Utilizing Lactic Acid Bacteria to Improve Hyperlipidemia: A Comprehensive Analysis from Gut Microbiota to Metabolic Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Culture
2.2. Reagents
2.3. Cholesterol-Lowering Activity
2.4. Animal Treatment
2.5. Serum Biochemistry
2.6. Metabonomic Analysis
2.7. Microbiota Analysis
2.8. Statistics
3. Results
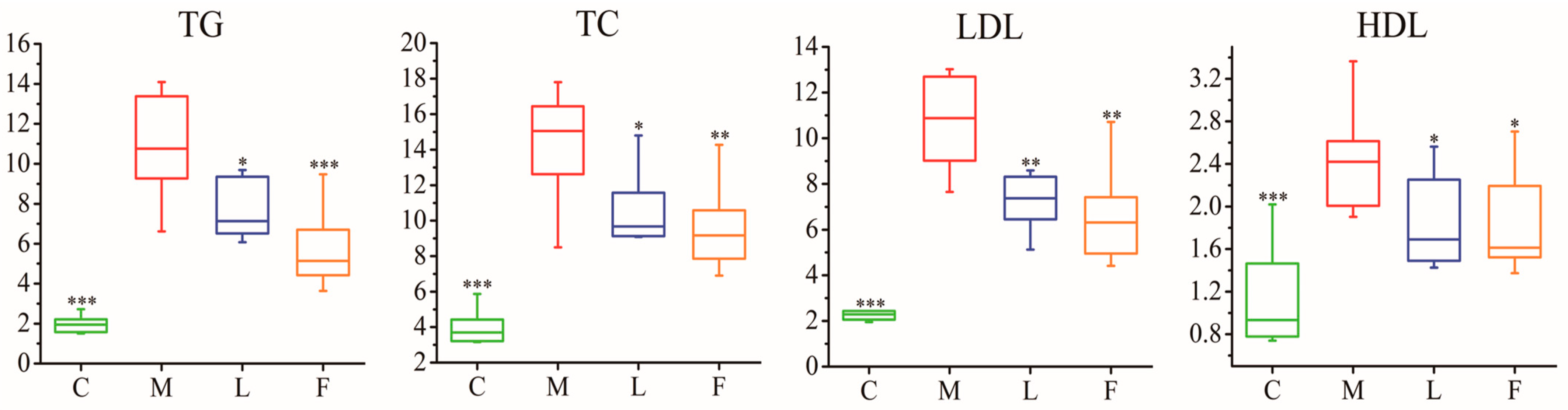
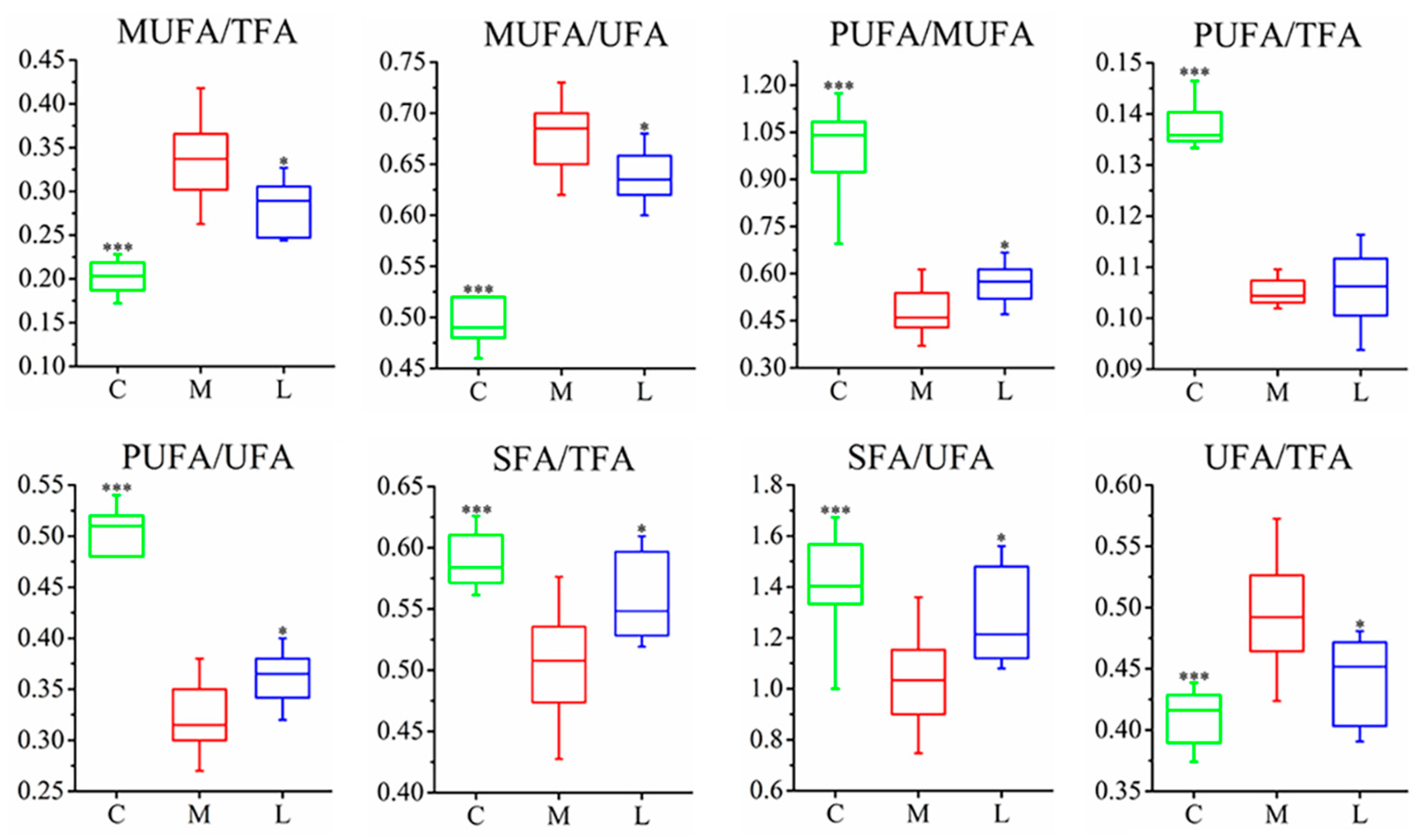
3.1. Measurement of Pathological Characteristics
3.2. H NMR-Based Metabonomics Reveals Metabolic Changes in Hyperlipidemia Hamsters
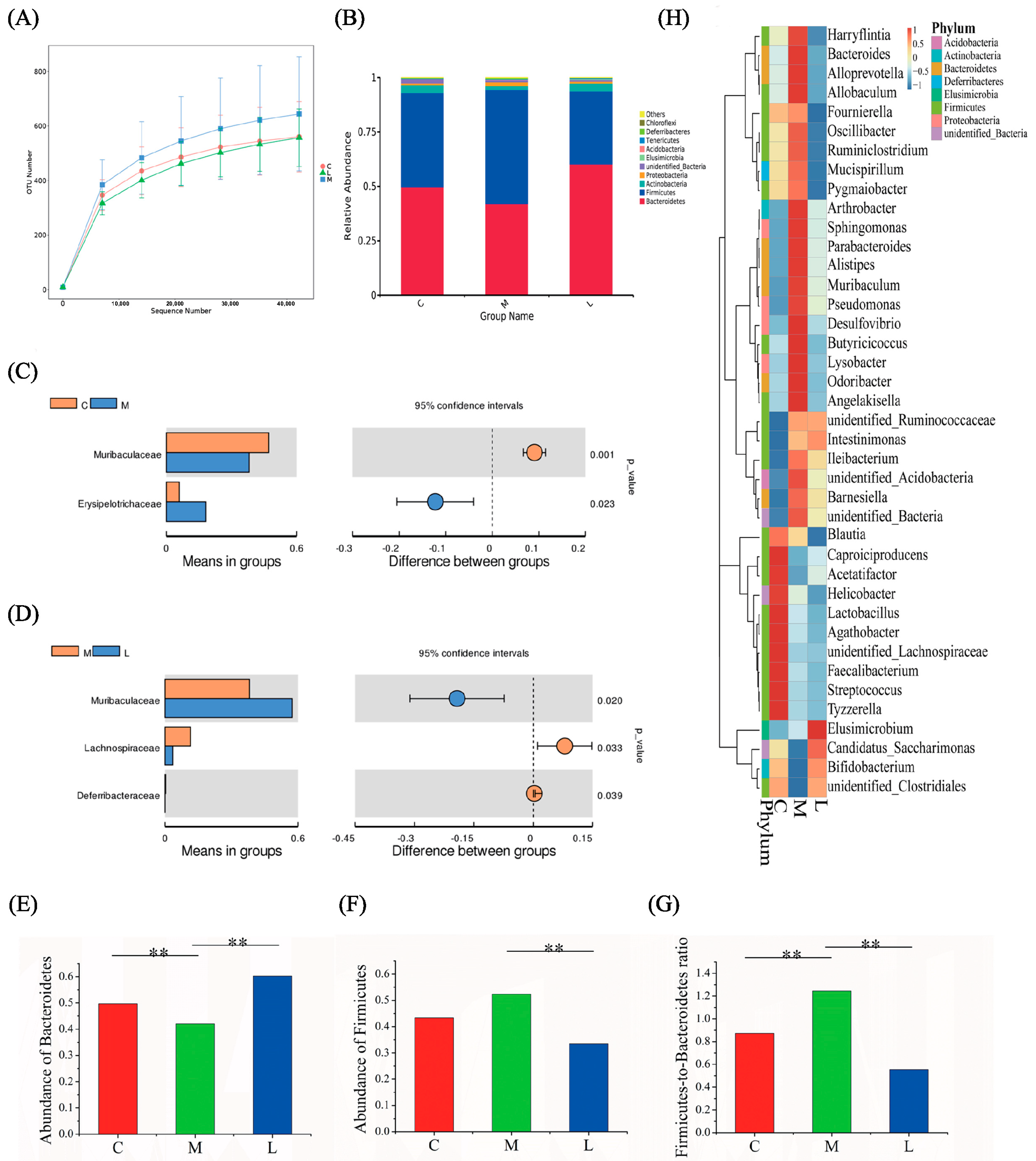
3.3. Analysis of Differences in the Gut Microbiome Composition
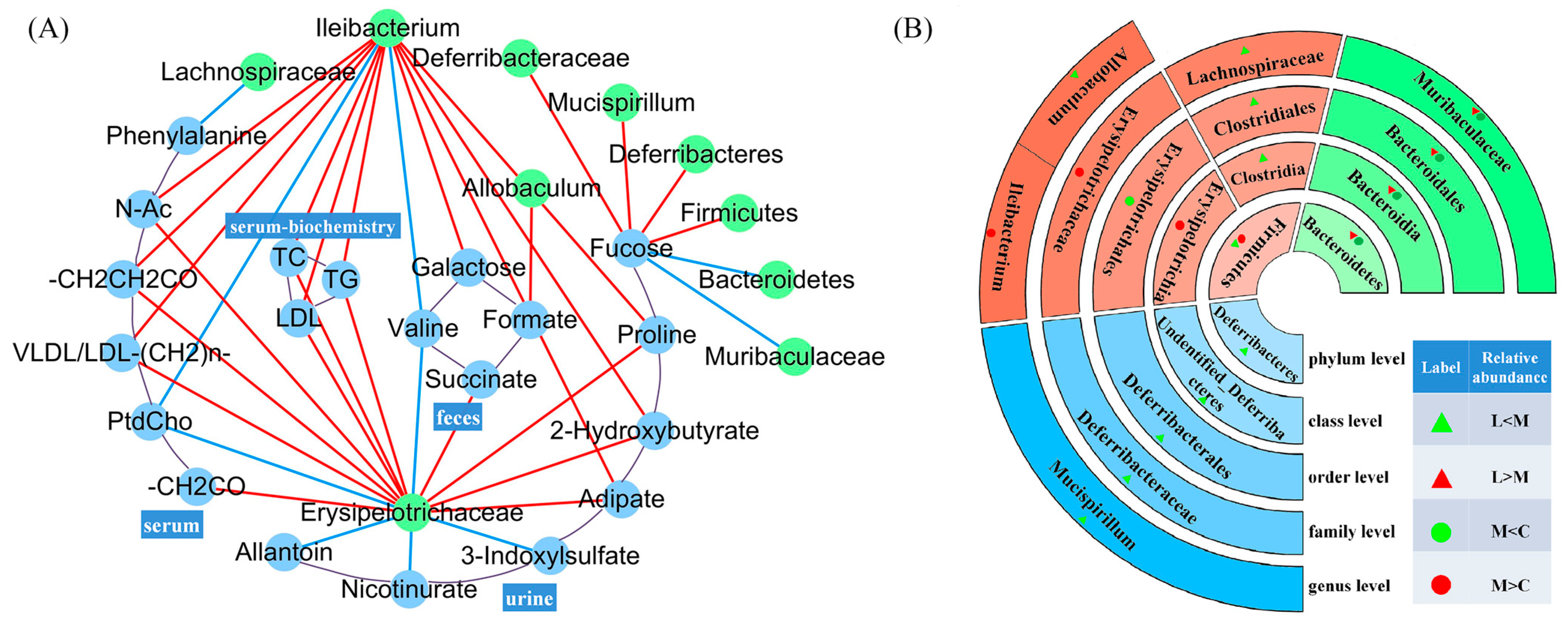
3.4. Correlation Between Metabolites and Intestinal Microbiota
4. Discussion
4.1. L. casei CAAS36 Treatment Induced Changes in Lipid Metabolism of HFD-Fed Hamsters
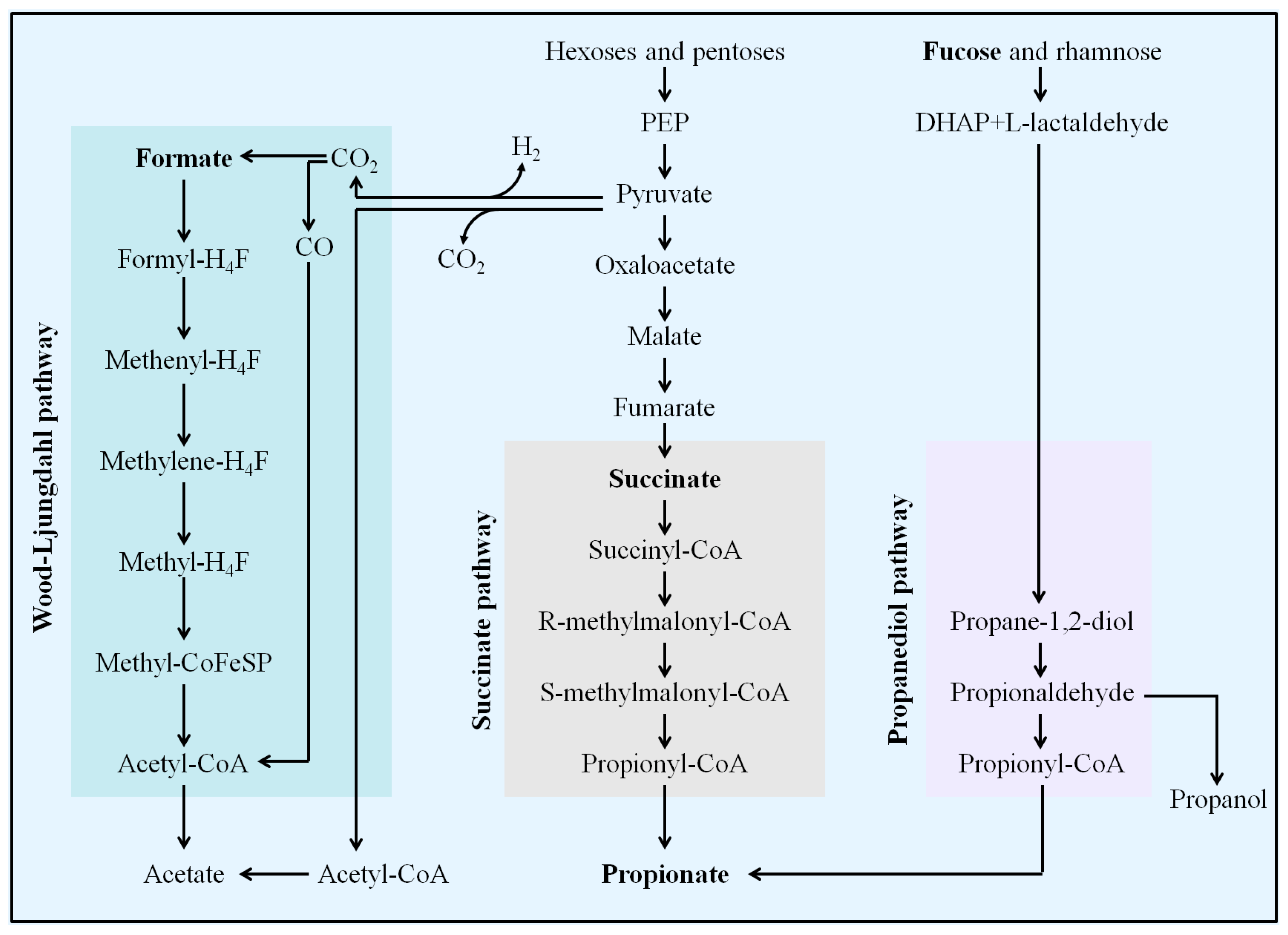
4.2. L. casei CAAS36 Treatment Induced Changes in SCFAs Metabolism of HFD-Fed Hamsters
4.3. L. casei CAAS36 Treatment Induced Changes in Proteolytic and Other Metabolites of HFD-Fed Hamsters
4.4. L. casei CAAS36 Treatment Induced Changes in Gut Microbiota Structure of HFD-Fed Hamsters and Correlation of Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fredrickson, D.S.; Levy, R.I.; National Heart and Lung Institute. Office of Heart Information. Hyperlipoproteinemia [Types] I, II, III, IV, V: [Diagnosis and Treatment]; National Institutes of Health: Bethesda, MD, USA, 1970; 16p. [Google Scholar]
- Lai, M.; Peng, H.; Wu, X.; Chen, X.; Wang, B.; Su, X. IL-38 in modulating hyperlipidemia and its related cardiovascular diseases. Int. Immunopharmacol. 2022, 108, 108876. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cheng, Y.; Zhang, G.; Wang, B. Novel insights into the pathological mechanisms of metabolic related dyslipidemia. Mol. Biol. Rep. 2021, 48, 5675–5687. [Google Scholar] [CrossRef]
