Targeting Spore-Forming Bacteria: A Review on the Antimicrobial Potential of Selenium Nanoparticles
Abstract
1. Introduction
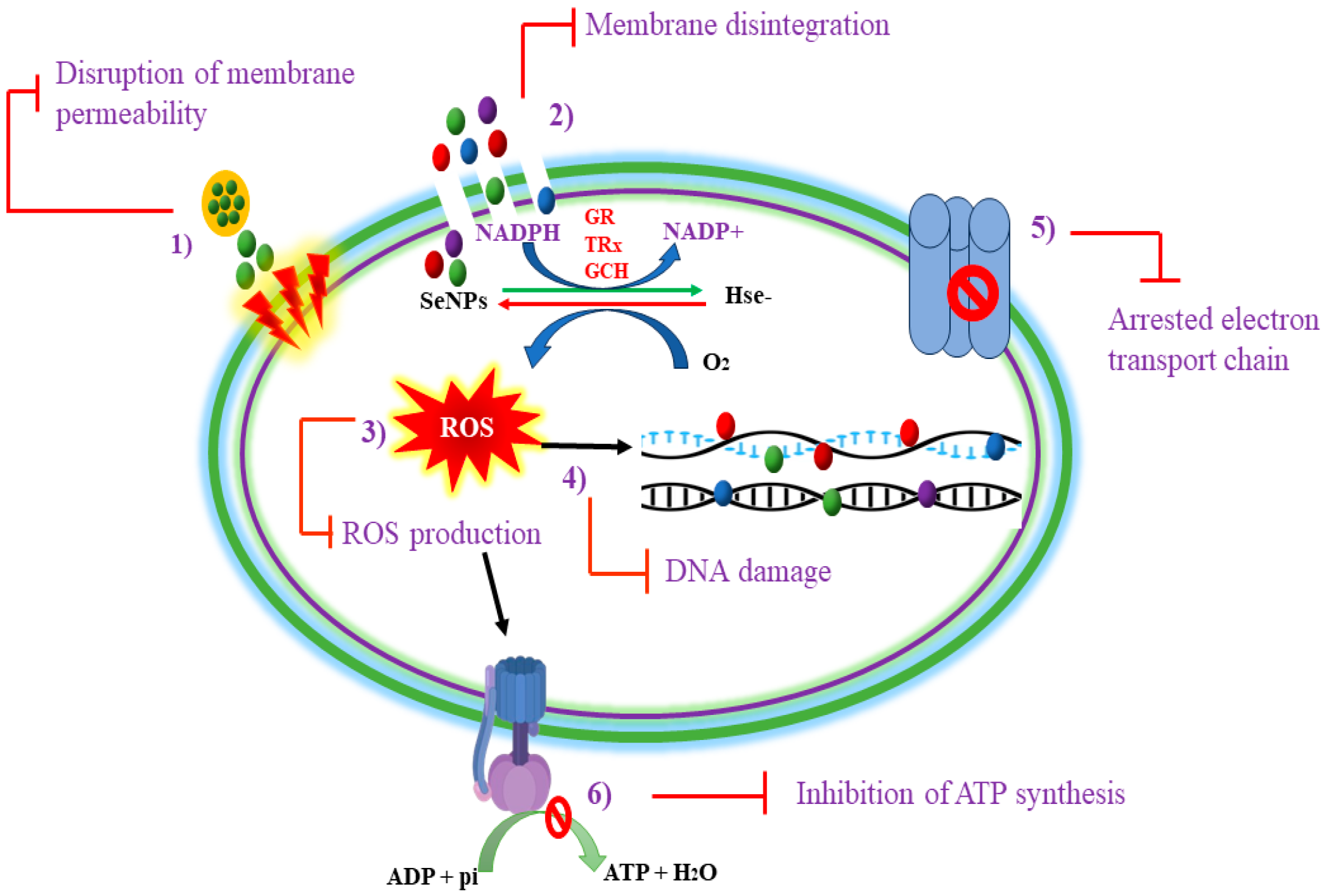
2. Mechanism of Action of SeNPs Against Spore-Forming Bacteria
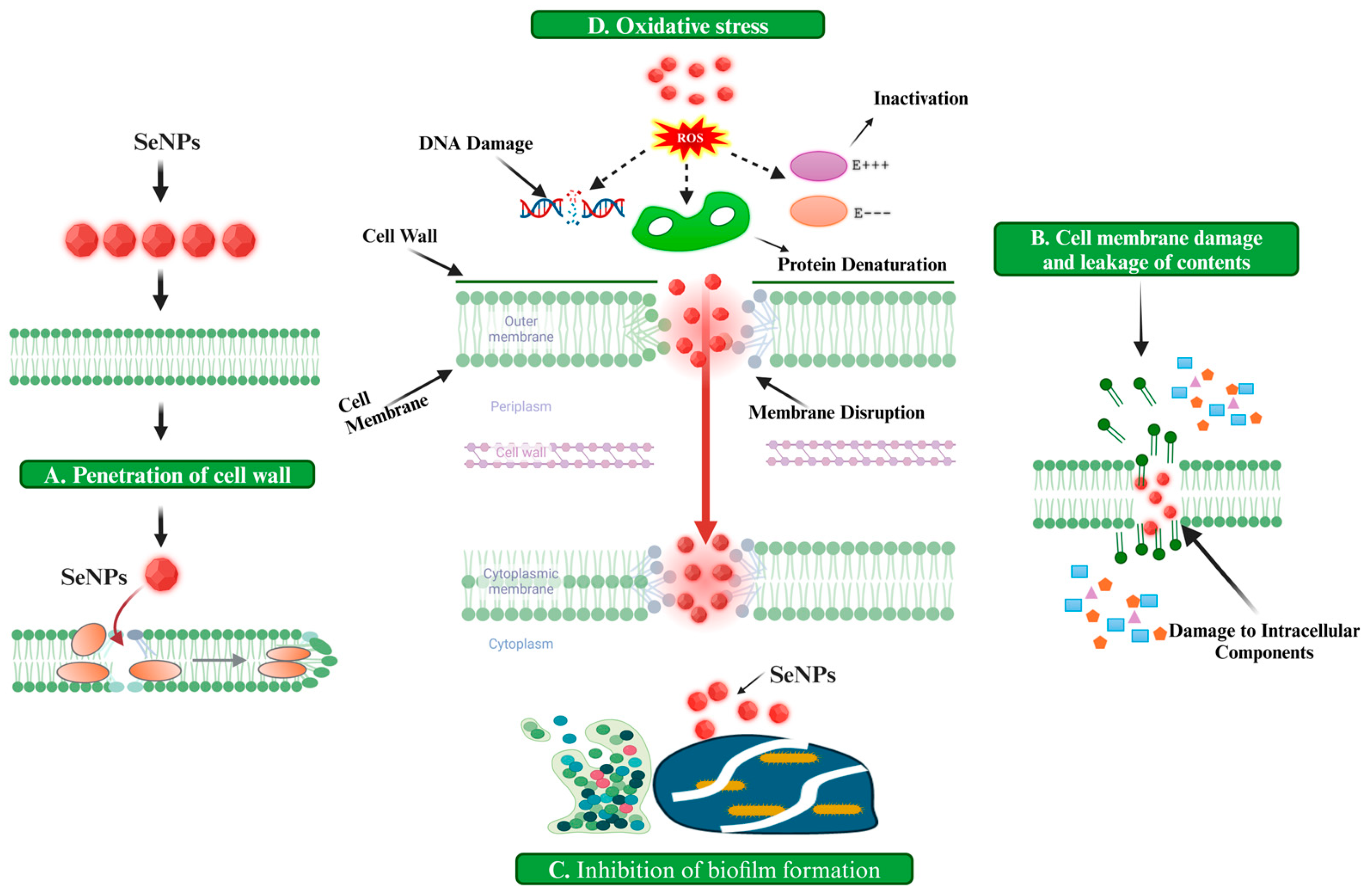
2.1. Oxidative Stress
2.2. Cell Wall Disruption
2.3. Damage to Intracellular Components
2.4. Inhibition of Adenosine Triphosphate (ATP) Synthesis
3. Therapeutic Approaches of SeNPs Against Several Spore-Forming Bacteria Causing Health Issues
3.1. The Antagonistic Effect of SeNP-Loaded Bifidobacterium Breve Against C. difficile
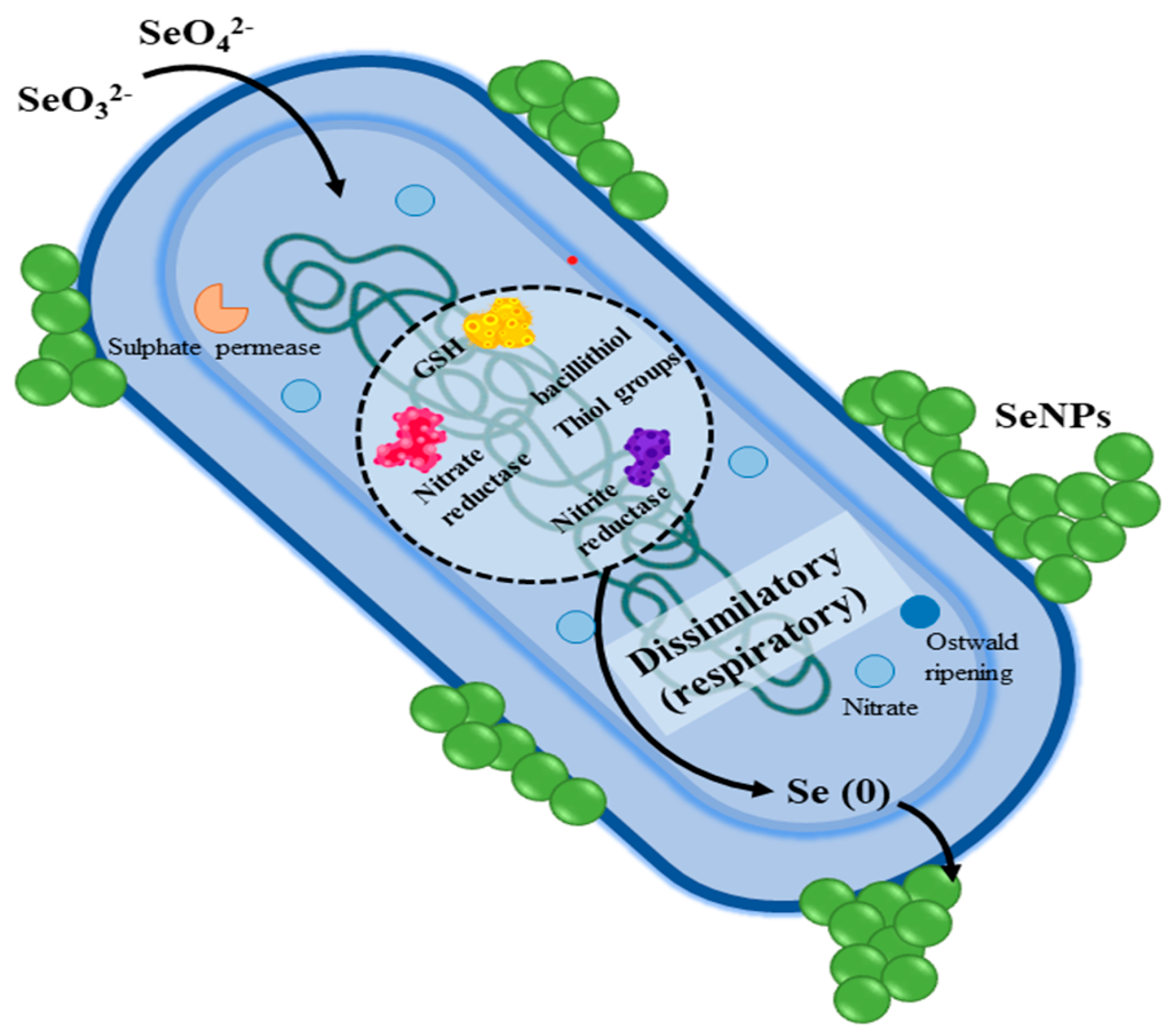
3.2. Biogenic SeNPs Against Gram-Positive and Gram-Negative Bacteria
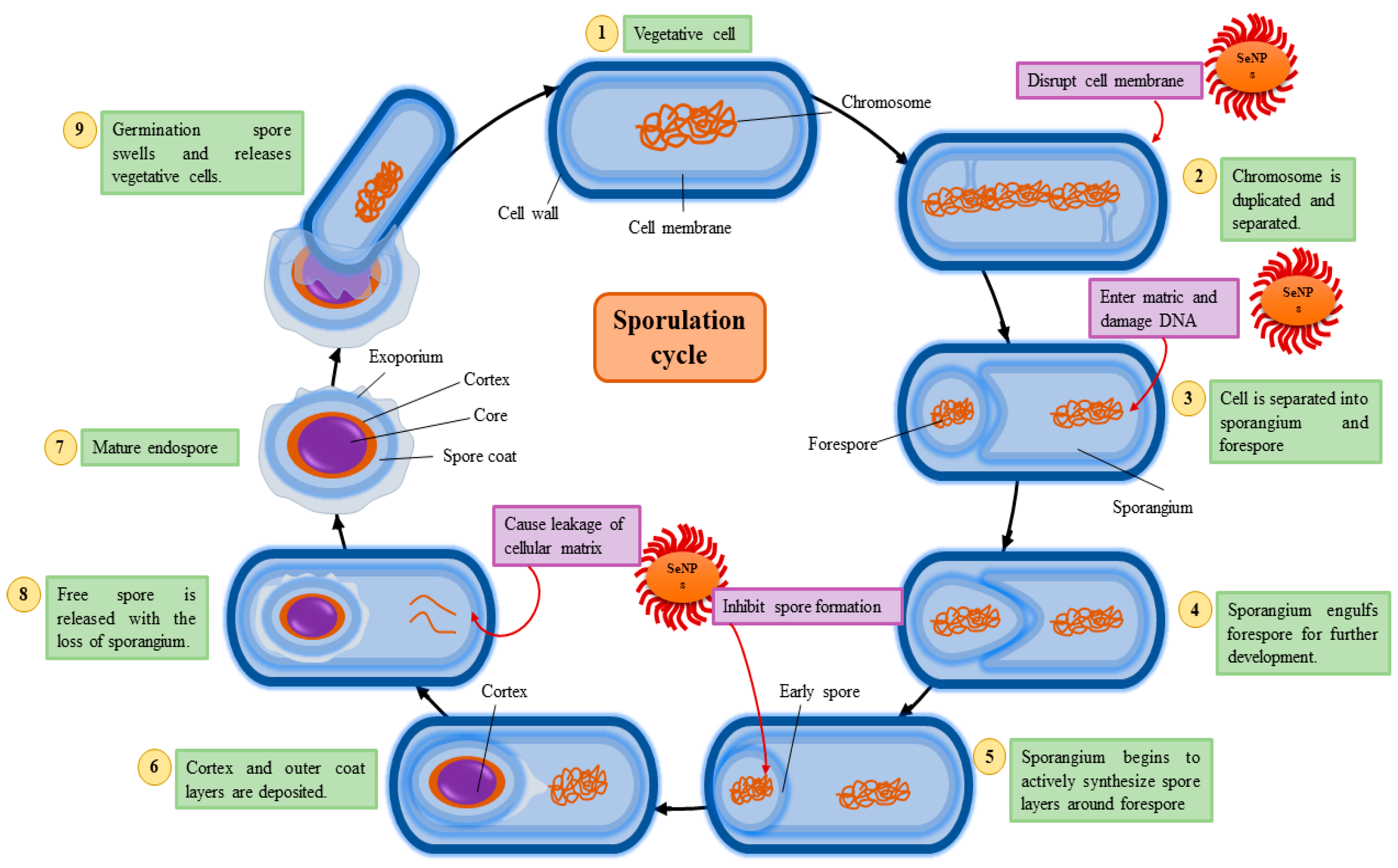
4. Sporulation Cycle and Antibacterial Mechanisms of SeNPs
5. Efficacy of SeNPs Against Spore-Forming Bacteria
6. Synthesis of SeNPs and Their Impact on Antimicrobial Efficacy
6.1. Chemical Synthesis of SeNPs
6.2. Physical Synthesis of SeNPs
6.3. Biological Synthesis of SeNPs
7. Applications of SeNPs in Food Preservation
7.1. Use of SeNP in Food Packaging
7.2. Direct Food Additive Applications
8. Applications of SeNPs in Healthcare
8.1. Anticancer Applications
8.2. Drug Delivery Systems
9. Environmental Factors Affecting SeNP Activity
10. Biocompatibility of SeNPs for Human Cells
11. Limitations and Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Emilie, G.; Anne, G.M.; Ivan, L.; Olivier, C.; Florence, P.; Veronique, B.; Louis, C. Knowledge of the physiology of spore-forming bacteria can explain the origin of spores in the food environment. Res. Microbiol. 2017, 168, 369–378. [Google Scholar]
- Steve, O.V.; Maximilienne, N.; Blaise, P.B.; François-Xavier, E. The Problem of Spore-Forming Bacteria in Food Preservation and Tentative Solutions. In Foodborne Pathogens and Antibiotic Resistance; Wiley: Hoboken, NJ, USA, 2016; pp. 139–151. [Google Scholar]
- Zongshuai, Z.; Anthony, P.B.; Tianran, H.; Yali, Z.; Iftikhar, A.K.; Ming, H. The formation, germination, and cold plasma inactivation of bacterial spore. Food Chem. Adv. 2022, 1, 100056. [Google Scholar]
- Jalalpour, S. Foodborne diseases bacteria; frequency antibiotic resistance bacteria in Iranian foods. Afr. J. Microbiol. Res. 2012, 6, 719–723. [Google Scholar]
- Brown, K. Control of bacterial spores. Br. Med. Bull. 2000, 56, 158–171. [Google Scholar] [CrossRef]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Rückert, A.; Ronimus, R.S.; Morgan, H.W. A RAPD-based survey of thermophilic bacilli in milk powders from different countries. Int. J. Food Microbiol. 2004, 96, 263–272. [Google Scholar] [CrossRef]
- André, S.; Zuber, F.; Remize, F. Thermophilic spore-forming bacteria isolated from spoiled canned food and their heat resistance. Results of a French ten-year survey. Int. J. Food Microbiol. 2013, 165, 134–143. [Google Scholar] [CrossRef]
- Osimani, A.; Aquilanti, L. Spore-forming bacteria in insect-based foods. Curr. Opin. Food Sci. 2021, 37, 112–117. [Google Scholar]
- Mathot, A.G.; Postollec, F.; Leguerinel, I. Bacterial spores in spices and dried herbs: The risks for processed food. Compr. Rev. Food Sci. Food Saf. 2021, 20, 840–862. [Google Scholar] [CrossRef]
- Ghosh, B.; Dhar, J.; Mukhopadhyay, M.; Bhattacharya, D. Bacillus cereus biofilm: Implications for food and diseases. Microbe 2024, 4, 100129. [Google Scholar] [CrossRef]
- Frederic, C.; Marie, G.; Caroline, C.; Roselyne, P.; Agnes, B.; Christophe, N. Spore-forming bacteria in commercial cooked, pasteurised and chilled vegetable purées. Food Microbiol. 2000, 17, 153–165. [Google Scholar]
- Rongxue, S.; An, V.; Anneleen, D.W.; Peter, V.; Frank, D. Identification and characterization of acid-tolerant spore-forming spoilage bacteria from acidified and low-acid pasteurized sauces. LWT 2021, 152, 112378. [Google Scholar]
- Setlow, P.; Christie, G. New Thoughts on an Old Topic: Secrets of Bacterial Spore Resistance Slowly Being Revealed. Microbiol. Mol. Biol. Rev. 2023, 87, e00080-22. [Google Scholar] [CrossRef]
