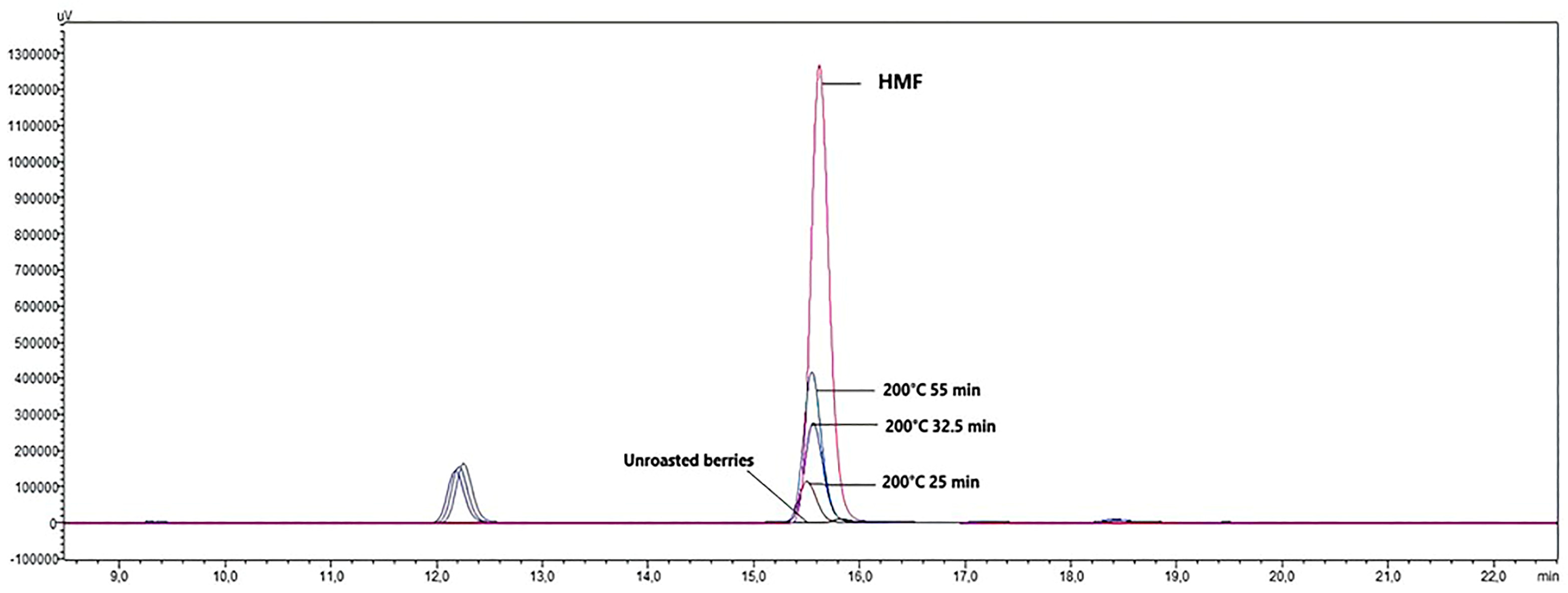
| HMF | nd | nd | nd | nd | nd | nd | nd | 0.01 ± 0.00 a | 0.13 ± 0.00 b | nd | 0.14 ± 0.01 c | 0.27 ± 0.00 d | 0.39 ± 0.01 e | ** | ** | ** |
3.2. Results of Antioxidant Activity (AA) and Total Phenolic Content (TPC) Analysis
3.3. Results of Hydroxymethylfurfural (HMF) Analysis
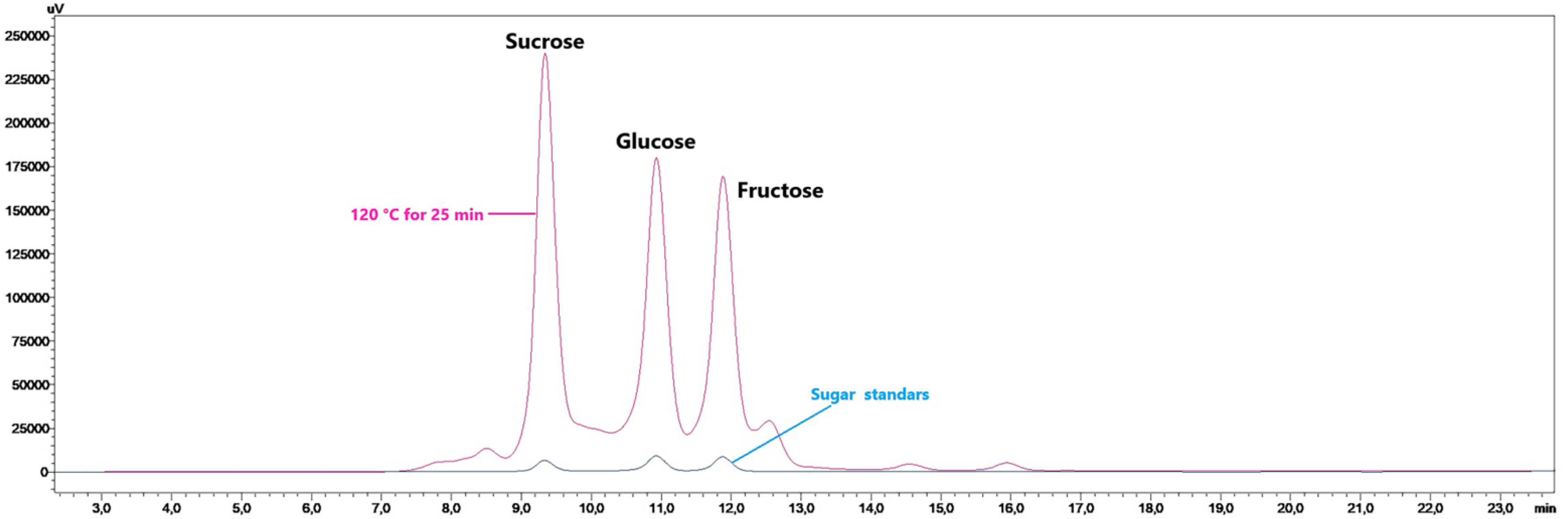
3.4. Results of Sugar Content Analysis
| 120 °C | 160 °C | 200 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugar Contents | Unroasted Fruits | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | T | Tp | T × Tp |
| Sucrose | 5.01 ± 0.05 b | 12.31 ± 0.05 d | 19.83 ± 0.13 h | 13.95 ± 0.11 e | 15.10 ± 1.24 fg | 12.78 ± 0.06 d | 14.61 ± 0.49 ef | 15.70 ± 0.64 g | 9.45 ± 0.18 c | 12.49 ± 0.39 d | 9.46 ± 0.27 c | 5.77 ± 0.09 b | 3.68 ± 0.05 a | ** | ** | ** |
| Glucose | 4.91 ± 0.03 a | 12.69 ± 0.30 d | 18.82 ± 0.22 g | 14.08 ± 0.13 ef | 14.99 ± 1.46 f | 12.97 ± 0.11 de | 14.03 ± 0.44 ef | 14.49 ± 0.66 f | 12.56 ± 0.48 d | 12.25 ± 0.26 d | 10.30 ± 0.32 c | 7.22 ± 0.05 b | 5.61 ± 0.04 a | ** | ** | ** |
| Fructose | 3.64 ± 0.02 a | 9.59 ± 1.18 efg | 12.78 ± 0.31 h | 9.10 ± 0.03 ef | 10.25 ± 0.47 fg | 8.66 ± 0.23 de | 10.03 ± 0.87 fg | 10.38 ± 0.57 g | 9.14 ± 0.11 ef | 7.74 ± 0.33 cd | 6.78 ± 0.49 c | 5.65 ± 0.23 b | 4.47 ± 0.16 a | ** | ** | ** |
| Total sugar | 13.55 ± 0.11 a | 34.59 ± 1.52 ef | 51.42 ± 0.04 i | 37.12 ± 0.27 fg | 40.33 ± 3.17 h | 34.41 ± 0.40 ef | 38.68 ± 1.79 gh | 40.56 ± 1.87 h | 31.15 ± 0.55 d | 32.47 ± 0.45 de | 26.54 ± 1.08 c | 18.63 ± 0.37 b | 13.75 ± 0.24 a | ** | ** | ** |
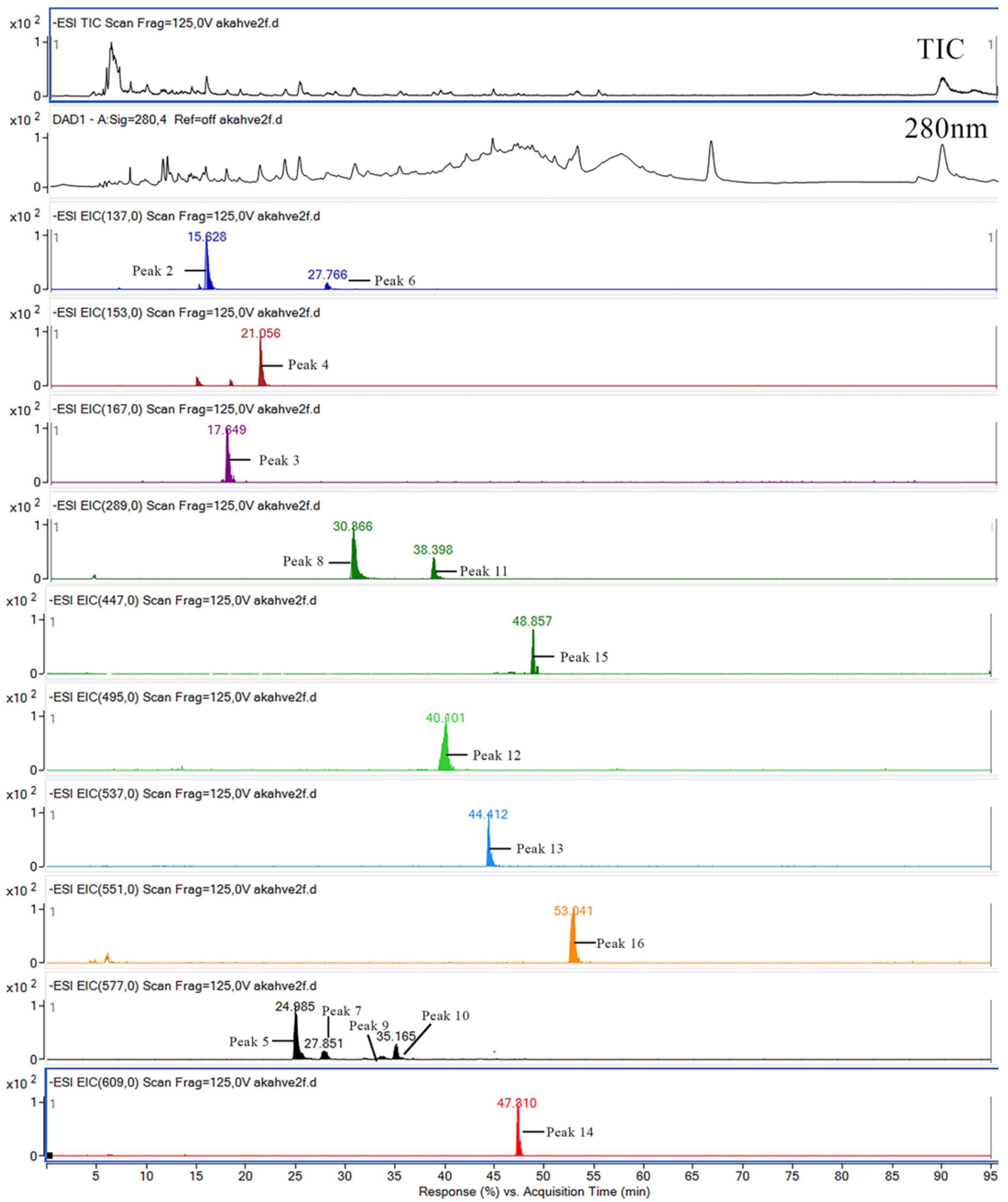
3.5. Results of Phenolic Compound Analysis
| 120 °C | 160 °C | 200 °C | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No | Compounds | RT | Precursor Ion (m/z) M− | Product Ions (m/z) | Unroasted Fruits | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | 10 min | 25 min | 32.5 min | 55 min | T | Tm | T × Tm |
| 1 | Gallic acid | 11.71 | 169 | 125 | 3.41 ± 0.13 d | 6.01 ± 0.22 e | 9.17 ± 0.09 h | 7.39 ± 0.06 g | 6.56 ± 0.13 f | 6.58 ± 0.04 f | 9.09 ± 0.05 h | 11.59 ± 0.13 j | 10.81 ± 0.35 i | 3.44 ± 0.16 d | 2.82 ± 0.08 c | 2.23 ± 0.02 b | 1.17 ± 0.06 a | ** | ** | ** |
| 2 | Tyrosol | 15.63 | 137 | 119 | 8.31 ± 0.14 a | 24.20 ± 0.45 e | 26.30 ± 1.00 f | 22.92 ± 0.12 e | 20.53 ± 1.09 d | 18.25 ± 0.02 c | 22.77 ± 0.02 e | 26.76 ± 0.78 f | 30.88 ± 0.69 g | 12.79 ± 0.71 b | 23.68 ± 0.96 e | 35.02 ± 0.55 h | 55.95 ± 0.90 i | ** | ** | ** |
| 3 | Vanillic acid | 17.65 | 167 | 152, 123, 108 | 6.03 ± 0.17 ab | 21.91 ± 0.57 efg | 23.98 ± 7.50 fgh | 28.86 ± 0.19 h | 24.66 ± 1.83 gh | 9.74 ± 0.02 bc | 14.36 ± 0.09 cd | 18.58 ± 0.37 de | 19.22 ± 0.48 def | 10.23 ± 1.01 bc | 9.56 ± 0.62 bc | 8.71 ± 0.47 ab | 4.04 ± 0.11 a | ** | * | ** |
