Efficacy of Hovenia dulcis Fruit Extract in Hangover Mitigation: Double-Blind Randomized Clinical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Inclusion/Exclusion Criteria
2.2. Study Design
2.3. Interventions
2.4. Outcome Measures
2.4.1. Acute Hangover Scale (AHS)
2.4.2. Alcohol and Acetaldehyde Analysis During Blood Sample Handling and Collection
2.4.3. Analysis of Alcohol and Acetaldehyde in Blood
2.5. Safety Assessments
2.6. ADH and ALDH Enzyme Activity Assay
2.7. Statistical Analysis
2.7.1. Validation Variables
2.7.2. Safety Evaluation Variables
3. Results and Discussion
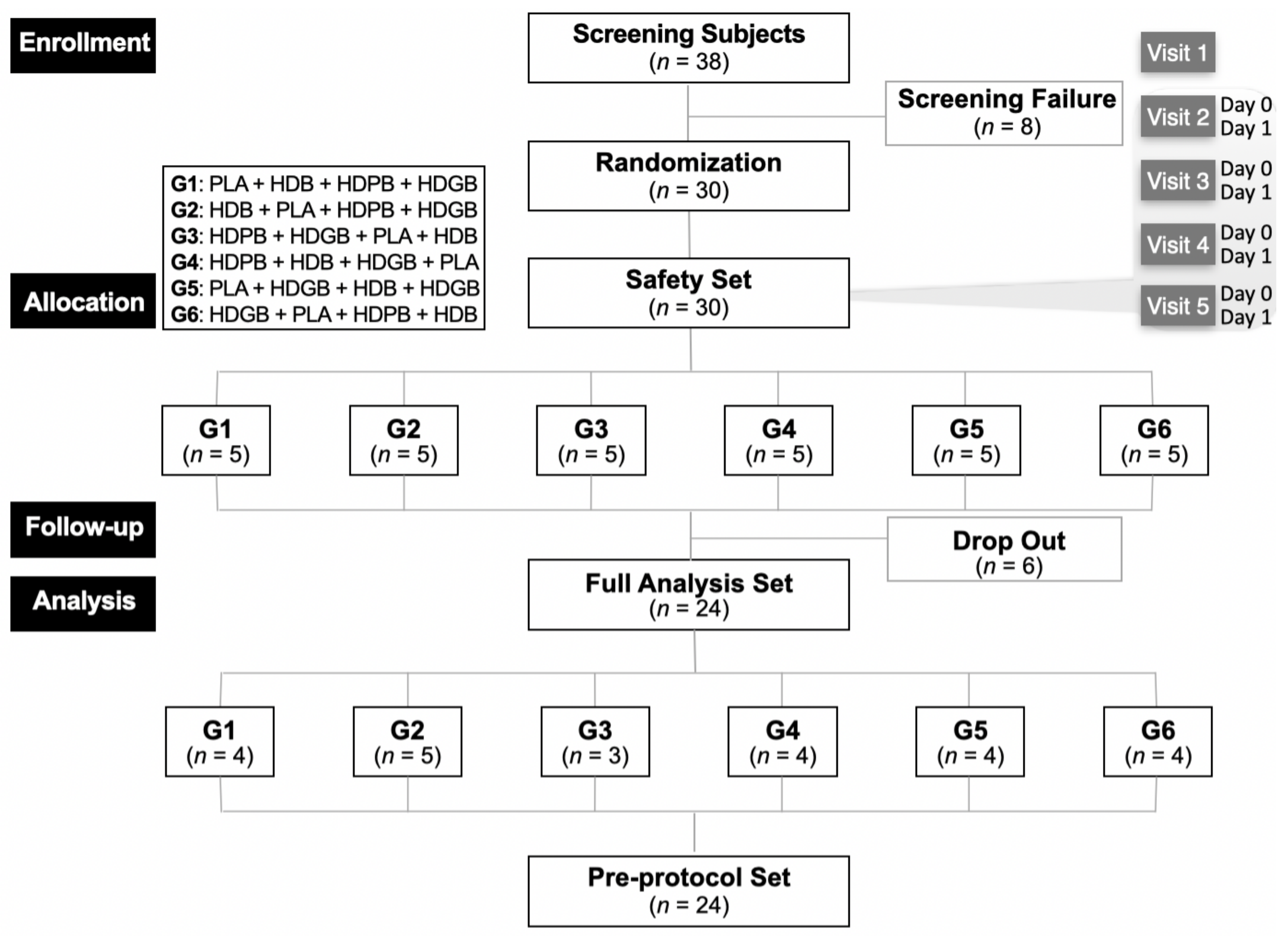
3.1. Enrollment
3.2. General Participant Characteristics
3.3. Clinical Pathology Assessment for Hangover Relief
3.4. Survey of the Symptoms of a Hangover
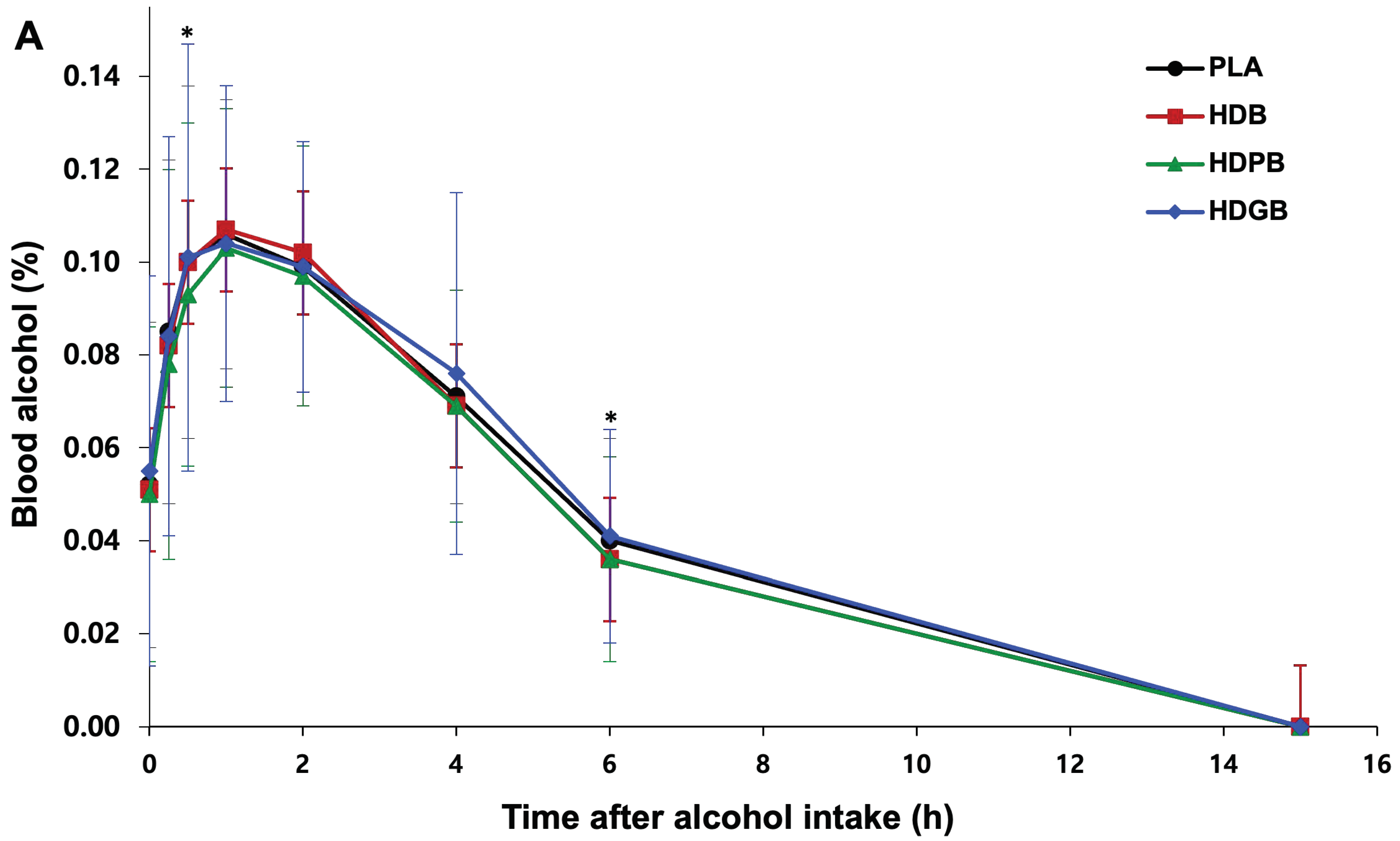
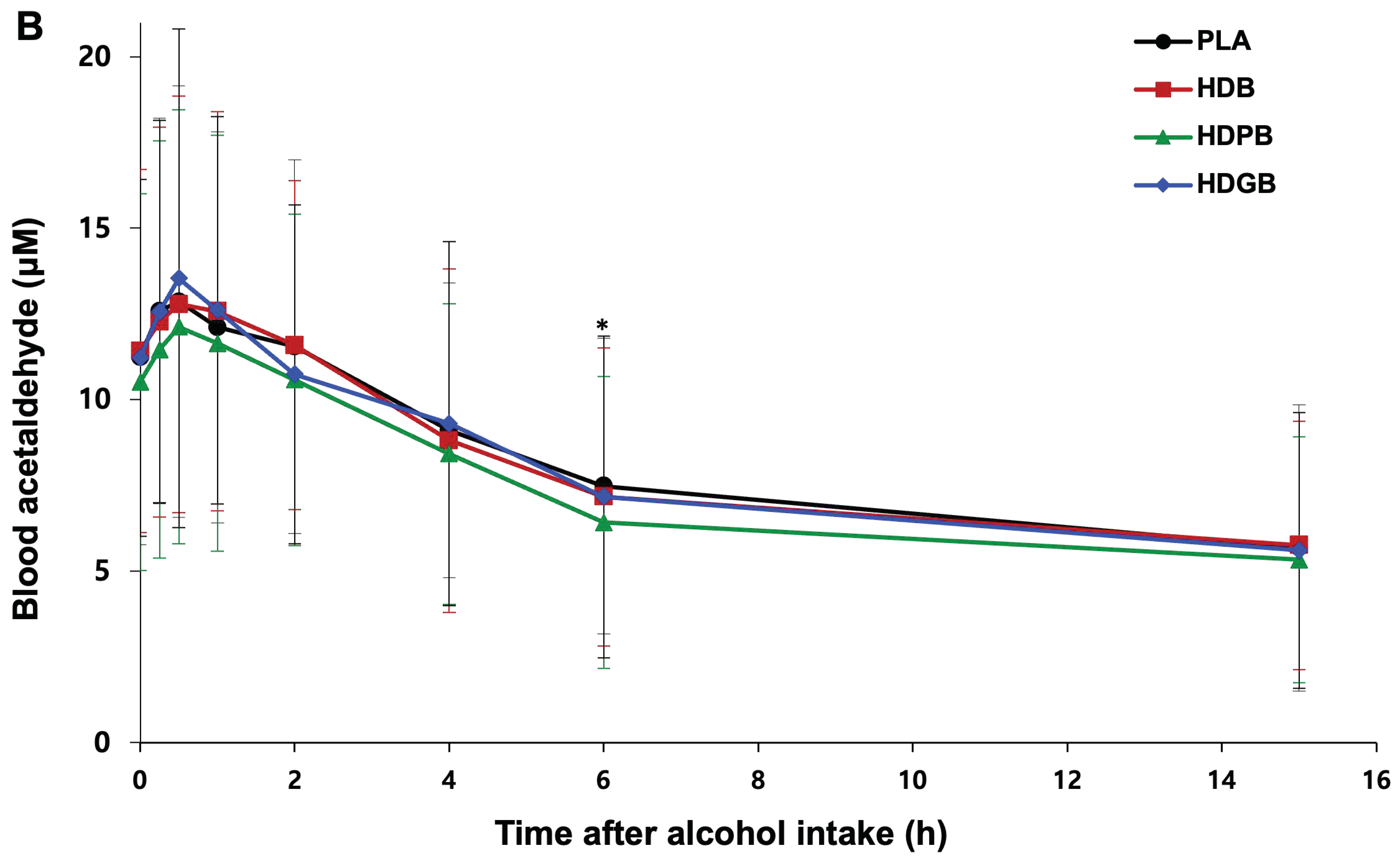
3.5. Changes in Blood Alcohol and Acetaldehyde Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Schrojenstein Lantman, M.; van de Loo, J.A.E.; Mackus, M.; Verster, J.C. Development of a definition for the alcohol hangover: Consumer descriptions and expert consensus. Curr. Drug Abuse Rev. 2016, 9, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Scholey, A.; van de Loo, A.J.A.E.; Benson, S.; Stock, A.-K. Updating the definition of the alcohol hangover. J. Clin. Med. 2020, 9, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Nutt, P.D.; Liu, Y.-C.; Chen, M.-S.; Wang, D.-L.; Palmer, E.; Tyacke, R. Alcohol hangover: The health impact with a historic and Chinese perspective. Drug Sci. Policy Law 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Bereda, G. Mental and physical symptoms of alcohol hangover. Neuropsychopharmacol. Disord. 2022, 1, 1–3. [Google Scholar] [CrossRef]
- Penning, R.; van Nuland, M.; Fliervoet, L.A.; Olivier, B.; Verster, J.C. The pathology of alcohol hangover. Curr. Drug Abuse Rev. 2010, 3, 68–75. [Google Scholar] [CrossRef]
- Sin, H.J.; Choung, S.Y.; Kang, S.; Kwon, H.T.; Kim, B.H. Anti-alcohol and anti-aldehyde hangover effect of aldehyde dehydrogenase related compounds in rat. J. Environ. Health Sci. 2023, 49, 99–107. [Google Scholar] [CrossRef]
- Yun, M.-K.; Jeong, H.C.; Lee, S.-J.; Lee, S.J. Effect of Lactobacillus fermented garlic extract powder on alcohol and acetaldehyde metabolism. J. Korean Soc. Food Sci. Nutr. 2023, 52, 357–362. [Google Scholar] [CrossRef]
- Tsai, J.; Ford, E.S.; Li, C.; Zhao, G. Past and current alcohol consumption patterns and elevations in serum hepatic enzymes among US adults. Alcohol. Behav. 2012, 37, 78–84. [Google Scholar] [CrossRef]
- Nivukoski, U.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O. Liver enzymes in alcohol consumers with or without binge drinking. Alcohol 2019, 78, 13–19. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, H.; Hong, K.-B.; Suh, H.J.; Ahn, Y. Hangover-relieving effect of ginseng berry kombucha fermented by Saccharomyces cerevisiae and Gluconobacter oxydans in ethanol-treated cells and mice model. Antioxidants 2023, 12, 774. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, Y.H.; Lee, E.K.; Park, S.-D.; Shim, J.-J.; Lee, J.-L.; Yoo, H.H. Effects of plant-based extract mixture on alcohol metabolism and hangover improvement in humans: A randomized, double-blind, paralleled, placebo-controlled clinical trial. J. Clin. Med. 2023, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, H.D.; Yu, S.N.; Kim, S.H.; Park, S.K.; Ahn, S.C. Biological activities of Mesembryanthemum crystallinum (ice plant) extract. J. Life Sci. 2015, 25, 638–645. [Google Scholar] [CrossRef]