- Setlow, P. The bacterial spore: From molecules to systems. In Spore Resistance Properties; Wiley: Hoboken, NJ, USA, 2016; pp. 201–215. [Google Scholar] [CrossRef]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.-Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- Abdel-Moneim, E.A.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.-F.; Pi, J. The advancing of selenium nanoparticles against infectious diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Senthil Kumar, P.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- El-Gameel, E.A.; Amin, S.R. Environmental Impact Assessment of Food Irradiation Technology as A Comparative Study with Some Other Food Preservation Methods. Egypt. J. Radiat. Sci. Appl. 2021, 34, 79–85. [Google Scholar] [CrossRef]
- Rudy, M.; Kucharyk, S.; Duma-Kocan, P.; Stanisławczyk, R.; Gil, M. Unconventional Methods of Preserving Meat Products and Their Impact on Health and the Environment. Sustainability 2020, 12, 5948. [Google Scholar] [CrossRef]
- Sharif, Z.I.M.; Mustapha, F.A.; Jai, J.; Mohd, Y.N.; Zaki, N.A.M. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bull. 2017, 19, 145. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Schaefer, S.; Hübner, R.; Fahmy, K.; Merroun, M.L. Exploring Antibacterial Activity and Bacterial-Mediated Allotropic Transition of Differentially Coated Selenium Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 29958–29970. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, T.; Che, J.; Yi, J.; Wei, L.; Li, H. Evaluation of the antimicrobial mechanism of biogenic selenium nanoparticles against Pseudomonas fluorescens. Biofouling 2023, 39, 157–170. [Google Scholar] [CrossRef]
- Escobar-Ramírez, M.C.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Rodríguez-Serrano, G.M.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Añorve-Morga, J.; Jaimez-Ordaz, J.; González-Olivares, L.G. Antimicrobial activity of Se-nanoparticles from bacterial biotransformation. Fermentation 2021, 7, 130. [Google Scholar] [CrossRef]
- Sahoo, B.; Panigrahi, L.L.; Jena, S.; Jha, S.; Arakha, M. Oxidative stress generated due to photocatalytic activity of biosynthesized selenium nanoparticles triggers cytoplasmic leakage leading to bacterial cell death. RSC Adv. 2023, 13, 11406–11414. [Google Scholar] [CrossRef]
- Wu, X.; Yang, M.; Kim, J.S.; Wang, R.; Kim, G.; Ha, J.; Kim, H.; Cho, Y.; Nam, K.T.; Yoon, J. Reactivity Differences Enable ROS for Selective Ablation of Bacteria. Angew. Chem. 2022, 61, e202200808. [Google Scholar]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free. Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ. Res. 2021, 194, 110630. [Google Scholar] [CrossRef] [PubMed]
- Tendenedzai, J.T.; Chirwa, E.M.N.; Brink, H.G. Enterococcus spp. Cell-Free Extract: An Abiotic Route for Synthesis of Selenium Nanoparticles (SeNPs), Their Characterisation and Inhibition of Escherichia coli. Nanomaterials 2022, 12, 658. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, H.; Lin, X.; Ali, A.S.M.; Viscardi, A.; Guo, Z.; Qiao, L.; He, Y.; Han, J. Selenium nanoparticles: Properties, preparation methods, and therapeutic applications. Chem.-Biol. Interact. 2023, 378, 110483. [Google Scholar]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar]
- Galić, E.; Ilić, K.; Hartl, S.; Tetyczka, C.; Kasemets, K.; Kurvet, I.; Milić, M.; Barbir, R.; Pem, B.; Erceg, I.; et al. Impact of surface functionalization on the toxicity and antimicrobial effects of selenium nanoparticles considering different routes of entry. Food Chem. Toxicol. 2020, 144, 111621. [Google Scholar]
- Timoshok, N.; Kharchuk, M.S.; Kaplunenko, V.G.; Bityutskyy, V.S.; Tsekhmistrenko, S.I.; Tsekhmistrenko, O.S.; Spivak, M.; Melnichenko, O.M. Evaluation of effects of selenium nanoparticles on Bacillus subtilis. Regul. Mech. Biosyst. 2019, 10, 544–552. [Google Scholar]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater. Res. Express 2019, 6, 0850i2. [Google Scholar]
- Rui, W.; Gu, C.; Zhang, H.; Liao, X.; Zhao, X.; Xu, Y.; Yang, J. Antagonistic activity of selenium-enriched Bifidobacterium breve against Clostridioides difficile. Appl. Microbiol. Biotechnol. 2022, 106, 6181–6194. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol. Rep. 2021, 30, e00615. [Google Scholar]
- Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Acosta-Gío, E.; Álvarez-Pérez, M.A. In vitro antimicrobial activity evaluation of metal oxide nanoparticles. In Nanoemulsions—Properties, Fabrications and Applications; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Lamarche, C.; Da Silva, C.; Demol, G.; Dague, E.; Rols, M.-P.; Pillet, F. Electrical discharges in water induce spores’ DNA damage. PLoS ONE 2018, 13, e0201448. [Google Scholar]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Han, H.-W.; Patel, K.D.; Kwak, J.-H.; Jun, S.-K.; Jang, T.-S.; Lee, S.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. Selenium nanoparticles as candidates for antibacterial substitutes and supplements against multidrug-resistant bacteria. Biomolecules 2021, 11, 1028. [Google Scholar] [CrossRef]