| 4 | Dihydroxy benzoic acid | 21.06 | 153 | 109 | 8.26 ± 0.09 a | 29.85 ± 0.24 de | 53.85 ± 0.39 i | 47.84 ± 0.27 h | 41.63 ± 1.03 g | 23.54 ± 0.68 c | 28.89 ± 0.18 de | 31.23 ± 3.21 ef | 33.41 ± 0.61 f | 22.06 ± 0.89 c | 23.89 ± 1.12 c | 28.17 ± 0.34 d | 17.01 ± 0.21 b | ** | ** | ** |
| 5 | Procyanidin dimer | 24.98 | 577 | 289, 245 | 108.11 ± 0.58 a | 354.06 ± 3.16 h | 411.80 ± 0.76 j | 374.23 ± 0.47 i | 349.75 ± 1.21 h | 331.03 ± 0.15 g | 330.92 ± 1.30 g | 334.20 ± 2.33 g | 312.26 ± 0.37 f | 272.71 ± 3.76 e | 231.59 ± 0.83 d | 189.32 ± 0.47 c | 175.01 ± 5.45 b | ** | ** | ** |
| 6 | 4-Hydroxy benzoic acid | 27.66 | 137 | 93 | 4.22 ± 0.05 a | 9.51 ± 0.25 c | 16.20 ± 0.28 g | 10.79 ± 0.33 d | 12.20 ± 0.14 e | 8.90 ± 0.03 c | 14.92 ± 0.22 f | 19.89 ± 1.02 i | 18.85 ± 0.30 h | 7.68 ± 0.04 b | 16.52 ± 0.74 g | 26.93 ± 0.71 j | 18.79 ± 0.63 h | ** | ** | ** |
| 7 | Procyanidin dimer | 27.85 | 577 | 289, 245 | 6.05 ± 0.81 a | 13.56 ± 0.58 f | 16.47 ± 0.11 g | 10.80 ± 0.42 c | 10.97 ± 0.60 cd | 8.95 ± 0.10 b | 11.64 ± 0.50 cde | 14.04 ± 0.68 f | 12.02 ± 0.08 de | 12.39 ± 0.38 e | 11.17 ± 0.58 cd | 10.54 ± 0.69 c | 8.82 ± 0.36 b | ** | ** | ** |
| 8 | Catechin | 30.21 | 289 | 245, 205, 179 | 8.95 ± 0.40 b | 29.11 ± 0.79 h | 33.66 ± 0.51 i | 34.76 ± 0.49 i | 24.35 ± 1.15 f | 26.74 ± 0.56 g | 26.62 ± 0.22 g | 25.10 ± 0.97 f | 16.02 ± 0.46 c | 22.27 ± 0.75 e | 18.26 ± 0.55 d | 15.06 ± 0.71 c | 4.48 ± 0.02 a | ** | ** | ** |
| 9 | Procyanidin dimer | 33.77 | 577 | 289, 245 | 1.81 ± 0.03 ab | 4.88 ± 0.28 gh | 3.14 ± 0.01 de | 1.73 ± 0.04 a | 1.82 ± 0.08 ab | 2.69 ± 0.05 bcd | 3.42 ± 0.02 def | 4.08 ± 0.01 fg | 2.94 ± 0.05 cd | 5.65 ± 1.36 h | 3.88 ± 0.11 ef | 3.22 ± 0.01 def | 2.11 ± 0.02 abc | ** | ** | ** |
| 10 | Procyanidin dimer | 35.16 | 577 | 289, 245 | 3.92 ± 0.06 a | 9.56 ± 0.33 fg | 13.88 ± 0.24 i | 13.51 ± 0.06 i | 9.58 ± 0.56 fg | 12.67 ± 0.08 h | 10.04 ± 0.13 g | 7.37 ± 0.25 de | 6.35 ± 0.03 c | 8.99 ± 0.39 f | 7.50 ± 0.43 e | 6.86 ± 0.05 cd | 5.08 ± 0.06 b | ** | ** | ** |
| 11 | Epicatechin | 38.39 | 289–245 | 245, 205, 179 | 2.66 ± 0.07 cd | 2.51 ± 0.54 bcd | 5.61 ± 0.16 f | 4.86 ± 0.08 f | 3.77 ± 0.14 e | 1.80 ± 0.02 abc | 3.08 ± 0.12 de | 4.91 ± 1.06 f | 5.63 ± 0.36 f | 2.55 ± 0.57 bcd | 1.68 ± 0.09 ab | 1.35 ± 0.01 a | 0.98 ± 0.09 a | ** | ** | ** |
| 12 | Digalloylquinic acid | 40.10 | 495 | 343, 191 | 13.31 ± 0.48 a | 38.02 ± 0.28 g | 49.63 ± 0.82 i | 41.54 ± 4.20 h | 29.50 ± 1.90 cd | 36.21 ± 1.01 fg | 37.02 ± 0.13 fg | 37.89 ± 0.33 g | 35.23 ± 0.47 f | 26.85 ± 0.98 b | 27.88 ± 0.91 bc | 30.25 ± 0.91 d | 33.11 ± 0.88 e | ** | ** | ** |