- Yamazaki, T.; Hosono, T.; Matsushita, Y.; Kawashima, K.; Someya, M.; Nakajima, Y.; Narui, K.; Hibi, Y.; Ishizaki, M.; Kinjo, J. Pharmacological studies on Puerariae Flos. IV: Effects of Pueraria thomsonii dried flower extracts on blood ethanol and acetaldehyde levels in humans. Int. J. Clin. Pharmacol. Res. 2002, 22, 23–28. [Google Scholar] [PubMed]
- Song, G.; Han, H.; Park, S.; Sa, S.; Chung, W.; Lee, B.Y. Effects of GSH on alcohol metabolism and hangover improvement in humans: A randomized double-blind placebo-controlled crossover clinical trial. Foods 2024, 16, 3262–3275. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kim, G.-H.; Seong, B.-J.; Kim, H.-H.; Kim, M.-Y.; Kim, M.-R. Effects of aqueous medicinal herb extracts and aqueous fermented extracts on alcohol-metabolizing enzyme activities. Food Sci. Preserv. 2009, 16, 259–265. [Google Scholar]
- Sung-Hee, K.; Jin-Yeul, M. A study on the extraction and efficacy of bioactive compound from Hovenia dulcis. Korean J. Pharm. 2006, 21, 11–15. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Park, Y.; Ryu, S.; Yeon, S.; Turk, A.; Lee, H.; Hwang, B.; Lee, M. Quantitation of antioxidant and α-glucosidase inhibitory flavonoids in the fruits, fruit stalks and seeds of Hovenia dulcis. J. Pharm. 2023, 54, 242–248. [Google Scholar] [CrossRef]
- Kong, L.; Li, J.; Zhang, X. Prevention and cure of acute alcohol intoxication in mice by administration of compound of Japanese raisintree fruit, lobed kudzuvine flower bud and lightyellow sophora root. Adv. Sci. Technol. 2014, 15, 874–880. [Google Scholar]
- Fang, H.-L.; Lin, H.-Y.; Chan, M.-C.; Lin, W.-L.; Lin, W.-C. Treatment of chronic liver injuries in mice by oral administration of ethanolic extract of the fruit of Hovenia dulcis. Asian J. Clin. Med. 2007, 35, 693–703. [Google Scholar] [CrossRef]
- Choi, R.-Y.; Woo, M.-J.; Ham, J.R.; Lee, M.-K. Anti-steatotic and anti-inflammatory effects of Hovenia dulcis Thunb. extracts in chronic alcohol-fed rats. Biochem. Pharmacol. 2017, 90, 393–401. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.J.; Jeong, H.Y.; Kim, J.Y.; Choi, E.-K.; Chae, S.W.; Kwon, O. A standardized extract of the fruit of Hovenia dulcis alleviated alcohol-induced hangover in healthy subjects with heterozygous ALDH2: A randomized, controlled, crossover trial. J. Ethnopharmacol. 2017, 209, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhong, G.; Li, A.; Li, S.; Wu, L. Influence of Hovenia dulcis on alcohol concentration in blood and activity of alcohol dehydrogenase (ADH) of animals after drinking. Zhongguo Zhong Yao Za Zhi 2006, 31, 1094–1096. [Google Scholar] [PubMed]