- Rath, S.; Das, S. Oxidative stress-induced DNA damage and DNA repair mechanisms in mangrove bacteria exposed to climatic and heavy metal stressors. Environ. Pollut. 2023, 339, 122722. [Google Scholar]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar]
- Urrutia Mera, M.; Kemper, M.; Doyle, R.; Beveridge, T.J. The membrane-induced proton motive force influences the metal binding ability of Bacillus subtilis cell walls. Appl. Environ. Microbiol. 1992, 58, 3837–3844. [Google Scholar]
- Hu, Y.; Jin, X.; Gao, F.; Lin, T.; Zhu, H.; Hou, X.; Yin, Y.; Kan, S.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 effectively ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2022, 13, 955112. [Google Scholar]
- Huang, T.; Holden, J.A.; Reynolds, E.C.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Multifunctional antimicrobial polypeptide-selenium nanoparticles combat drug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 55696–55709. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Selenium Nanoparticles: Green Synthesis and Biomedical Application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Pérez-Larios, A.; González Silva, N.; Becerra-Ruiz, J.S. A Review of the Antimicrobial Activity of Selenium Nanoparticles. J. Nanosci. Nanotechnol. 2021, 21, 5383–5398. [Google Scholar]
- Martínez Esquivias, F.; Méndez-Robles, M.D.; Villagómez-Vega, A.; Segura-Almendárez, M.S.; Cruz-Ahumada, C.J.; Guzmán-Flores, J.M. Medicinal Applications of Selenium Nanoparticles Synthesized by Green Methods. Lett. Org. Chem. 2024, 21, 40–54. [Google Scholar]
- Ameen, F.; Almalki, N.S.; Alshalan, R.; Sakayanathan, P. Green Synthesis of Selenium Nanoparticles Utilizing Drimia indica: Insights into Anticancer and Antimicrobial Activities. Microsc. Res. Tech. 2024; early view. [Google Scholar]
- Alavi, M.; Rai, M.; Martinez, F.; Kahrizi, D.; Khan, H.; de Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M. The efficiency of metal, metal oxide, and metalloid nanoparticles against cancer cells and bacterial pathogens: Different mechanisms of action. Cell. Mol. Biomed. Rep. 2022, 2, 10–21. [Google Scholar]
- Yang, J.; Meng, L.; Li, Y.; Huang, H. Strategies for applying probiotics in the antibiotic management of Clostridioides difficile infection. Food Funct. 2023, 14, 8711–8733. [Google Scholar]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection. Nat. Rev. Dis. Primers 2016, 2, 1–20. [Google Scholar]
- Candel-Pérez, C.; Ros-Berruezo, G.; Martínez-Graciá, C. A review of Clostridioides [Clostridium] difficile occurrence through the food chain. Food Microbiol. 2019, 77, 118–129. [Google Scholar]
- Goldenberg, J.Z.; Mertz, D.; Johnston, B.C. Probiotics to prevent Clostridium difficile infection in patients receiving antibiotics. Jama 2018, 320, 499–500. [Google Scholar]
- Yang, J.; Yang, H. Effect of Bifidobacterium breve in combination with different antibiotics on Clostridium difficile. Front. Microbiol. 2018, 9, 2953. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, H. Recent development in Se-enriched yeast, lactic acid bacteria and bifidobacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Zambonino, M.C.; Mateo Quizhpe, E.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic selenium nanoparticles in biomedical sciences: Properties, current trends, novel opportunities and emerging challenges in theranostic nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.S.; Baker, D.H.A.; Ahmed, E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021, 203, 523–532. [Google Scholar] [CrossRef]
- Beheshti, N.; Soflaei, S.; Shakibaie, M.; Yazdi, M.H.; Ghaffarifar, F.; Dalimi, A.; Shahverdi, A.R. Efficacy of biogenic selenium nanoparticles against Leishmania major: In vitro and in vivo studies. J. Trace Elem. Med. Biol. 2013, 27, 203–207. [Google Scholar] [CrossRef]
- Shakibaie, M.; Mohazab, N.S.; Mousavi, S.A.A. Antifungal activity of selenium nanoparticles synthesized by Bacillus species Msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8, e26381. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef]