- Gisterå, A.; Ketelhuth, D.F.J. Lipid-driven immunometabolic responses in atherosclerosis. Curr. Opin. Lipidol. 2018, 29, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xie, X.; Liao, W.; Chen, S.; Zhong, R.; Qin, J.; He, P.; Xie, J. Ferroptosis in cardiovascular disease. Biomed. Pharmacother. 2024, 170, 116057. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef]
- Wang, J.K.; Li, Y.; Zhao, X.L.; Liu, Y.B.; Tan, J.; Xing, Y.Y.; Adi, D.; Wang, Y.T.; Fu, Z.Y.; Ma, Y.T.; et al. Ablation of Plasma Prekallikrein Decreases Low-Density Lipoprotein Cholesterol by Stabilizing Low-Density Lipoprotein Receptor and Protects Against Atherosclerosis. Circulation 2022, 145, 675–687. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Gómez-Díaz, R.A.; Corral, P. New Therapies for Primary Hyperlipidemia. J. Clin. Endocrinol. Metab. 2021, 107, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Neverovskyi, A.; Chernyavskyi, V.; Shypulin, V.; Hvozdetska, L.; Tishchenko, V.; Nechypurenko, T.; Mikhn Ova, N. Probiotic Lactobacillus plantarum may reduce cardiovascular risk: An experimental study. ARYA Atheroscler. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Tarrah, A.; Dos Santos Cruz, B.C.; Sousa Dias, R.; da Silva Duarte, V.; Pakroo, S.; Licursi de Oliveira, L.; Gouveia Peluzio, M.C.; Corich, V.; Giacomini, A.; Oliveira de Paula, S. Lactobacillus paracasei DTA81, a cholesterol-lowering strain having immunomodulatory activity, reveals gut microbiota regulation capability in BALB/c mice receiving high-fat diet. J. Appl. Microbiol. 2021, 131, 1942–1957. [Google Scholar] [CrossRef]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, T.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. Gut Microbiota and Coronary Artery Disease: Current Therapeutic Perspectives. Metabolites 2023, 13, 256. [Google Scholar] [CrossRef]
- Zafar, H.; Ain, N.U.; Alshammari, A.; Alghamdi, S.; Raja, H.; Ali, A.; Siddique, A.; Tahir, S.D.; Akbar, S.; Arif, M.; et al. Lacticaseibacillus rhamnosus FM9 and Limosilactobacillus fermentum Y57 Are as Effective as Statins at Improving Blood Lipid Profile in High Cholesterol, High-Fat Diet Model in Male Wistar Rats. Nutrients 2022, 14, 1654. [Google Scholar] [CrossRef] [PubMed]
