Quality Characteristics and Flavor Analysis of Five Mulberry Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mulberry Sample Preparation
2.2. Determination of Physicochemical Properties
2.3. Nutrional Index Measurements
2.3.1. Determination of Total Phenol Content
2.3.2. Determination of Total Flavonoid Content
2.4. Flavor Component Analysis
2.4.1. Determination of Soluble Sugar
2.4.2. Determination of Organic Acid
2.4.3. Determination of Amino Acid
2.4.4. Extraction and Determination of Volatile Components
2.5. Statistical Analysis
3. Results and Discussion
3.1. Titratable Acidity, Soluble Solid, and Total Sugar
3.2. Total Phenols and Total Flavonoids
3.3. Soluble Sugar and Organic Acid
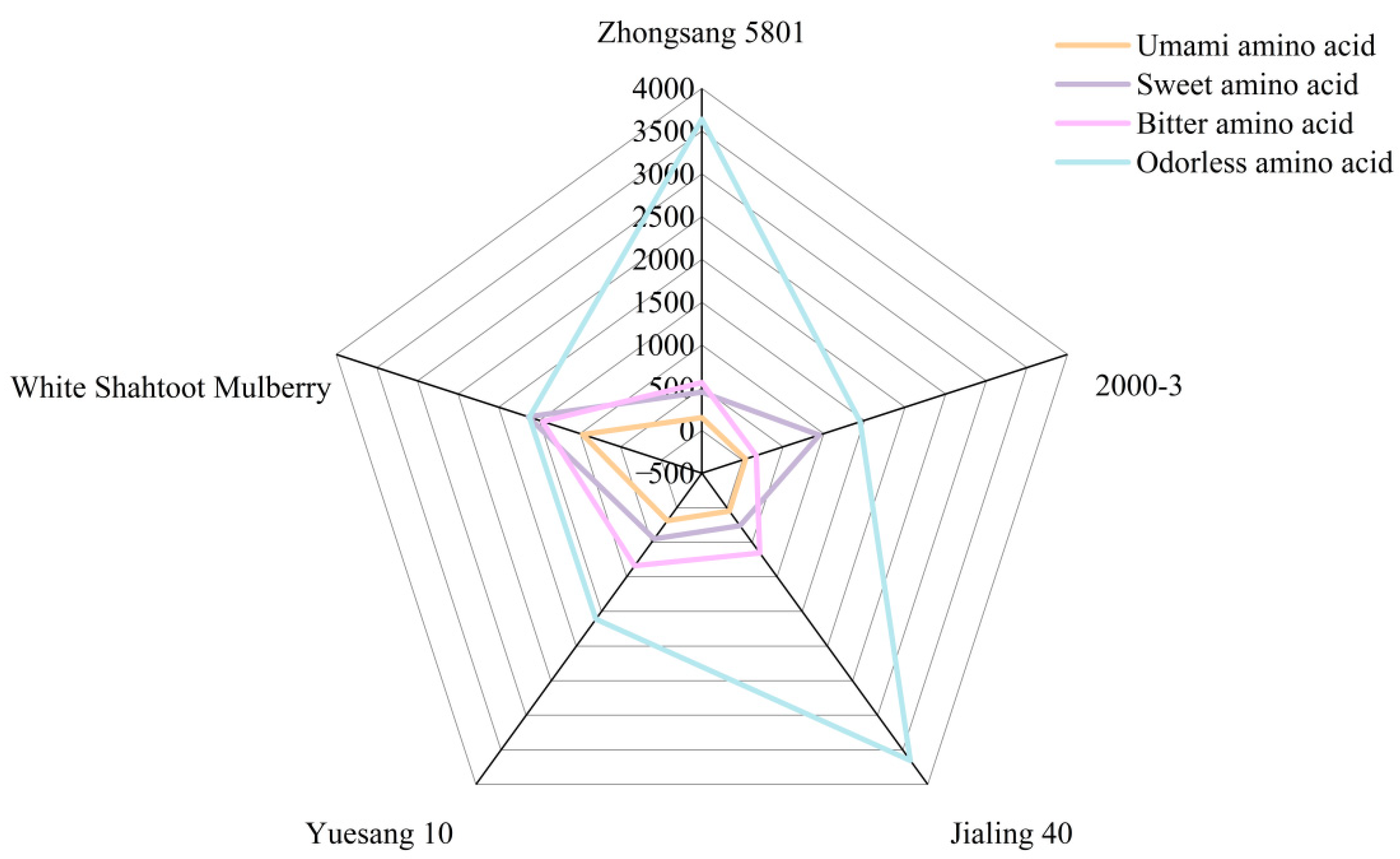
3.4. Amino Acid
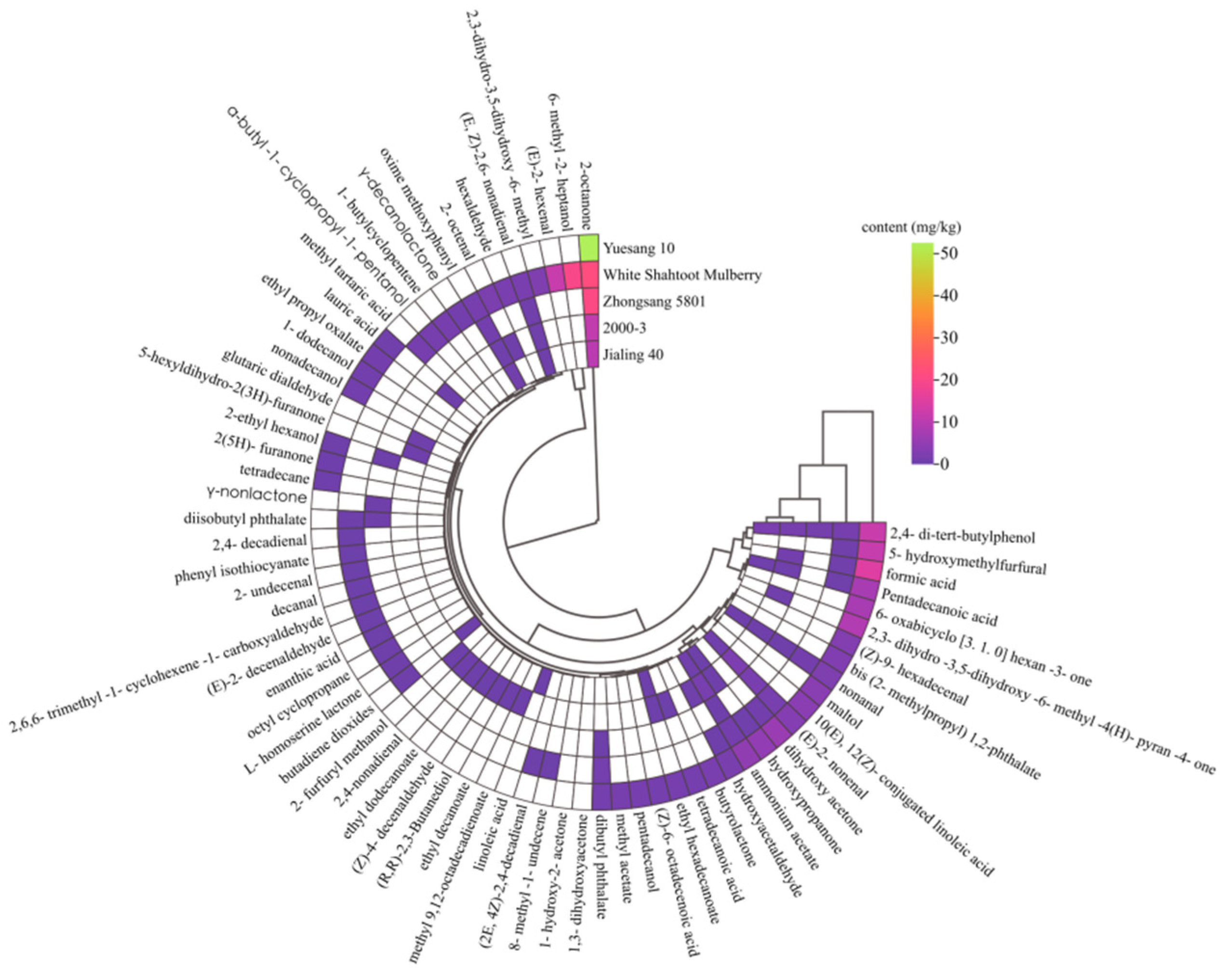
3.5. Volatile Components
3.6. Correlation Analysis Between Amino Acids and Volatile Substances (OAV > 1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Zhang, X.; Jin, Q.; Yang, L.; Li, J.; Chen, F. Free and bound volatile chemicals in mulberry (morus atropurpurea roxb.). J. Food Sci. 2015, 80, C975–C982. [Google Scholar] [CrossRef] [PubMed]
- Kattil, A.; Hamid, D.K.; Shams, R.; Sharma, S. Nutritional composition, phytochemical extraction, and pharmacological potential of mulberry: A comprehensive review. Future Foods 2024, 9, 100295. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.; Li, C. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Xiong, L. Medicinal parts of mulberry (leaf, twig, root bark, and fruit) and compounds thereof are excellent traditional chinese medicines and foods for diabetes mellitus. J. Funct. Foods 2023, 106, 105619. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Y.; Xu, J.; He, X.; Wang, Y. Anti-neuroinflammatory and antioxidant phenols from mulberry fruit (morus alba L.). J. Funct. Foods 2020, 68, 103914. [Google Scholar] [CrossRef]
- Peng, C.; Liu, L.; Chuang, C.; Chyau, C.; Huang, C.; Wang, C. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food. Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef]
- Zheng, Y.; He, H.; Wei, X.; Ge, S.; Lu, Y. Comparison of regulation mechanisms of five mulberry ingredients on insulin secretion under oxidative stress. J. Agric. Food. Chem. 2016, 64, 8763–8772. [Google Scholar] [CrossRef]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-o-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef]
- Chan, K.; Ho, H.; Lin, M.; Wu, C.; Huang, C.; Chang, W.; Wang, C. Mulberry water extracts inhibit rabbit atherosclerosis through stimulation of vascular smooth muscle cell apoptosis via activating p53 and regulating both intrinsic and extrinsic pathways. J. Agric. Food. Chem. 2014, 62, 5092–5101. [Google Scholar] [CrossRef]