- Na, C.-S.; Yoon, S.Y.; Kim, J.B.; Na, D.-S.; Dong, M.-S.; Lee, M.-Y.; Hong, C.Y. Anti-fatigue activity of Hovenia dulcis on a swimming mouse model through the inhibition of stress hormone expression and antioxidation. Asian J. Clin. Med. 2013, 41, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, Y.; Chen, S.; Ma, W.; Xu, X.; Hu, S.; Jin, L.; Sun, J.; Mao, J.; Shen, C. The positive influence of polyphenols extracted from Pueraria lobata root on the gut microbiota and its antioxidant capability. Front. Nutr. 2022, 9, 868188–868200. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Effects of puerarin on the prevention and treatment of cardiovascular diseases. Front. Pharmacol. 2021, 12, 771793–771800. [Google Scholar] [CrossRef]
- Gao, E.; Wang, W.; Huang, Y.; Luo, Z.; Chen, B.; Xiao, S.; Li, D. Puerariae lobatae Radix: Progress in extraction, separation methods and pharmacological activities research. Separations 2024, 11, 195–210. [Google Scholar] [CrossRef]
- Li, Z.; Cao, W.; Zhang, Y.; Lai, S.; Ye, Y.; Bao, J.; Fu, A. Puerarin ameliorates non-alcoholic fatty liver disease by inhibiting lipid metabolism through FMO5. Front. Pharmacol. 2024, 15, 1423634–1423645. [Google Scholar] [CrossRef]
- Gao, R.; Huang, Q.; Zeng, Y.; Chen, D.; Jia, Z.; Han, B.; Huang, X.; Wang, Q.; Hu, X.; Liao, M.; et al. Pueraria lobata–Prunus mume complex alleviates alcoholic liver disease by regulating lipid metabolism and inhibiting inflammation: A transcriptome and gut microbiota analysis. Foods 2024, 13, 2431–2440. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Li, X.; Liu, Y.; Chen, F. Antioxidant properties of glutathione-enriched yeast extract in vitro and in vivo. Food Chem. 2022, 370, 130978–130985. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Park, J.; Choi, H.; Kim, S. Immunomodulatory effects of glutathione-enriched yeast extract on macrophage activation. J. Funct. Foods 2023, 98, 105295. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, L.; Li, Y.; Zhao, Y. Neuroprotective effects of glutathione-enriched yeast extract against oxidative stress-induced neuronal damage. Neurochem. Int. 2024, 152, 105276–105285. [Google Scholar]
- Liu, L.; Zhu, S.; Zhang, Y.; Zhu, Z.; Xue, Y.; Liu, X. Hovenia dulcis fruit peduncle polysaccharides reduce intestinal dysbiosis and hepatic fatty acid metabolism disorders in alcohol-exposed mice. Foods 2024, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, W.; Zhang, H.; Chen, F.; Liu, Y. Protective effects of glutathione-enriched yeast extract on alcohol-induced liver injury in rats. Food Funct. 2023, 14, 4567–4578. [Google Scholar]
- Chang, A.-K.; Kim, S.-H. Predictors of weight-control behavior in healthy weight and overweight Korean middle-aged women. Int. J. Environ. Res. Public Health 2022, 19, 7546–7555. [Google Scholar] [CrossRef]
- Kinoshita, T.; Shinoda, M.; Nishizaki, Y.; Shiraki, K.; Hirai, Y.; Kichikawa, Y.; Tsushima, K.; Shinkai, M.; Komura, N.; Yoshida, K.; et al. A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study). BMC Med. 2022, 20, 342–350. [Google Scholar] [CrossRef]