| 13 | Amentoflavone | 44.41 | 537 | 493, 443, 417, 374 | 27.89 ± 1.09 a | 109.08 ± 0.52 i | 120.58 ± 1.08 j | 134.26 ± 1.64 k | 109.83 ± 2.62 i | 75.59 ± 2.64 f | 82.83 ± 0.24 g | 87.74 ± 2.18 h | 73.62 ± 0.75 f | 70.64 ± 0.83 e | 64.98 ± 1.22 d | 61.81 ± 0.27 c | 56.82 ± 0.51 b | ** | ** | ** |
| 14 | Rutin | 47.31 | 609 | 301, 273, 257, 179, 151 | 0.93 ± 0.04 a | 2.27 ± 0.02 cde | 2.63 ± 0.02 e | 2.71 ± 0.02 e | 2.35 ± 0.43 de | 1.98 ± 0.06 cd | 2.02 ± 0.03 cd | 1.92 ± 0.20 cd | 1.39 ± 0.02 ab | 1.80 ± 0.06 bc | 1.33 ± 0.09 ab | 0.98 ± 0.05 a | 1.42 ± 0.55 ab | ** | ns | ** |
| 15 | Quercetin-rhamnosyl | 48.85 | 447 | 301, 151 | 1.29 ± 0.06 ef | 1.17 ± 0.21 cde | 1.70 ± 0.72 e | 1.32 ± 0.02 ef | 1.34 ± 0.07 ef | 1.25 ± 0.04 def | 1.12 ± 0.03 de | 0.89 ± 0.16 abcde | 0.51 ± 0.02 a | 1.03 ± 0.05 bcde | 0.76 ± 0.07 abcd | 0.54 ± 0.03 ab | 0.67 ± 0.03 abc | ** | * | ns |
| 16 | Methyl-biflavone | 53.04 | 551 | - | 31.541.00 a | 98.63 ± 0.67 g | 128.37 ± 0.78 k | 88.88 ± 0.32 e | 90.70 ± 1.16 e | 101.73 ± 0.86 h | 109.69 ± 0.44 i | 115.26 ± 1.62 j | 95.68 ± 0.89 f | 88.98 ± 1.48 e | 84.86 ± 1.07 d | 80.68 ± 0.76 c | 77.06 ± 1.06 b | ** | ** | ** |
| Total | 236.7 ± 5.20 a | 754.3 ± 8.59 i | 917.0 ± 2.59 k | 826.4 ± 2.25 j | 739.6 ± 9.81 h | 667.7 ± 3.37 f | 708.4 ± 0.70 g | 741.5 ± 6.22 hi | 674.8 ± 5.94 f | 570.1 ± 0.46 e | 530.4 ± 9.47 d | 501.7 ± 6.05 c | 462.5 ± 6.85 b | ** | ** | ** | ||||

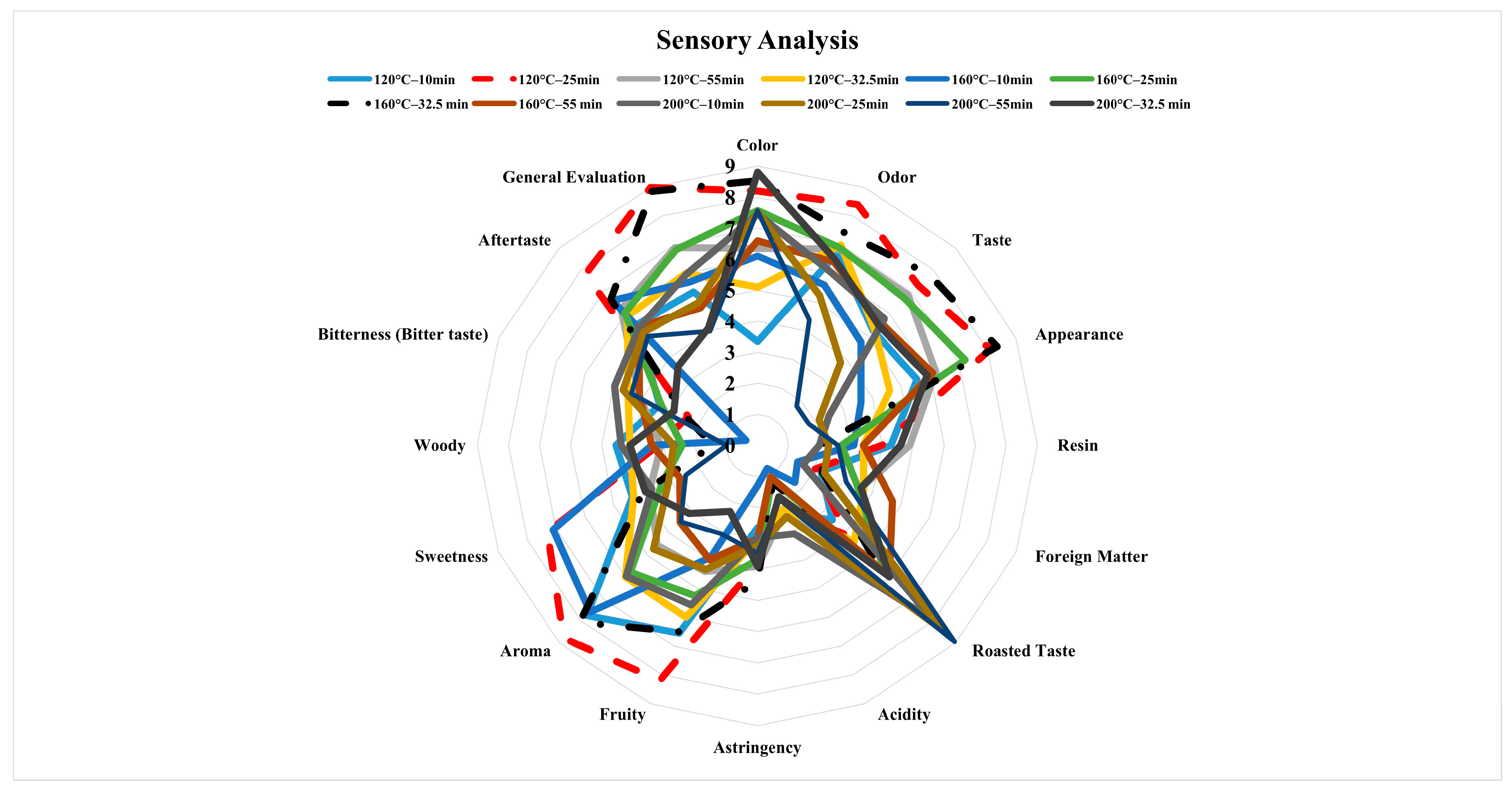
3.6. Results of Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekeroglu, N.; Senol, F.S.; Orhan, I.E.; Gulpinar, A.R.; Kartal, M.; Sener, B. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Food Res. Int. 2012, 45, 197–203. [Google Scholar] [CrossRef]
- Durmaz, G.; Gökmen, V. Changes in oxidative stability, antioxidant capacity and phytochemical composition of Pistacia terebinthus oil with roasting. Food Chem. 2011, 128, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol, F.S.; Gulpinar, A.R.; Sekeroglu, N.; Kartal, M.; Sener, B. Neuroprotective potential of some terebinth coffee brands and the unprocessed fruits of Pistacia terebinthus L. and their fatty and essential oil analyses. Food Chem. 2012, 130, 882–888. [Google Scholar] [CrossRef]
- Miceli, N.; Trovato, A.; Marino, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Güvenç, A.; et al. Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey. Food Chem. Toxicol. 2011, 49, 2600–2608. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yavuz, Z. Seed dormancy and cone and seed morphology of syrian juniper (Juniperus drupacea Labill.) in the eastern mediterranean region of Turkey. Šumar. List. 2017, 141, 257–262. [Google Scholar] [CrossRef]