- Chan, K.; Ho, H.; Lin, M.; Yen, C.; Huang, C.; Huang, H.; Wang, C. Mulberry water extracts inhibit atherosclerosis through suppression of the integrin-β3/focal adhesion kinase complex and downregulation of nuclear factor κb signaling in vivo and in vitro. J. Agric. Food. Chem. 2014, 62, 9463–9471. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, W.; Bai, S.; Ye, L.; Du, J.; Zhong, M.; Liu, J.; Zhao, R.; Shen, B. White mulberry fruit polysaccharides enhance endothelial nitric oxide production to relax arteries in vitro and reduce blood pressure in vivo. Biomed. Pharmacother. 2019, 116, 109022. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zheng, X. Anthocyanin-rich mulberry fruit improves insulin resistance and protects hepatocytes against oxidative stress during hyperglycemia by regulating ampk/acc/mtor pathway. J. Funct. Foods 2017, 30, 270–281. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The mulberry (morus alba L.) Fruit—A review of characteristic components and health benefits. J. Agric. Food. Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.A.; Ayepa, E.; Fometu, S.S.; Shittu, S.; Davids, J.S.; Wang, J. Mulberry fruit post-harvest management: Techniques, composition and influence on quality traits—A review. Food Control 2022, 140, 109126. [Google Scholar] [CrossRef]
- Vijayan, K.; Jayarama Raju, P.; Tikader, A.; Saratchnadra, B. Biotechnology of mulberry (Morus L.)—A review. Emir. J. Food Agric. 2014, 26, 472–496. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Gibertini, G.; Benvenuti, S.; Cappellozza, S.; Giovannini, D.; Saviane, A.; Sirri, S.; Pinetti, D.; Assirelli, A.; et al. Phytochemical and functional characterization of cultivated varieties of morus alba L. Fruits grown in italy. Food. Chem. 2024, 431, 137113. [Google Scholar] [CrossRef]
- Uyak, C.; Aglar, E.; Ozturk, B.; Doğan, A.; Tekin, O. Biochemical characterization of mulberry (Morus spp.) genotypes from the Turkiye (Hizan, Bitlis): A comprehensive analysis of fruit properties and bioactive compounds. Food Sci. Nutr. 2024, 12, 6425–6441. [Google Scholar] [CrossRef]
- Ma, D.; Lin, T.; Zhao, H.; Li, Y.; Wang, X.; Di, S.; Liu, Z.; Liu, M.; Qi, P.; Zhang, S.; et al. Development and comprehensive SBSE-GC/Q-TOF-MS analysis optimization, comparison, and evaluation of different mulberry varieties volatile flavor. Food Chem. 2024, 443, 138578. [Google Scholar] [CrossRef]
- Ali Redha, A.; Freije, A.; Kodikara, C.; Rondanelli, M.; Aqeel, E.; Zafar, W.; Albunni, H.; Merza, H.; Khonji, A.; Aljar, M.; et al. Comparison of fatty acid profile and mineral content of black mulberry (Morus nigra), white mulberry (Morus alba) and red mulberry (Morus rubra) grown in Bahrain. Appl. Food Res. 2023, 3, 100358. [Google Scholar] [CrossRef]