- Romão da Silva, L.F.; de Oliveira, Y.; de Souza, E.L.; de Luna Freire, M.O.; Braga, V.A.; Magnani, M.; de Brito Alves, J.L. Effects of probiotic therapy on cardio-metabolic parameters and autonomic modulation in hypertensive women: A randomized, triple-blind, placebo-controlled trial. Food Funct. 2020, 11, 7152–7163. [Google Scholar] [CrossRef]
- Lin, S.Y.; Ayres, J.W.; Winkler, W., Jr.; Sandine, W.E. Lactobacillus effects on cholesterol: In vitro and in vivo results. J. Dairy Sci. 1989, 72, 2885–2899. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Kazemi, A.; UP Bartolomaeus, T.; Martami, F.; Noormohammadi, M.; Salari, A.; Löber, U.; Balou, H.A.; Forslund, S.K.; Mahdavi-Roshan, M. The effect of probiotic and synbiotic supplementation on lipid parameters among patients with cardiometabolic risk factors: A systematic review and meta-analysis of clinical trials. Cardiovasc. Res. 2023, 119, 933–956. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Qin, L.; Wu, L. Effects of probiotic administration on overweight or obese children: A meta-analysis and systematic review. J. Transl. Med. 2023, 21, 525. [Google Scholar] [CrossRef]
- Mirjalili, M.; Salari Sharif, A.; Sangouni, A.A.; Emtiazi, H.; Mozaffari-Khosravi, H. Effect of probiotic yogurt consumption on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled trial. Clin. Nutr. ESPEN 2023, 54, 144–149. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Muller, E.; Algavi, Y.M.; Borenstein, E. The gut microbiome-metabolome dataset collection: A curated resource for integrative meta-analysis. npj Biofilms Microbiomes 2022, 8, 79. [Google Scholar] [CrossRef]