- Özdemir, F.; Topuz, A.; Gölükçü, M.; Şahin, H. Andız (Juniperus drupacea) Pekmezi Üretim Tekniğinin Geliştirilmesi Üzerine bir Araştırma. Gıda 2004, 29, 33–40. [Google Scholar]
- Sezik, E.; Kocakulak, E.; Ozek, T.; Baser, K.H.C. Essential oil composition of Juniperus drupacea Lab. leaf from Turkey. Acta Pharm. Sci. 2009, 51, 109–120. [Google Scholar]
- Akbulut, H.F.; Akbulut, M. Mineral composition, the profile of phenolic compounds, organic acids, sugar and in vitro antioxidant capacity, and antimicrobial activity of organic extracts of Juniperus drupacea fruits. Food Sci. Nutr. 2023, 11, 6435–6446. [Google Scholar] [CrossRef]
- Boratynski, A.; Donmez, A.A.; Bou Dagher-Kharrat, M.; Romo, Á.; Tan, K.; Ok, T.; Iszkulo, G.; Sobierajska, K.; Marcysiak, K. Biology and ecology of Juniperus drupacea Labill. Dendrobiology 2023, 90, 1–29. [Google Scholar] [CrossRef]
- Satıl, F.; Akan, H.; Karaaslan, M.; Maruf Balos, M.; Başyiğit, B. Ethnobotanical and Chemical Studies on Gezo Molasses from Quercus brantii Lindl. Acorns in Turkey. Acta Soc. Bot. Pol. 2021, 90, 1–14. [Google Scholar] [CrossRef]
- Konuk Takma, D.; Çin, S.; Şahin Nadeem, H. Active Biopackaging Films Enriched With Andız (Juniperus drupacea L.) Shell Extract for Fresh Meat Packaging. Packag. Technol. Sci. 2024, 37, 989–1002. [Google Scholar] [CrossRef]
- Akbulut, M.; Bilgiçli, N. Effects of different pekmez (fruit molasses) types used as a natural sugar source on the batter rheology and physical properties of cakes. J. Food Process Eng. 2010, 33, 272–286. [Google Scholar] [CrossRef]
- Walas, Ł.; Sobierajska, K.; Ok, T.; Dönmez, A.A.; Kanoğlu, S.S.; Dagher-Kharrat, M.B.; Douaihy, B.; Romo, A.; Stephan, J.; Jasińska, A.K.; et al. Past, present, and future geographic range of an oro-Mediterranean Tertiary relict: The juniperus drupacea case study. Reg. Environ. Chang. 2019, 19, 1507–1520. [Google Scholar] [CrossRef]
- Ramakrishnan, P. HPLC and HPTLC determination of caffeine in raw and roasted date seeds (Phoenix dactylifera L). J. Chromatogr. Sep. Tech. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván D’Alessandro, L.; Manrique, G.D. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int. J. Food Sci. Technol. 2016, 51, 1018–1025. [Google Scholar] [CrossRef]
- Chbani, M.; Matthäus, B.; Charrouf, Z.; El Monfalouti, H.; Kartah, B.; Gharby, S.; Willenberg, I. Characterization of Phenolic Compounds Extracted from Cold Pressed Cactus (Opuntia ficus-indica L.) Seed Oil and the Effect of Roasting on Their Composition. Foods 2020, 9, 1098. [Google Scholar] [CrossRef]
- Park, S.-H.; Jo, A.; Lee, K.-G. Effect of various roasting, extraction and drinking conditions on furan and 5-hydroxymethylfurfural levels in coffee. Food Chem. 2021, 358, 129806. [Google Scholar] [CrossRef]