- Sefidi, M.D.; Bakhshi, B. Enhanced Antibacterial and Antibiofilm Effects of Thiolated Chitosan-Encapsulated Nisin and Selenium Nanoparticles: A Potential Nanotechnology-Based Solution for Antibiotic-Resistant Bacterial Infections. 2023; preprint. [Google Scholar]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. 2017, 45, 1490–1495. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Fasihi Harandi, M.; Shakibaie, M.; Aflatoonian, M.R.; ZiaAli, N.; Makki, M.S.; Jahanbakhsh, S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 2014, 12, 399–403. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.H.; Ali, A.; Usman, M.; Ali, N.; et al. Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Khatoon, N.; Khan, M.A.; Husain, S.A.; Saravanan, M.; Sardar, M. Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J. Clust. Sci. 2020, 31, 1003–1011. [Google Scholar] [CrossRef]
- El-Deeba, B.; Al-Talhib, A.; Mostafac, N.; Abou-assyd, R. Biological synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial properties. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 45, 135–170. [Google Scholar]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-targeted isoniazid–selenium nanoparticles promote antimicrobial immunity and synergize bactericidal destruction of tuberculosis bacilli. Angew. Chem. 2020, 132, 3252–3260. [Google Scholar] [CrossRef]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on antibacterial to combat resistance: From the ancient era of plants and metals to present and future perspectives of green nanotechnological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Alqaraleh, S.Y.; Al-Zereini, W.A.; Mwafi, N.R.; Jaffal, S.M.; Al-Qtaitat, A.I. The Green Synthesis of Selenium Nanoparticles: A Comprehensive Review on Methodology, Characterization and Biomedical Applications. Res. J. Pharm. Technol. 2024, 17, 4054–4062. [Google Scholar] [CrossRef]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Shen, A.; Edwards, A.N.; Sarker, M.R.; Paredes-Sabja, D. Sporulation and Germination in Clostridial Pathogens. Microbiol. Spectr. 2019, 7, 1–30. [Google Scholar] [CrossRef]
- Callejón-Leblic, B.; Selma-Royo, M.; Collado, M.C.; Abril, N.; García-Barrera, T. Impact of antibiotic-induced depletion of gut microbiota and selenium supplementation on plasma selenoproteome and metal homeostasis in a mice model. J. Agric. Food Chem. 2021, 69, 7652–7662. [Google Scholar] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Synthesis and Characterization of Bimetallic Platinum/Selenium (Pt/Se) Nanoparticles for Synergistic Antibacterial Activity. BioNanoScience 2024, 14, 630–642. [Google Scholar]
- Varak, K.; Priya, P.K. Synergistic Effect of Selenium Nanoparticles (Senps) With Various Antibiotics As An Antimicrobial Activity”. Think India J. 2019, 22, 1707–1712. [Google Scholar]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Naveen, K.V.; Wang, M.H. Enhancement of anti-bacterial potential of green synthesized selenium nanoparticles by starch encapsulation. Microb. Pathog. 2022, 167, 105544. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, S.; Tahan, M.; Sadeghian, H.; Nazari, R.; Behmadi, M.; Hosseini Bafghi, M. Effect of biosynthesized selenium nanoparticles using Nepeta extract against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Basic Microbiol. 2023, 63, 210–222. [Google Scholar] [CrossRef]
- Huang, S.; Yu, K.; Xiao, Q.; Song, B.; Yuan, W.; Long, X.; Cai, D.; Xiong, X.; Zheng, W. Effect of bio-nano-selenium on yield, nutritional quality and selenium content of radish. J. Food Compos. Anal. 2023, 115, 104927. [Google Scholar]
- Postollec, F.; Mathot, A.-G.; Bernard, M.; Divanac’h, M.-L.; Pavan, S.; Sohier, D. Tracking spore-forming bacteria in food: From natural biodiversity to selection by processes. Int. J. Food Microbiol. 2012, 158, 1–8. [Google Scholar]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Advances in green synthesis of selenium nanoparticles and their application in food packaging. Int. J. Food Sci. Technol. 2021, 56, 2640–2650. [Google Scholar]
- Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging. Coatings 2020, 10, 280. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Selenium nanoparticles–enhanced potato starch film for active food packaging application. Int. J. Food Sci. Technol. 2022, 57, 6512–6521. [Google Scholar]