- Ma, C. Effect and Mechanism of Lactobacillus Casei on Intestinal Flora and Lipid Metabolism of High-Fat Diet Hamsters. Ph.D. Dissertation, Institute of Food Science and Technology, Chinese Academy of Agricultural Science, Beijing, China, 2020. (In Chinese). [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Danielson, A.D.; Peo, E.R., Jr.; Shahani, K.M.; Lewis, A.J.; Whalen, P.J.; Amer, M.A. Anticholesteremic property of Lactobacillus acidophilus yogurt fed to mature boars. J. Anim. Sci. 1989, 67, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Khallou, J.; Riottot, M.; Parquet, M.; Verneau, C.; Lutton, C. Biodynamics of cholesterol and bile acids in the lithiasic hamster. Br. J. Nutr. 1991, 66, 479–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singhal, A.K.; Finver-Sadowsky, J.; McSherry, C.K.; Mosbach, E.H. Effect of cholesterol and bile acids on the regulation of cholesterol metabolism in hamster. Biochim. Biophys. Acta 1983, 752, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K.; Dietschy, J.M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 1983, 24, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Cuthbert, J.A.; Spady, D.K. Regulation of hepatic 7 alpha-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J. Biol. Chem. 1995, 270, 5381–5387. [Google Scholar] [CrossRef]
- Dong, Z.; Shi, H.; Zhao, M.; Zhang, X.; Huang, W.; Wang, Y.; Zheng, L.; Xian, X.; Liu, G. Loss of LCAT activity in the golden Syrian hamster elicits pro-atherogenic dyslipidemia and enhanced atherosclerosis. Metabolism 2018, 83, 245–255. [Google Scholar] [CrossRef]
- Li, T.; Sun, S.; Zhang, J.; Qu, K.; Yang, L.; Ma, C.; Jin, X.; Zhu, H.; Wang, Y. Beneficial Metabolic Effects of 2′,3′,5′-Triacetyl-N(6)-(3-hydroxylaniline) adenosine in Multiple Biological Matrices and Intestinal Flora of Hyperlipidemic Hamsters. J. Proteome Res. 2018, 17, 2870–2879. [Google Scholar] [CrossRef]