- Patterson, E.; Tan, H.T.T.; Groeger, D.; Andrews, M.; Buckley, M.; Murphy, E.F.; Groeger, J.A. Bifidobacterium longum 1714 improves sleep quality and aspects of well-being in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2024, 14, 3725–3735. [Google Scholar] [CrossRef]
- Efird, J. Blocked randomization with randomly selected block sizes. Int. J. Environ. Res. Public Health 2011, 8, 15–20. [Google Scholar] [CrossRef]
- Rohsenow, D.J.; Howland, J.; Minsky, S.J.; Greece, J.; Almeida, A.; Roehrs, T.A. The acute hangover scale: A new measure of immediate hangover symptoms. Addict. Behav. 2007, 32, 1314–1320. [Google Scholar] [CrossRef]
- Thiel, A.; Rümbeli, R.; Mair, P.; Yeman, H.; Beilstein, P. 3-NOP: ADME studies in rats and ruminating animals. Food Chem. Toxicol. 2019, 125, 528–539. [Google Scholar] [CrossRef]
- Wankhede, S.; Mohan, V.; Thakurdesai, P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: A randomized controlled pilot study. J. Sport Health Sci. 2016, 5, 176–182. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.P.; Park, E.O.; Jung, S.J.; Chae, S.W.; Park, Y.S. Alleviation of hangover effects by DA-5521: A randomized, double-blind, placebo-controlled, crossover trial. Food Eng. Prog. 2024, 28, 20–30. [Google Scholar] [CrossRef]
- Lu, C.L.; Yang, L.Q.; Liu, X.H.; Jin, X.Y.; Wang, F.X.; Friedemann, T.; Robinson, N.; Schröder, S.; Lu, H.Z.; Liu, J.P. Chinese herbal medicine Shufeng Jiedu Capsule for patients with mild to moderate coronavirus disease 2019 (COVID-19): Protocol for a randomized, blinded, placebo control trial. Eur. J. Integr. Med. 2023, 62, 102286–102295. [Google Scholar] [CrossRef]
- Tripepi, G.; Chesnaye, N.C.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology 2020, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.U.; Lee, J.H.; Song, Y.J.; Kim, K.R.; Kang, M.J.; Choi, S.H.; Lee, J.S.; Lee, Y.J.; Kim, Y.I.; Jung, Y.C. A randomized, multicenter, treatment-as-usual controlled clinical trial to evaluate the safety and efficacy of digital cognitive behavioral therapy for eating disorders in South Korea: Study protocol. Res. Sq. 2024, 1, 1–10. [Google Scholar] [CrossRef]
- Seppä, K.; Sillanaukee, P. Binge drinking and ambulatory blood pressure. Hypertens 1999, 33, 79–82. [Google Scholar] [CrossRef]
- Kawano, Y.; Abe, H.; Kojima, S.; Ashida, T.; Yoshida, K.; Imanishi, M.; Yoshimi, H.; Kimura, G.; Kuramochi, M.; Omae, T. Acute depressor effect of alcohol in patients with essential hypertension. Hypertens 1992, 20, 219–226. [Google Scholar] [CrossRef]
- Penning, R.; McKinney, A.; Verster, J.C. Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol Alcohol. 2012, 47, 248–252. [Google Scholar] [CrossRef]
- Verster, J.C.; Severeijns, N.R.; Sips, A.S.; Saeed, H.M.; Benson, S.; Scholey, A.; Bruce, G. Alcohol hangover across the lifespan: Impact of sex and age. Alcohol Alcohol. 2021, 50, 589–598. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, H.; Jones, A.W.; Wang, F.; Zhang, Y.; Rao, Y. Evaluation and review of ways to differentiate sources of ethanol in postmortem blood. Int. J. Legal Med. 2020, 134, 2081–2093. [Google Scholar] [CrossRef]