- Cemeroğlu, B. Gıda Analizlerinde Genel Yöntemler: Gıda Analizleri; Gıda Teknolojisi Dernegi: Ankara, Türkiye, 2014. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Saafi, E.B.; El Arem, A.; Issaoui, M.; Hammami, M.; Achour, L. Phenolic content and antioxidant activity of four date palm (Phoenix dactylifera L.) fruit varieties grown in Tunisia. Int. J. Food Sci. Technol. 2009, 44, 2314–2319. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.; Olano, A.; Villamiel, M. Determination of hydroxymethylfurfural in commercial jams and in fruit-based infant foods. Food Chem. 2002, 79, 513–516. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Coates, G.A. Quantitative study of free sugars and myo-inositol in citrus juices by HPLC and a literature compilation. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2123–2141. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Elmaci, T.A.O.Y. Gidalarda Duyusal Değerlendirme; Sidas Medya: Izmir, Türkiye, 2011. [Google Scholar]
- Özkan, K.; Karadag, A.; Sagdic, O. Determination of the in vitro bioaccessibility of phenolic compounds and antioxidant capacity of Juniper berry (Juniperus drupacea Labill.) pekmez. Turk. J. Agric. For. 2021, 45, 290–300. [Google Scholar] [CrossRef]
- León, K.; Mery, D.; Pedreschi, F.; León, J. Color measurement in L∗a∗b∗ units from RGB digital images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Hermann, C. The International Commission on Illumination—CIE: What It Is and How It Works. Symp.-Int. Astron. Union 2001, 196, 60. [Google Scholar] [CrossRef]
- İzgi, N. Composition, Rheological Properties, Antioxidant and Antimicrobial Activities of Homemade Andiz Molasses; Namık Kemal University: Tekirdag, Türkiye, 2011. [Google Scholar]
- Akbulut, M.; Çoklar, H.; Özen, G. Rheological Characteristics of Juniperus drupacea Fruit Juice (pekmez) Concentrated by Boiling. Food Sci. Technol. Int. 2008, 14, 321–328. [Google Scholar] [CrossRef]
- Seninde, D.R.; Chambers, E.; Chambers, D. Determining the impact of roasting degree, coffee to water ratio and brewing method on the sensory characteristics of cold brew Ugandan coffee. Food Res. Int. 2020, 137, 109667. [Google Scholar] [CrossRef]
- Ayseli, M.T. The Effect of Two Different Roasting Processes on Aroma and Aroma-Active Compounds of Turkish Coffee; Cukurova University: Adana, Türkiye, 2015. [Google Scholar]