- Du, J.; Huang, P.; Qian, Y.; Yang, X.; Cui, S.; Lin, Y.; Gao, C.; Zhang, P.; He, Y.; Xiao, Q.; et al. Fecal and Blood Microbial 16s rRNA Gene Alterations in Chinese Patients with Multiple System Atrophy and Its Subtypes. J. Park. Dis. 2019, 9, 711–721. [Google Scholar] [CrossRef]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Song, M.; Park, S.; Lee, H.; Min, B.; Jung, S.; Park, S.; Kim, E.; Oh, S. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J. Dairy Sci. 2015, 98, 1492–1501. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Tang, H.R.; Nicholson, J.K.; Lindon, J.C. Use of H-1 NMR-determined diffusion coefficients to characterize lipoprotein fractions in human blood plasma. Magn. Reson. Chem. 2002, 40, S83–S88. [Google Scholar] [CrossRef]
- Tannock, L.R.; De Beer, M.C.; Ji, A.L.; Shridas, P.; Noffsinger, V.P.; den Hartigh, L.; Chait, A.; De Beer, F.C.; Webb, N.R. Serum amyloid A3 is a high density lipoprotein-associated acute-phase protein. J. Lipid Res. 2018, 59, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, S.; Hamaguchi, K.; Seike, M.; Himeno, K.; Sakata, T.; Yoshimatsu, H. Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. Exp. Biol. Med. 2004, 229, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Chen, M.; Huang, Z.R.; Guo, W.L.; Ai, L.Z.; Bai, W.D.; Yu, X.D.; Liu, Y.L.; Rao, P.F.; Ni, L. Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res. Int. 2021, 139, 109956. [Google Scholar] [CrossRef] [PubMed]
- Zartl, B.; Silberbauer, K.; Loeppert, R.; Viernstein, H.; Praznik, W.; Mueller, M. Fermentation of non-digestible raffinose family oligosaccharides and galactomannans by probiotics. Food Funct. 2018, 9, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Tazakori, Z.; Zare, M.; Jafarabadi, M.A. Probiotic yogurt effect on macronutrients ingredients, blood glucose and lipid profile in type 2 diabetes. J. Pak. Med. Assoc. 2017, 67, 1123. [Google Scholar] [PubMed]