- Huo, J.; Ni, Y.; Li, D.; Qiao, J.; Huang, D.; Sui, X.; Zhang, Y. Comprehensive structural analysis of polyphenols and their enzymatic inhibition activities and antioxidant capacity of black mulberry (Morus nigra L.). Food Chem. 2023, 427, 136605. [Google Scholar] [CrossRef]
- Bao, T.; Xu, Y.; Gowd, V.; Zhao, J.; Xie, J.; Liang, W.; Chen, W. Systematic study on phytochemicals and antioxidant activity of some new and common mulberry cultivars in china. J. Funct. Foods 2016, 25, 537–547. [Google Scholar] [CrossRef]
- Lin, M.; Li, Y.; Gao, Q.; Shi, L.; He, W.; Li, W.; Liang, Y.; Zhang, Z. Dynamic changes in physicochemical and transcriptional expression profiles of mulberry (morus alba L.) Fruit during ripening. Food Biosci. 2024, 57, 103606. [Google Scholar] [CrossRef]
- Pu, X.; Ye, P.; Sun, J.; Zhao, C.; Shi, X.; Wang, B.; Cheng, W. Investigation of dynamic changes in quality of small white apricot wine during fermentation. LWT 2023, 176, 114536. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wu, D.; Xiao, J.; Lu, J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chem. 2021, 340, 128179. [Google Scholar] [CrossRef]
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R.M.; Li, Z.; Feng, X. Evaluation of antioxidant capacity and flavor profile change of pomegranate wine during fermentation and aging process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Zhao, J.; Wang, Q. Phenolic, flavonoid, and lutein ester content and antioxidant activity of 11 cultivars of chinese marigold. J. Agric. Food. Chem. 2007, 55, 8478–8484. [Google Scholar] [CrossRef]
- Han, B.; Tian, S.; Fan, R.; Chen, R.; Wang, Y.; Gong, H.; Bian, M. Selection of drying technology based on dynamic effects on physicochemical properties and flavours of mulberry. Czech J. Food Sci. 2023, 41, 295–303. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, F.; Yu, D.; Liu, X.; Huang, W.; Zhan, J. Dynamic changes in phenolic compounds, colour and antioxidant activity of mulberry wine during alcoholic fermentation. J. Funct. Foods 2015, 18, 254–265. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food. Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Yativ, M.; Harary, I.; Wolf, S. Sucrose accumulation in watermelon fruits: Genetic variation and biochemical analysis. J. Plant Physiol. 2010, 167, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.; Wu, H.; Wei, H.; Lu, F.; Geng, T.; Lin, P.; Wang, S. Analysis of Main Components of Five Mulberry Varieties in Tropics. Plants 2024, 13, 2763. [Google Scholar] [CrossRef] [PubMed]