- Mackus, M.; Van de Loo, A.J.A.E.; Garssen, J.; Kraneveld, A.D.; Verster, J.C. The association between ethanol elimination rate and hangover severity. Int. J. Environ. Res. Public Health 2020, 17, 4324–4332. [Google Scholar] [CrossRef]
- Jamal, M.; Ameno, K.; Tanaka, N.; Ito, A.; Takakura, A.; Kumihashi, M.; Kinoshita, H. Ethanol and acetaldehyde after intraperitoneal administration to Aldh2-knockout mice-reflection in blood and brain levels. Neurochem. Res. 2016, 41, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Xu, G.; Paik, D.H.; Shim, Y.Y.; Reaney, M.J.T.; Park, I.; Lee, S.-H.; Park, J.-Y.; Park, E.; Lee, S.-B.; et al. Clinical evaluation of Hovenia dulcis extract combinations for effective hangover relief in humans. Foods 2024, 13, 4021. [Google Scholar] [CrossRef]
- Peana, A.T.; Sánchez-Catalán, M.J.; Hipólito, L.; Rosas, M.; Porru, S.; Bennardini, F.; Romualdi, P.; Caputi, F.F.; Candeletti, S.; Polache, A.; et al. Mystic acetaldehyde: The never-ending story on alcoholism. Front. Behav. Neurosci. 2017, 11, 81–90. [Google Scholar] [CrossRef]
- Chen, L.; Smith, G.D.; Harbord, R.M.; Lewis, S.J. Alcohol intake and blood pressure: A systematic review implementing a Mendelian randomization approach. PLoS Med. 2008, 5, e52. [Google Scholar] [CrossRef]
- Chen, X.; Dong, X.; Zhu, R.; Xue, Q.; Zhang, D.; Liu, X.; Zheng, L.; Jiang, Y. Abnormally high blood acetaldehyde concentrations suggest potential postmortem ethanol generation. J. Anal. Toxicol. 2021, 45, 748–755. [Google Scholar] [CrossRef]
- Ialongo, C. Blood alcohol concentration in the clinical laboratory: A narrative review of the preanalytical phase in diagnostic and forensic testing. Biochem. Med. 2024, 34, 10501. [Google Scholar] [CrossRef]
- Thomes, P.G.; Rasineni, K.; Saraswathi, V.; Kharbanda, K.K.; Clemens, D.L.; Sweeney, S.A.; Kubik, J.L.; Donohue, T.M., Jr.; Casey, C.A. Natural recovery by the liver and other organs after chronic alcohol use. Alcohol Res. Curr. Rev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.S.; Sim, J.; Seo, S.; Seo, W. Alcoholic liver disease: A new insight into the pathogenesis of liver disease. Arch. Pharm. Res. 2022, 45, 447–459. [Google Scholar] [CrossRef]
- Guo, R.; Ren, J. Alcohol and acetaldehyde in public health: From marvel to menace. Int. J. Environ. Res. Public Health 2010, 7, 1285–1301. [Google Scholar] [CrossRef]
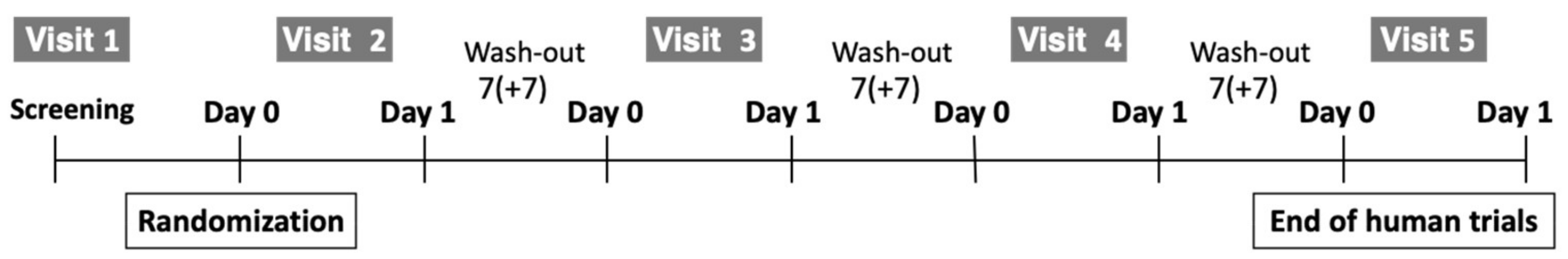
| Period | Screening 1 | Active Treatment 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit | 1 | 2 | 3 | 4 | 5 | |||||
| Day | −14 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | |
| Window Period (Day) 3 | +7 | +7 | +7 | |||||||
| Written consent | ✓ | |||||||||
| Demographics 4 | ✓ | |||||||||
| Lifestyle research 5 | ✓ | |||||||||
| Medical and surgical history 6 | ✓ | ✓ | ||||||||
| Medication history, non-medication history 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Physical examination | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Vital signs (blood pressure, pulse) 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Body instrumentation 9 | Height and BMI | ✓ | ||||||||
| Weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Clinical pathology 10 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Pregnancy reaction test 10 | ✓ | |||||||||