- Verma, P.V.R.K. A review on synthesis and the antibacterial activity of Silver and Selenium nanoparticles against biofilm-forming Staphylococcus aureus. World J. Pharm. Pharmaceut. Sci. 2015, 4, 652–677. [Google Scholar]
- Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Imani, M.M.; Sharifi, R.; Golshah, A. Optimization of green synthesis of selenium nanoparticles and evaluation of their antifungal activity against oral Candida albicans infection. Adv. Mater. Sci. Eng. 2022, 2022, 1376998. [Google Scholar]
- Varlamova, E.G.; Baimler, I.V.; Gudkov, S.V.; Turovsky, E.A. Comparative Study of the Anticancer Effects of Selenium Nanoparticles and Selenium Nanorods: Regulation of Ca2+ Signaling, ER Stress and Apoptosis. Appl. Sci. 2023, 13, 10763. [Google Scholar] [CrossRef]
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M.S. Biocontrol Efficacy of Mycosynthesized Selenium Nanoparticle Using Trichoderma sp. on Insect Pest Spodoptera litura. J. Clust. Sci. 2022, 33, 1645–1653. [Google Scholar]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar]
- Sun, Y.; Shi, Y.; Jia, H.; Ding, H.; Yue, T.; Yuan, Y. Biosynthesis of selenium nanoparticles of Monascus purpureus and their inhibition to Alicyclobacillus acidoterrestris. Food Control 2021, 130, 108366. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, X.C.; Tran, T.N.M.; Nguyen, N.T.T.; Pham, B.N.; Vu, D. Nano selenium–alginate edible coating extends hydroponic strawberry shelf life and provides selenium fortification as a micro-nutrient. Food Biosci. 2023, 53, 102597. [Google Scholar] [CrossRef]
- Medina Cruz, D.; Mi, G.; Webster, T.J. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J. Biomed. Mater. Res. Part A 2018, 106, 1400–1412. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar]
- Nayak, V.; Singh, K.R.B.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Salavati-Niasari, M.; Hosseinpour-Mashkani, S.M. Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J. Alloys Compd. 2014, 617, 627–632. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. The Influence of Synthesis Conditions on the Antioxidant Activity of Selenium Nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Quintana, M.; Haro-Poniatowski, E.; Morales, J.; Batina, N. Synthesis of selenium nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2002, 195, 175–186. [Google Scholar] [CrossRef]
- Haro-Poniatowski, E.; Escobar-Alarcón, L.; Hernández-Pozos, J.L.; Mendoza-Luna, L.G.; Guarin, C.A. Synthesis and characterization of selenium nanoparticles obtained by femtosecond pulsed laser ablation in liquid media. Appl. Phys. A 2022, 128, 827. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar]
- Senthamarai, M.D.; Hillary, V.E.; Rajan, M.R.; Ceasar, S.A.; Ceasar, S.A. Biosynthesis of selenium nanoparticles and its biological applications: A systematic review. Nano-Struct. Nano-Objects 2024, 39, 101261. [Google Scholar]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostructure Chem. 2021, 12, 467–480. [Google Scholar]
- Dwivedi, C.; Shah, C.P.; Singh, K.; Kumar, M.; Bajaj, P.N. An organic acid-induced synthesis and characterization of selenium nanoparticles. J. Nanotechnol. 2011, 2011, 651971. [Google Scholar] [CrossRef]
- Jwied, D.H.; Nayef, U.M.; Mutlak, F.A.-H. Synthesis of C: Se nanoparticles ablated on porous silicon for sensing NO2 and NH3 gases. Optik 2021, 241, 167013. [Google Scholar]
- Vera, P.; Echegoyen, Y.; Canellas, E.; Nerín, C.; Palomo, M.; Madrid, Y.; Cámara, C. Nano selenium as an antioxidant agent in a multilayer food packaging material. Anal. Bioanal. Chem. 2016, 408, 6659–6670. [Google Scholar] [CrossRef] [PubMed]
- El Gohary, H.G.; Asnag, G.M.; Tarabiah, A.E.; Qahtan, T.F.; Abdelrazek, E.M.; Cevik, E.; Al-Hakimi, A.N.; Mohammed Abdulwahed, J.A.; Madkhli, A.Y.; Alajmi, F.; et al. Modification and development of optical, thermal, dielectric properties and antibacterial activity of PVA/SA blend by Ag/Se nanofillers: Nanocomposites for energy storage devices and food packaging applications. Polym. Test. 2023, 129, 108258. [Google Scholar]
- Stabnikova, O.; Khonkiv, M.; Kovshar, I.; Stabnikov, V. Biosynthesis of selenium nanoparticles by lactic acid bacteria and areas of their possible applications. World J. Microbiol. Biotechnol. 2023, 39, 230. [Google Scholar]