- Baccichet, I.; Chiozzotto, R.; Bassi, D.; Gardana, C.; Cirilli, M.; Spinardi, A. Characterization of fruit quality traits for organic acids content and profile in a large peach germplasm collection. Sci. Hortic. 2021, 278, 109865. [Google Scholar] [CrossRef]
- Dong, M.; Xin, R.; Li, Z.; Li, Y.; Huang, X.; Dong, X.; Zhu, B.; Qin, L. Simultaneously quantification of organic acids metabolites by hplc mass spectrometry to reveal the postharvest quality change in cherry tomato. J. Food Compos. Anal. 2023, 117, 105105. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, X.; Zhao, Y.; Wang, C.; Sun, Q.; Hu, D. Optimization of apple fruit flavor by mdvhp1-2 via modulation of soluble sugar and organic acid accumulation. Plant Physiol. Biochem. 2024, 206, 108227. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.I.G.; Yilmaz, H. Determination of fruit chemical properties of morus nigra l., morus alba L. and morus rubra L. by hplc. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhou, G.; Wei, Q.; Hu, H.; Peng, S. Identification of organic acid-related genes and their expression profiles in two pear (pyrus pyrifolia) cultivars with difference in predominant acid type at fruit ripening stage. Sci. Hortic. 2011, 129, 680–687. [Google Scholar] [CrossRef]
- Arena, M.E.; Zuleta, A.; Dyner, L.; Constenla, D.; Ceci, L.; Curvetto, N. Berberis buxifolia fruit growth and ripening: Evolution in carbohydrate and organic acid contents. Sci. Hortic. 2013, 158, 52–58. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Li, G.; Zeng, T.; Chen, L.; Xu, W.; Gu, T.; Tao, Z.; Du, X.; Lu, L. Microbial fermented feed affects flavor amino acids and yolk trimethylamine of duck eggs via cecal microbiota–yolk metabolites crosstalk. Food Chem. 2024, 430, 137008. [Google Scholar] [CrossRef]
- Lian, W.; Lei, J.; Han, C.; Wu, J.; Liu, Z.; Liu, W.; Jiapaer, A.; Su, H.; Xu, Y.; Chen, Y.; et al. Effect of Different Yeasts on the Higher Alcohol Content of Mulberry Wine. Foods 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sheng, M.; Wang, S.; Chen, X.; Leng, A.; Lin, S. Dynamic changes and formation of key contributing odorants with amino acids and reducing sugars as precursors in shiitake mushrooms during hot air drying. Food Chem. 2023, 424, 136409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Matsuoka, R.; Yanagisawa, T.; Xi, Y.; Zhang, L.; Wang, X. Effect of different drying methods on free amino acid and flavor nucleotides of scallop (patinopecten yessoensis) adductor muscle. Food Chem. 2022, 396, 133620. [Google Scholar] [CrossRef]