| Alcohol degradation genetic testing 11 | ✓ | |||||||||
| Breath alcohol test 12 | ✓ | ✓ | ✓ | ✓ | ||||||
| Drinking habits survey | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Validity evaluation | Blood Alcohol, acetaldehyde concentrations 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Alcohol Hangover Scale (AHS) 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Evaluating human subject suitability | ✓ | ✓ | ||||||||
| Randomization | ✓ | |||||||||
| Consumption of human investigational foods/alcohol 15 | ✓ | ✓ | ✓ | ✓ | ||||||
| Checking for adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Human subject training 16 | ✓ | ✓ | ✓ | ✓ | ||||||
| Ingredients (g) | Beverage Group Contents (%) | |||
|---|---|---|---|---|
| HDB | HDPB | HDGB | PLA | |
| HD 1 | 0.475 | 0.475 | 0.475 | 0 |
| PL 2 | 0 | 0.1 | 0 | 0 |
| GY 3 | 0 | 0 | 0.02 | 0 |
| Sodium bicarbonate | 0.023 | 0.023 | 0.023 | 0 |
| Vitamin C | 0.020 | 0.020 | 0.020 | 0 |
| Glycine | 0.010 | 0.010 | 0.010 | 0 |
| Flavors | 0.105 | 0.105 | 0.105 | 0.070 |
| Caramel pigment powder | 0 | 0 | 0 | 0.012 |
| Purified water | 99.367 | 99.267 | 99.347 | 99.918 |
| Variables | G1 (n = 4) | G2 (n = 5) | G3 (n = 3) | G4 (n = 4) | G5 (n = 4) | G6 (n = 4) | p-Value 1 | |
|---|---|---|---|---|---|---|---|---|
| Gender n (%) | Male | 3 (75.00) | 3 (60.00) | 1 (33.33) | 2 (50.00) | 2 (50.00) | 1 (25.00) | 0.8946 (F) |
| Female | 1 (25.00) | 2 (40.00) | 2 (66.67) | 2 (50.00) | 2 (50.00) | 3 (75.00) | ||
| Age | Mean ± SD | 30.50 ± 5.26 | 32.80 ± 4.38 | 26.33 ± 1.53 | 28.25 ± 3.30 | 28.25 ± 6.55 | 29.00 ± 2.16 | 0.2075 (K) |
| Min, Max | 26.00, 38.00 | 28.00, 40.00 | 25.00, 28.00 | 24.00, 32.00 | 24.00, 38.00 | 27.00, 32.00 | ||
| Whether you smoke, n (%) | Yes | 1 (25.00) | 2 (40.00) | 1 (33.33) | 1 (25.00) | 1 (25.00) | 0 (0.00) | 0.9525 (F) |
| No | 3 (75.00) | 3 (60.00) | 2 (66.67) | 3 (75.00) | 3 (75.00) | 4 (100.00) | ||
| Smoking amount (cigarette/day) | Mean ± SD | 10.00 | 6.50 ± 4.95 | 7.00 | 1.00 | 5.00 | NS 2 | 0.5252(K) |
| Min, Max | 10.00 | 3.00, 10.00 | 7.00 | 1.00 | 5.00 | NS | ||
| Exercise or not n (%) | No | 1 (25.00) | 2 (40.00) | 0 (0.00) | 1 (25.00) | 1 (25.00) | 0 (0.00) | 0.7085 (F) |
| 1–2 times/week | 3 (75.00) | 1 (20.00) | 1 (33.33) | 1 (25.00) | 1 (25.00) | 3 (75.00) | ||
| 3–4 times/week | 0 (0.00) | 2 (40.00) | 2 (66.67) | 2 (50.00) | 1 (25.00) | 1 (25.00) | ||
| 5–6 times/week | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (25.00) | 0 (0.00) | ||
| Daily | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Total sleep time (h/day) | Mean ± SD | 7.75 ± 0.50 | 7.60 ± 0.89 | 6.67 ± 0.58 | 7.25 ± 0.96 | 7.50 ± 0.58 | 7.50 ± 1.00 | 0.4311(K) |
| Min, Max | 7.00, 8.00 | 6.00, 8.00 | 6.00, 7.00 | 6.00, 8.00 | 7.00, 8.00 | 6.00, 8.00 | ||
| Height (cm) | Mean ± SD | 174.03 ± 11.11 | 169.46 ± 10.83 | 167.43 ± 1.59 | 172.33 ± 9.24 | 175.33 ± 10.56 | 166.63 ± 3.20 | 0.8130 (K) |
| Min, Max | 158.30, 184.30 | 157.30, 183.80 | 165.60, 168.50 | 164.20, 182.50 | 164.20, 184.60 | 164.70, 171.40 | ||