- Vera, P.; Canellas, E.; Nerín, C. New Antioxidant Multilayer Packaging with Nanoselenium to Enhance the Shelf-Life of Market Food Products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.-H.; Gontard, N.; Gott, D.; Grilli, S.; et al. Selenium-enriched yeast as a source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 766, 1–42. [Google Scholar]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar]
- Silano, V.; Bolognesi, C.; Chipman, K.; Cravedi, J.-P.; Engel, K.-H.; Fowler, P.A.; Franz, R.; Grob, K.; Gürtler, R.; Husøy, T.; et al. Safety assessment of the active substance selenium nanoparticles, for use in active food contact materials. EFSA J. 2018, 16, e05115. [Google Scholar]
- Martínez-Esquivias, F.; Gutiérrez-Angulo, M.; Pérez-Larios, A.; Sánchez-Burgos, J.A.; Becerra-Ruiz, J.S.; Guzmán-Flores, J.M. Anticancer activity of selenium nanoparticles in vitro studies. Anti-Cancer Agents Med. Chem. 2022, 22, 1658–1673. [Google Scholar] [CrossRef]
- Debnath, S.; Agarwal, A.; Kumar, N.R.; Bedi, A. Selenium-based drug development for antioxidant and anticancer activity. Future Pharmacol. 2022, 2, 595–607. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Ibrahim, A.; Eltarahony, M. Anticancer activity of lactoferrin-coated biosynthesized selenium nanoparticles for combating different human cancer cells via mediating apoptotic effects. Sci. Rep. 2023, 13, 9579. [Google Scholar] [CrossRef] [PubMed]
- Birhan, Y.S.; Tsai, H.-C. Recent developments in selenium-containing polymeric micelles: Prospective stimuli, drug-release behaviors, and intrinsic anticancer activity. J. Mater. Chem. B 2021, 9, 6770–6801. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, J.; Shi, R.; Wei, J.; Lei, X.; Dou, Y.; Li, Y.; Zuo, Y.; Li, J. Loading and releasing behaviourbehaviour of selenium and doxorubicin hydrochloride in hydroxyapatite with different morphologies. ACS Omega 2021, 6, 8365–8375. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Johari, B.; Mousazadeh, N.; Rahimi, B.; Parvinzad Leilan, M.; Eslami, S.S.; Sharafi, A. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep. 2020, 47, 6517–6529. [Google Scholar] [CrossRef]
- Ullah, A.; Mirani, Z.A.; Binbin, S.; Wang, F.; Chan, M.W.H.; Aslam, S.; Yonghong, L.; Hasan, N.; Naveed, M.; Hussain, S.; et al. An elucidative study of the anti-biofilm effect of selenium nanoparticles (SeNPs) on selected biofilm producing pathogenic bacteria: A disintegrating effect of SeNPs on bacteria. Process Biochem. 2023, 126, 98–107. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Rajeshkumar, S.; Rosy, P.J.; Shanmugam, V.K. Investigating the Antimicrobial Activities of the Biosynthesized Selenium Nanoparticles and Its Statistical Analysis. BioNanoScience 2020, 10, 122–135. [Google Scholar] [CrossRef]
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium nanoparticles: Synthesis, characterization, and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 2019, 6, 0850d8. [Google Scholar] [CrossRef]
- Ao, B.; Du, Q.; Liu, D.; Shi, X.; Tu, J.; Xia, X. A review on synthesis and antibacterial potential of bio-selenium nanoparticles in the food industry. Front. Microbiol. 2023, 14, 1229838. [Google Scholar] [CrossRef]
- Chellappa, L.R.; Samuel, S.R.; Shanmugam, R. Biogenic nano selenium nanoselenium nano selenium synthesis, its antimicrobial, antioxidant activity and toxicity. Bioinspired Biomim. Nanobiomaterials 2020, 9, 184–189. [Google Scholar] [CrossRef]
- Nouri, Z.H.M.; Tafvizi, F.; Amini, K.; Khandandezfully, N.; Kheirkhah, B. Enhanced Induction of Apoptosis and Cell Cycle Arrest in MCF-7 Breast Cancer and HT-29 Colon Cancer Cell Lines via Low-Dose Biosynthesis of Selenium Nanoparticles Utilizing Lactobacillus casei. Biol. Trace Elem. Res. 2024, 202, 1288–1304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, R.; Zhou, F.; Wu, Y.; Li, S.; Huo, G.; Ye, J.; Hua, C.; Wang, Z. Preparation, physicochemical characterization, and cytotoxicity of selenium nanoparticles stabilized by Oudemansiella raphanipies polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Mu, J.; Wang, F.; Chan, M.W.H.; Yin, X.; Liao, Y.; Mirani, Z.A.; Sebt-E-Hassan, S.; Aslam, S.; Naveed, M.; et al. Biogenic selenium nanoparticles and their anticancer effect probiotic bacteria—A Review. Antioxidants 2022, 11, 1916. [Google Scholar] [PubMed]
- Ashraf, H.; Cossu, D.; Ruberto, S.; Noli, M.; Jasemi, S.; Simula, E.R.; Sechi, L.A. Latent Potential of Multifunctional Selenium Nanoparticles in Neurological Diseases and Altered Gut Microbiota. Materials 2023, 16, 699. [Google Scholar] [CrossRef]