- Ereli, E. Determination of the Effect of Different Andiz Molasses Production Methods on Phenolic Compounds and Some Quality Parameters; Alparslan Türkes Science and Technology University: Adana, Türkiye, 2021. [Google Scholar]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Surmacz, K.; Błoniarz, P.; Chmielarz, P. Coffee Beverage: A New Strategy for the Synthesis of Polymethacrylates via ATRP. Molecules 2022, 27, 840. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Acrylamide formation and antioxidant activity in coffee during roasting—A systematic study. Food Chem. 2021, 343, 128514. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Pelvan, E.; Topal, B. Effects of roasting on oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.). Int. J. Food Sci. Nutr. 2010, 61, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Slatnar, A.; Veberic, R.; Stampar, F.; Solar, A. Roasting Affects Phenolic Composition and Antioxidative Activity of Hazelnuts (Corylus avellana L.). J. Food Sci. 2011, 76, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Setianto, W.B.; Yohanes, H.; Atmaji, G.; Susanto, M.; Astin, E.P. The effect of fluidized bed roaster temperature on the roasted coffee beans antioxidant activity and its sensory evaluation. IOP Conf. Ser. Earth Environ. Sci. 2023, 1246, 012005. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, L.; Wu, X.; Rao, L.; Ai, Q. Comparison Studies on Antioxidant Properties of Black Sesame Seed (Sesamum indicum L.) Extracts Prepared by Thermal Processing Methods. Adv. Mater. Res. 2012, 554–556, 918–921. [Google Scholar] [CrossRef]
- Gumral, N.; Kumbul, D.D.; Aylak, F.; Saygin, M.; Savik, E. Juniperus communis Linn oil decreases oxidative stress and increases antioxidant enzymes in the heart of rats administered a diet rich in cholesterol. Toxicol. Ind. Health 2013, 31, 85–91. [Google Scholar] [CrossRef]
- Minh, N. Influence of thermal treatment on Anthocyanin, total phenolic content and antioxidant capacity of Pigmented Maize (Zea mays L.). Plant Sci. Today 2021, 8, 863. [Google Scholar] [CrossRef]
- Li, X.; Rui, P.; Ye, T.; Yao, X.; Zhou, R.; Li, D.; Wang, S.; Carter, J.H.; Hutchings, G.J. Hydrogenolysis of 5-hydroxymethylfurfural by in situ produced hydrogen from water on an iron catalyst. Catal. Sci. Technol. 2023, 13, 3366–3374. [Google Scholar] [CrossRef]
- Marić, A.; Jovanov, P.; Gadžurić, S.; Trtić-Petrović, T.; Sakač, M.; Tot, A.; Bertić, M.; Vraneš, M. Application of biodegradable cholinium ionic liquids for the extraction of 5-hydroxymethylfurfural (HMF) from honey. RSC Adv. 2023, 13, 32714–32721. [Google Scholar] [CrossRef]
- Murkovic, M.; Bornik, M.-A. Formation of 5-hydroxymethyl-2-furfural (HMF) and 5-hydroxymethyl-2-furoic acid during roasting of coffee. Mol. Nutr. Food Res. 2007, 51, 390–394. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Laukalēja, I.; Kruma, Z. Influence of the Roasting Process on Bioactive Compounds and Aroma Profile in Specialty Coffee: A Review. In Proceedings of the Baltic Conference on Food Science and Technology and North and East European Congress on Food, Jelgava, Latvia, 2–3 May 2019; pp. 7–12. [Google Scholar]