| Weight (kg) | Mean ± SD Min, Max | 66.48 ± 13.79 | 64.26 ± 11.28 | 55.77 ± 1.66 | 68.13 ± 12.18 | 70.38 ± 12.53 | 63.10 ± 6.25 | 0.6024 (A) |
| 48.00, 78.60 | 50.30, 76.60 | 54.20, 57.50 | 57.60, 81.50 | 57.60, 83.90 | 57.20, 69.50 | |||
| Parameters 1 | HDB (n = 27) | HDPB (n = 28) | HDGB (n = 27) | PLA (n = 27) | p-Value 2 |
|---|---|---|---|---|---|
| WBC (103/μL) | 6.2 ± 1.7 | 6.0 ± 1.4 | 6.6 ± 1.8 | 6.3 ± 1.7 | 0.7388 (K) |
| RBC (106/μL) | 4.63 ± 0.46 | 4.6 ± 0.48 | 4.61 ± 0.49 | 4.62 ± 0.47 | 0.9941 (A) |
| Hb (g/dL) | 14.0 ± 1.4 | 14.0 ± 1.5 | 14.0 ± 1.5 | 14.0 ± 1.4 | 1.0000 (A) |
| Hct (%) | 43.5 ± 4.1 | 43 ± 4.1 | 43.5 ± 4.3 | 43.2 ± 4.0 | 0.9962 (A) |
| Platelet (103/μL) | 277 ± 64 | 262 ± 54 | 270 ± 63 | 274 ± 56 | 0.8224 (A) |
| Neutrophil (%) | 45.7 ± 8.4 | 48 ± 8.3 | 47.9 ± 9.4 | 45.3 ± 8.4 | 0.6103 (A) |
| Lymphocyte (%) | 42.8 ± 8.7 | 41.1 ± 9.1 | 41.3 ± 9.5 | 42.9 ± 9.0 | 0.8278 (A) |
| Monocyte (%) | 7.1 ± 1.7 | 7.2 ± 1.7 | 6.6 ± 1.2 | 7.3 ± 1.3 | 0.2959 (K) |
| Eosinophil (%) | 3.8 ± 1.9 | 3.5 ± 1.6 | 3.5 ± 2.2 | 3.8 ± 2.3 | 0.9384 (K) |
| Basophil (%) | 0.64 ± 0.34 | 0.73 ± 0.30 | 0.68 ± 0.34 | 0.63 ± 0.31 | 0.6845 (A) |
| AST (GOT) (U/L) | 19.4 ± 7.3 | 18.8 ± 5.9 | 19.2 ± 7.2 | 17.3 ± 4.8 | 0.6886 (K) |
| ALT (GPT) (U/L) | 19 ± 15 | 17 ± 12 | 15 ± 11 | 15 ± 11 | 0.8195 (K) |
| γ-GTP (U/L) | 23 ± 23 | 21 ± 18 | 21 ± 17 | 23 ± 24 | 0.9917 (K) |
| Total protein (g/dL) | 7.4 ± 0.39 | 7.16 ± 0.29 | 7.22 ± 0.33 | 7.22 ± 0.23 | 0.9486 (K) |
| BUN (mg/dL) | 12.9 ± 2.7 | 12.8 ± 3.1 | 13.4 ± 3.3 | 13.2 ± 2.4 | 0.9703 (K) |
| Creatinine (mg/dL) | 0.81 ± 0.15 | 0.80 ± 0.16 | 0.81 ± 0.14 | 0.78 ± 0.16 | 0.8200 (A) |
| Uric acid (mg/dL) | 5.8 ± 1.1 | 6.0 ± 1.3 | 5.9 ± 1.3 | 5.7 ± 1.3 | 0.7620 (A) |
| ALP (U/L) | 62 ± 22 | 61 ± 25 | 62 ± 22 | 61 ± 22 | 0.9848 (K) |
| Bilirubin (mg/dL) | 0.77 ± 0.32 | 0.72 ± 0.24 | 0.78 ± 0.29 | 0.77 ± 0.29 | 0.9394 (K) |
| Glucose (mg/dL) | 74 ± 5.4 | 76.2 ± 6.6 | 74.6 ± 5.0 | 74.2 ± 5.7 | 0.4531 (A) |
| Total cholesterol (mg/dL) | 201 ± 38 | 197 ± 44 | 193 ± 46 | 201 ± 42 | 0.5444 (K) |
| HDL cholesterol (mg/dL) | 65 ± 14 | 64 ± 12 | 65 ± 14 | 67 ± 14 | 0.8874 (A) |
| LDL cholesterol (mg/dL) | 117 ± 34 | 113 ± 42 | 111 ± 43 | 117 ± 41 | 0.6057 (K) |
| Triglyceride (mg/dL) | 104 ± 37 | 113 ± 46 | 104 ± 41 | 106 ± 36 | 0.8643 (K) |
| Parameters | HDB (n = 27) | HDPB (n = 28) | HDGB (n = 27) | PLA (n = 27) | p-Value 1 | |
|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | Baseline (before ingestion) | 116 ± 13 | 115 ± 11 | 116 ± 13 | 117 ± 13 | 0.9602 (A) |
| 15 h after drinking | 112 ± 12 | 111 ± 12 | 114 ± 11 | 114 ± 12 | ||
| Change from baseline | −4.0 ± 6.9 | −4.0 ± 9.3 | −1.7 ± 8.7 | −2.8 ± 10 | 0.4753 (K) | |
| p-value 2 | 0.0060 ** | 0.0309 * | 0.3416 | 0.1709 | ||
| Diastolic blood pressure (mmHg) | Baseline (before ingestion) | 71 ± 14 | 71 ± 13 | 70 ± 11 | 73 ± 13 | 0.8774 (K) |
| 15 h after drinking | 69 ± 12 | 66 ± 12 | 69 ± 11 | 69 ± 11 | ||
| Change from baseline | −2.6 ± 12.4 | −5.4 ± 11.7 | −0.15 ± 9.12 | −4.0 ± 10.0 | 0.3476 (A) | |
| p-value 2 | 0.2807 | 0.0227 * | 0.9321 | 0.0462 * | ||
| Pulse (Times/min) | Baseline (before ingestion) | 82 ± 12 | 83 ± 12 | 83 ± 11 | 82 ± 10 | 0.9688 (A) |