- Cid-Barrio, L.; Bouzas-Ramos, D.; Salinas-Castillo, A.; Ogra, Y.; Ruiz Encinar, J.; Costa-Fernández, J.M. Quantitative assessment of cellular uptake and differential toxic effects of HgSe nanoparticles in human cells. J. Anal. At. Spectrom. 2020, 35, 1979–1988. [Google Scholar]

| Aspects | SeNPs | Thermal Processing (e.g., Pasteurization, Sterilization) | Chemical Preservatives (e.g., Sodium Benzoate, Nitrates) | Natural Antimicrobials (e.g., Essential Oils, Bacteriocins) | Ref. |
|---|---|---|---|---|---|
| Mechanism of Action | Generates ROS and disrupts microbial cell membranes, proteins, and DNA. Effective against spores. | Kills microbes by denaturing proteins and disrupting cellular processes through heat application. | Alters microbial metabolism and inhibits enzymatic activity through chemical interaction. | Disrupts cell membranes and metabolic pathways; depends on active compounds such as phenols and peptides. | [22] |
| Target Range | Broad-spectrum activity, including spore-forming bacteria (Clostridium botulinum, Bacillus cereus). | Effective against most vegetative bacteria but less effective against spores without high temperatures. | Effective against broad range of bacteria, but spores often require additional treatments. | Limited to specific strains and less effective against spores. | [23] |
| Effect on Food Quality | Minimal effect on sensory and nutritional properties due to low concentrations needed. | Significant nutrient and texture loss due to high temperatures; impacts sensory attributes. | Potentially alters taste and color of food, depending on chemical used and its concentration. | Risk of altering taste and aroma of food; requires optimization to prevent off-flavors. | [24] |
| Environmental Impact | Green synthesis methods reduce environmental impact; some methods risk contamination with by-products. | Energy-intensive and contributes to greenhouse gas emissions in large-scale applications. | Residues can persist in environment, causing potential ecological damage. | Eco-friendly, but large-scale extraction of natural resources can impact ecosystems. | [25] |
| Health Considerations | Potential cytotoxicity and dysbiosis risks require precise dosing and safety assessments. | Generates potentially harmful compounds (e.g., acrylamide) at high temperatures. | Chemical residues in food are linked to health concerns such as allergies and chronic diseases. | Generally safe, but risk of allergies or sensitivities to specific natural compounds. | [26] |
| Stability | High stability in food matrices; retains antimicrobial activity under varying conditions. | Stability depends on temperature control and duration; prolonged processing risks overcooking or underprocessing. | Stability can decrease over time due to chemical degradation or reactions with food components. | Stability varies with exposure to temperature, light, and oxygen; often requires encapsulation for improved use. | [27] |
| Mechanism | Description | Key Steps | Applications | Ref. |
|---|---|---|---|---|
| 1. Oxidative Stress | SeNPs induce the production of ROS, leading to cellular damage. | SeNPs generate ROS upon interaction with bacterial membranes. ROS causes the oxidation of lipids, proteins, and DNA. The accumulation of ROS triggers apoptosis-like pathways. | Antibacterial coatings | [54] |
| 2. Cell Wall Disruption | SeNPs penetrate and disrupt bacterial cell walls, compromising structural integrity. | Electrostatic interactions facilitate SeNP adhesion. Transient pores form in the membrane. Increased permeability leads to the leakage of intracellular contents. | Targeted drug delivery | [55] |
| 3. Inhibition of ATP Synthesis | SeNPs inhibit ATP production by disrupting the proton motive force and interfering with ATP synthase. | SeNPs destabilize the proton gradient across the membrane. Direct interaction with ATP synthase reduces its activity. The induction of ROS damages components of the electron transport chain. | Cancer therapy | [56] |
| 4. Membrane Integrity Alteration | SeNPs affect membrane integrity, leading to ion imbalance and the loss of essential metabolites. | Membrane poration allows for uncontrolled ion flow. The loss of critical ions disrupts metabolic processes. The influx of SeNPs exacerbates membrane damage. | Food preservation | [57] |