- Baggerman, J.O.; Smith, Z.K.; Thompson, A.J.; Kim, J.; Hergenreder, J.E.; Rounds, W.; Johnson, B.J. Chromium propionate supplementation alters animal growth performance, carcass characteristics, and skeletal muscle properties in feedlot steers. Transl. Anim. Sci. 2020, 4, txaa146. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.M.; Li, D.D.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Su, Z.W.; Xuan, Y.; Zhang, K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018, 97, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Douard, V.; Edelblum, K.L.; Laubitz, D.; Zhao, Y.L.; Kiela, P.R.; Yap, G.S.; Gao, N. Paneth Cell Specific Lysozyme Regulates Intestinal Mucosal Immune Response by Shaping Gut Microbiota Landscape. J. Immunol. 2017, 198, 218.13. [Google Scholar] [CrossRef]
- Fu, T.; Huan, T.; Rahman, G.; Zhi, H.; Xu, Z.; Oh, T.G.; Guo, J.; Coulter, S.; Tripathi, A.; Martino, C.; et al. Paired microbiome and metabolome analyses associate bile acid changes with colorectal cancer progression. Cell Rep. 2023, 42, 112997. [Google Scholar] [CrossRef]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, J.; Chen, B.; Zhao, J.; Hu, L.; Huang, K.; Chen, Q.; Yao, J.; Lin, G.; Bao, L.; et al. The enrichment of the gut microbiota Lachnoclostridium is associated with the presence of intratumoral tertiary lymphoid structures in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1289753. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Y.; Huang, F.Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B.; et al. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. npj Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef]
- Liu, S.; Qin, P.; Wang, J. High-Fat Diet Alters the Intestinal Microbiota in Streptozotocin-Induced Type 2 Diabetic Mice. Microorganisms 2019, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, E.; Young, W.; Rosendale, D.; Reichert-Grimm, V.; Riedel, C.U.; Conrad, R.; Egert, M. RNA-Based Stable Isotope Probing Suggests Allobaculum spp. as Particularly Active Glucose Assimilators in a Complex Murine Microbiota Cultured In Vitro. Biomed. Res. Int. 2017, 2017, 1829685. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Mattijssen, F.; Alex, S.; Swarts, H.J.; Groen, A.K.; van Schothorst, E.M.; Kersten, S. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Mol. Metab. 2014, 3, 135–144. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Mattijssen, F.; de Wit, N.J.; Georgiadi, A.; Hooiveld, G.J.; van der Meer, R.; He, Y.; Qi, L.; Köster, A.; Tamsma, J.T.; et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010, 12, 580–592. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Pottanat, T.G.; Siegel, R.W.; Ehsani, M.; Qian, Y.W.; Roell, W.C.; Konrad, R.J. Angiopoietin-like protein 4(E40K) and ANGPTL4/8 complex have reduced, temperature-dependent LPL-inhibitory activity compared to ANGPTL4. Biochem. Biophys. Res. Commun. 2021, 534, 498–503. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum Involves in the Modulation of Intestinal ANGPTLT4 Expression in Mice Treated by High-Fat Diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.F.; Katiraei, S.; Bartosinska, B.; Eberhard, D.; Willems van Dijk, K.; Kersten, S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 2018, 61, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ou, Y.; Guo, Y.; Chen, M.; Lu, X.; Guo, Z.; Zheng, B. Gut microbiome-serum metabolic profiles: Insight into the hypoglycemic effect of Porphyra haitanensis glycoprotein on hyperglycemic mice. Food Funct. 2023, 14, 7977–7991. [Google Scholar] [CrossRef]
- Xiao, H.; Sun, X.; Lin, Z.; Yang, Y.; Zhang, M.; Xu, Z.; Liu, P.; Liu, Z.; Huang, H. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pan, Y.; Shao, W.; Wang, C.; Wang, R.; He, Y.; Zhang, M.; Wang, Y.; Li, T.; Wang, Z.; et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome 2022, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Palatinszky, M.; Pereira, F.C.; Nguyen, J.; Fernandez, V.I.; Mueller, A.J.; Menolascina, F.; Daims, H.; Berry, D.; Wagner, M.; et al. An automated Raman-based platform for the sorting of live cells by functional properties. Nat. Microbiol. 2019, 4, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhao, Y.; Huang, L.; Liu, J.; Wang, K.; Gao, X.; Zhao, X.; Wang, X. Remodeling of the gut microbiome by Lactobacillus johnsonii alleviates the development of acute myocardial infarction. Front. Microbiol. 2023, 14, 1140498. [Google Scholar] [CrossRef]