- Martins, F.C.O.L.; Alcantara, G.M.R.N.; Silva, A.F.S.; Melchert, W.R.; Rocha, F.R.P. The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chem. 2022, 395, 133539. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, G.; Yu, Y.; Xu, Y.; Wu, J.; Peng, J.; Li, L. Effects of high pressure and thermal processing on quality properties and volatile compounds of pineapple fruit juice. Food Control 2021, 130, 108293. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Chung, H.-S.; Chung, S.-K.; Youn, K.-S. Effects of roasting temperature and time on bulk density, soluble solids, browning index and phenolic compounds of corn kernels. J. Food Process. Preserv. 2011, 35, 832–839. [Google Scholar] [CrossRef]
- Taha, E.; Matthäus, B. Effect of Roasting Temperature on Safflower Seeds and Oil. J. Food Dairy Sci. 2018, 9, 103–109. [Google Scholar] [CrossRef]
- Duskaev, G.; Kurilkina, M.; Zavyalov, O. Growth-stimulating and antioxidant effects of vanillic acid on healthy broiler chickens. Vet. World 2023, 16, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sedej, I.; Milczarek, R.; Wang, S.C.; Sheng, R.; de Jesús Avena-Bustillos, R.; Dao, L.; Takeoka, G. Spray drying of a phenolic-rich membrane filtration fraction of olive mill wastewater: Optimisation and dried product quality. Int. J. Food Sci. Technol. 2016, 51, 1900–1909. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Shu, Y.; Tan, S.; Yin, L.; Guo, Y.; Tang, L. HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities. Molecules 2016, 21, 1234. [Google Scholar] [CrossRef]
- Pawar, S.; Shubham, K.; Patil, V.; Bhambar, R. Effect of Vanillic Acid on Nerve Conduction Velocity in Chronic Constriction Injury Model of Neuropathy. Indian J. Pharm. Educ. Res. 2019, 54, 108–113. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Maisch, N.A.; Bereswill, S.; Heimesaat, M.M. Antibacterial effects of vanilla ingredients provide novel treatment options for infections with multidrug-resistant bacteria—A recent literature review. Eur. J. Microbiol. Immunol. 2022, 12, 53–62. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1α Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension1. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Manhivi, V.E.; Akinola, S.A.; Garcia, C.; Remize, F.; Shoko, T.; Sivakumar, D. Changes in phenolics and antioxidant capacity during fermentation and simulated in vitro digestion of mango puree fermented with different lactic acid bacteria. J. Food Process. Preserv. 2021, 45, e15937. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, G.W.; Oh, H.; Yoo, S.Y.; Kim, Y.O.; Oh, M.S. Influence of roasting on the antioxidant activity of small black soybean (Glycine max L. Merrill). LWT-Food Sci. Technol. 2011, 44, 992–998. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B.; Gliszczyńska-Świgło, A.; Niedźwiedzińska, K. Effect of thermal pre-treatment on the phenolic and protein profiles and oil oxidation dynamics of golden flaxseeds. Int. J. Food Sci. Technol. 2020, 55, 1272–1280. [Google Scholar] [CrossRef]
- Corina, B.; Pavaloiu, R.; Pirvu, L. HPTLC Profiles and Antioxidant Activities from Leaves to Green and Roasted Beans of Coffea Arabica. Malays. J. Med. Biol. Res. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Y.; Li, J.; Long, Q.; Zhong, H. Effects of Seed Coat on Oxidative Stability and Antioxidant Activity of Apricot (Prunus armeniaca L.) Kernel Oil at Different Roasting Temperatures. J. Am. Oil Chem. Soc. 2018, 95, 1297–1306. [Google Scholar] [CrossRef]
- Cai, L.; Cao, A.; Aisikaer, G.; Ying, T. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur. J. Lipid Sci. Technol. 2013, 115, 556–563. [Google Scholar] [CrossRef]
- Karaman, E.E.; Çetinkaya, N. The Role of Senses in Food Preference: Determination of the Difference of Sense of Taste According to Demographic Characteristics with Taste Test. J. Atatürk Univ. Inst. Soc. Sci. 2020, 24, 883–898. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelebek, H.; Carikcioglu, M.; Kadiroglu, P.; Ereli, E.; Uzlasir, T.; Selli, S. Phenolic Characterization and Quality Evaluation of Herbal Coffee from Roasted Juniper Berry Fruits (Juniperus drupacea L.): Elucidating the Impact of Roasting. Foods 2024, 13, 3946. https://doi.org/10.3390/foods13233946
Kelebek H, Carikcioglu M, Kadiroglu P, Ereli E, Uzlasir T, Selli S. Phenolic Characterization and Quality Evaluation of Herbal Coffee from Roasted Juniper Berry Fruits (Juniperus drupacea L.): Elucidating the Impact of Roasting. Foods. 2024; 13(23):3946. https://doi.org/10.3390/foods13233946
Chicago/Turabian StyleKelebek, Hasim, Merve Carikcioglu, Pınar Kadiroglu, Esra Ereli, Turkan Uzlasir, and Serkan Selli. 2024. "Phenolic Characterization and Quality Evaluation of Herbal Coffee from Roasted Juniper Berry Fruits (Juniperus drupacea L.): Elucidating the Impact of Roasting" Foods 13, no. 23: 3946. https://doi.org/10.3390/foods13233946
APA StyleKelebek, H., Carikcioglu, M., Kadiroglu, P., Ereli, E., Uzlasir, T., & Selli, S. (2024). Phenolic Characterization and Quality Evaluation of Herbal Coffee from Roasted Juniper Berry Fruits (Juniperus drupacea L.): Elucidating the Impact of Roasting. Foods, 13(23), 3946. https://doi.org/10.3390/foods13233946