| 5. Induction of Genotoxicity | SeNPs cause DNA damage through oxidative stress and direct interactions with genetic material. | ROS induce strand breaks and mutations in bacterial DNA. Impaired DNA repair mechanisms lead to cell cycle arrest and death. The loss of genetic stability affects survival. | Antiviral applications | [58] |
| 6. Inhibition of Biofilm Formation | SeNPs disrupt biofilm development, enhancing susceptibility to antimicrobial agents. | The downregulation of genes involved in biofilm formation. A reduction in biofilm biomass increases the effectiveness of antibiotics against biofilm-associated bacteria. | Medical device coatings | [59] |
| Target Organisms | SeNPs Properties | Mechanism of Action | Effects of SeNP Exposure | References |
|---|---|---|---|---|
| Leishmania major | Spherical and amorphous SeNPs (80–220 nm) | DNA fragmentation | Inhibition of growth of cells and proliferation of cutaneous leishmaniasis | [68] |
| Aspergillus fumigatus Candida albicans | Spherical (80–220 nm) | Unknown | Enhanced antifungal activity and fungal growth inhibition | [69] |
| Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus | Spherical (100–400 nm) | Oxidative stress through production of reactive oxygen species | Inhibition of bacterial growth and eradication of already-produced biofilms | [70] |
| Multidrug-resistant enteric pathogens | Spherical (approx. 79 nm) | Oxidative stress | Inhibition of bacterial growth and biofilm formation | [71] |
| Multidrug-resistant bacteria and pathogenic fungi | Isotropic and poly-dispersed spheres (55 nm) | Alteration of membrane structure and oxidative stress | Growth inhibition | [72] |
| Fourth-instar larvae of mosquito vectors | Spherical and elongated (46–78 nm) | Denaturation of cellular components | Larvicidal activity against spread of mosquito vectors | [73] |
| Echinococcus granulosus | Amorphous (80–220 nm) | Membrane disruption and | Strong suicidal effects causing pathogenic killing | [74] |
| Escherichia coli Staphylococcus aureus | Spherical (25–40 nm) | Cytotoxicity and alteration in membrane structure | Growth inhibition | [75] |
| Multidrug-resistant bacterial species | Spherical (25–40 nm) | Oxidative stress and abnormal protein synthesis | Bacteriostatic and bactericidal effects | [47] |
| Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa | Amorphous and spherical (530 nm) | Oxidative stress | Growth inhibition | [76] |
| Escherichia coli Staphylococcus aureus Bacillus subtilis | Spherical (2–15 nm) | Oxidative stress | Inhibition and degradation of bacterial film | [77] |
| Gram-positive and Gram-negative bacteria | Hexagonal (3–50 nm) | Cell wall lysis and DNA unwinding | Growth inhibition and cell death | [78] |
| Stenotrophomonas bentonitica LysiniBacillus sphaericus | Amorphous and spherical (50–90) | Oxidative stress and DNA degradation | Reduced cellular growth and viability | [28] |
| Mycobacterium tuberculosis | Spherical (40–45 nm) | ROS generation | Growth inhibition and cell death | [79] |
| Staphylococcus aureus Escherichia coli Klebsiella pneumonia | Spherical and monodispersed (80 nm) | ATP reduction, ROS production, and membrane disruption | Enhanced cytotoxicity and cellular inhibition | [53] |
| Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus Staphylococcus epidermidis | Amorphous and crystalline (1–150 nm) | Membrane penetration and ROS generation | Inhibition of bacterial proliferation | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Zhang, D.; Tang, X.; Malakar, P.K. Targeting Spore-Forming Bacteria: A Review on the Antimicrobial Potential of Selenium Nanoparticles. Foods 2024, 13, 4026. https://doi.org/10.3390/foods13244026
Ahmed F, Zhang D, Tang X, Malakar PK. Targeting Spore-Forming Bacteria: A Review on the Antimicrobial Potential of Selenium Nanoparticles. Foods. 2024; 13(24):4026. https://doi.org/10.3390/foods13244026
Chicago/Turabian StyleAhmed, Faraz, Dingwu Zhang, Xiaoyang Tang, and Pradeep K. Malakar. 2024. "Targeting Spore-Forming Bacteria: A Review on the Antimicrobial Potential of Selenium Nanoparticles" Foods 13, no. 24: 4026. https://doi.org/10.3390/foods13244026
APA StyleAhmed, F., Zhang, D., Tang, X., & Malakar, P. K. (2024). Targeting Spore-Forming Bacteria: A Review on the Antimicrobial Potential of Selenium Nanoparticles. Foods, 13(24), 4026. https://doi.org/10.3390/foods13244026