| 15 h after drinking | 83 ± 14 | 80 ± 12 | 83 ± 15 | 85 ± 13 | ||
| Change from baseline | 1.3 ± 13.4 | −2.0 ± 12.8 | −0.04 ± 10.84 | 3.2 ± 9.3 | 0.4186 (A) | |
| p-value 2 | 0.6299 | 0.4082 | 0.9857 | 0.0888 | ||
| Symptoms | HDB (n = 24) | HDPB (n = 24) | HDGB (n = 24) | PLA (n = 24) | p-Value 1 |
|---|---|---|---|---|---|
| Total score | 3.8 ± 3.3 | 3.6 ± 3.3 | 3.2 ± 3.0 | 3.4 ± 2.7 | 0.3951 |
| Thirst | 2.1 ± 2.0 | 2.2 ± 2.0 | 2.3 ± 2.0 | 2.0 ± 1.9 | 0.7680 |
| Hangover | 0.50 ± 0.72 | 0.54 ± 0.93 | 0.25 ± 0.61 | 0.25 ± 0.61 | 0.1147 |
| Fatigue | 0.92 ± 1.06 | 0.63 ± 0.92 | 0.63 ± 1.17 | 0.75 ± 1.03 | 0.5307 |
| Headache | 0.08 ± 0.28 | 0.17 ± 0.64 | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.4491 |
| Dizziness/Fainting | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3982 |
| Anorexia | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | NA 2 |
| Gastrointestinal disorder | 0.08 ± 0.28 | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.04 ± 0.20 | 0.2645 |
| Nausea | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3982 |
| Heart palpitations | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3982 |
| Variables | Concentration 1 | HDB (n = 24) | HDPB (n = 24) | HDGB (n = 24) | PLA (n = 24) | p-Value 2 |
|---|---|---|---|---|---|---|
| Blood Alcohol (%) | AUC | 0.64 ± 0.22 | 0.62 ± 0.25 | 0.67 ± 0.26 | 0.66 ± 0.24 | 0.0800 |
| Cmax | 0.113 ± 0.034 | 0.109 ± 0.036 | 0.122 ± 0.050 | 0.112 ± 0.035 | 0.2263 | |
| Tmax | 1.09 ± 0.58 | 1.01 ± 0.57 | 1.10 ± 0.87 | 1.01 ± 0.51 | 0.7620 | |
| Blood Acetaldehyde (µM) | AUC | 119 ± 63 | 109 ± 60 | 118 ± 66 | 121 ± 63 | 0.1000 |
| Cmax | 14.0 ± 6.0 | 13.1 ± 6.2 | 14.8 ± 7.4 | 14.0 ± 6.5 | 0.4342 | |
| Tmax | 0.69 ± 0.51 | 1.0 ± 1.4 | 1.1 ± 1.2 | 1.3 ± 3.0 | 0.5489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, D.H.; Lee, K.W.; Shim, Y.Y.; Reaney, M.J.T.; Park, I.; Lee, S.-H.; Park, J.-Y.; Park, E.; Lee, S.-B.; Kim, I.A.; et al. Efficacy of Hovenia dulcis Fruit Extract in Hangover Mitigation: Double-Blind Randomized Clinical Evaluation. Foods 2024, 13, 4084. https://doi.org/10.3390/foods13244084
Paik DH, Lee KW, Shim YY, Reaney MJT, Park I, Lee S-H, Park J-Y, Park E, Lee S-B, Kim IA, et al. Efficacy of Hovenia dulcis Fruit Extract in Hangover Mitigation: Double-Blind Randomized Clinical Evaluation. Foods. 2024; 13(24):4084. https://doi.org/10.3390/foods13244084
Chicago/Turabian StylePaik, Dong Hyun, Ki Won Lee, Youn Young Shim, Martin J. T. Reaney, Ilbum Park, Sang-Hun Lee, Jong-Yul Park, Euddeum Park, Sung-Bum Lee, In Ah Kim, and et al. 2024. "Efficacy of Hovenia dulcis Fruit Extract in Hangover Mitigation: Double-Blind Randomized Clinical Evaluation" Foods 13, no. 24: 4084. https://doi.org/10.3390/foods13244084
APA StylePaik, D. H., Lee, K. W., Shim, Y. Y., Reaney, M. J. T., Park, I., Lee, S.-H., Park, J.-Y., Park, E., Lee, S.-B., Kim, I. A., Xu, G., Hong, J. Y., & Kim, Y. J. (2024). Efficacy of Hovenia dulcis Fruit Extract in Hangover Mitigation: Double-Blind Randomized Clinical Evaluation. Foods, 13(24), 4084. https://doi.org/10.3390/foods13244084