- Ke, W.; Flay, K.J.; Huang, X.; Hu, X.; Chen, F.; Li, C.; Yang, D.A. Polysaccharides from Platycodon grandiflorus attenuates high-fat diet induced obesity in mice through targeting gut microbiota. Biomed. Pharmacother. 2023, 166, 115318. [Google Scholar] [CrossRef] [PubMed]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota. mSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, E.J.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kim, J.Y.; Lee, D.; Kang, J.; Lee, H.J. Lactiplantibacillusplantarum ATG-K2 Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 12665. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Xu, C.; Zheng, M.; Zhang, S.; Liu, Q.; Lyu, J.; Pang, X.; Wang, Y. Utilizing Lactic Acid Bacteria to Improve Hyperlipidemia: A Comprehensive Analysis from Gut Microbiota to Metabolic Pathways. Foods 2024, 13, 4058. https://doi.org/10.3390/foods13244058
Ma C, Xu C, Zheng M, Zhang S, Liu Q, Lyu J, Pang X, Wang Y. Utilizing Lactic Acid Bacteria to Improve Hyperlipidemia: A Comprehensive Analysis from Gut Microbiota to Metabolic Pathways. Foods. 2024; 13(24):4058. https://doi.org/10.3390/foods13244058
Chicago/Turabian StyleMa, Changlu, Chen Xu, Mumin Zheng, Shuwen Zhang, Qifeng Liu, Jiaping Lyu, Xiaoyang Pang, and Yinghong Wang. 2024. "Utilizing Lactic Acid Bacteria to Improve Hyperlipidemia: A Comprehensive Analysis from Gut Microbiota to Metabolic Pathways" Foods 13, no. 24: 4058. https://doi.org/10.3390/foods13244058
APA StyleMa, C., Xu, C., Zheng, M., Zhang, S., Liu, Q., Lyu, J., Pang, X., & Wang, Y. (2024). Utilizing Lactic Acid Bacteria to Improve Hyperlipidemia: A Comprehensive Analysis from Gut Microbiota to Metabolic Pathways. Foods, 13(24), 4058. https://doi.org/10.3390/foods13244058