- Wang, K.; Qi, J.; Jin, Y.; Li, F.; Wang, J.; Xu, H. Influence of fruit maturity and lactic fermentation on physicochemical properties, phenolics, volatiles, and sensory of mulberry juice. Food Biosci. 2022, 48, 101782. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.; Schieberle, P. Re-investigation on odour thresholds of key aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Additional studies of flavor components of corm tortilla chips. J. Agric. Food Chem. 1998, 46, 2764–2769. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterization of headspace volatiles of fish miso, a Japanese fish meat based fermenented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Schnabel, K.O.; Belitz, H.D.; Von Ranson, C. Untersuchungen zur Struktur-Aktivität-Beziehung bei Geruchsstoffen. 1. Mitteilung: Wahrnehmungsschwellenwerte und Geruchsqualitäten von gesättigten aliphatischen und alicyclischen Verbindungen mit Sauerstoff-Funktion. Unters. Forsch. 1988, 187, 215–223. [Google Scholar] [CrossRef]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J.; Nakamoto, H.; Ishihara, M.; Yoshizawa, T.; Varanyanond, W. Characteristic Aroma Components of the Volatile Oil of Yellow Keaw Mango Fruits Determined by Limited Odor Unit Method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef]
- Burdack-Freitag, A.; Schieberle, P. Changes in the key odorants of Italian hazelnuts (Coryllus avellana L. var. tonda romana) induced by roasting. J. Agric. Food Chem. 2010, 58, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.; Judith, M. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flvour Fragr. J. 2006, 21, 207–213. [Google Scholar]
- Regina, S.; Peter, S. Comparison of the key aroma compounds in hand-squeezed and unpasteurised, commercial NFC juices prepared from Brazilian Pera Rio oranges. Eur. Food Res. Technol. 2011, 232, 995–1005. [Google Scholar]
- Melanie, A.; Melanie, A. Characterisation of the key aroma compounds in a freshly reconstituted orange juice from concentrate. Eur. Food Res. Technol. 2009, 229, 611–622. [Google Scholar]
- Melanie, A.; Melanie, A. Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Eur. Food Res. Technol. 2011, 232, 129–142. [Google Scholar]
- Rashash, D.M.C.; Dietrich, A.M.; Hoehn, R.C. FPA of selected odorous compounds. J. Am. Water Works Assoc. 1997, 89, 131–141. [Google Scholar] [CrossRef]
- Tamura, H.; Yang, R.H.; Sugisawa, H. Aroma Profiles of Peel Oils of Acid Citrus. ACS Symp. Ser. 1993, 525, 121–136. [Google Scholar]
- Tamura, H.; Nakamoto, H.; Yang, R.; Sugisawa, H. Characteristic aroma compounds in green algae (Ulva pertusa) volatiles. Nippon. Shokuhin Kagaku Kogaku Kaishi. 1995, 42, 887–891. [Google Scholar] [CrossRef]
- Kossa, T.; Bahri, D.; Tressl, R. Aromastoffe des Malzes und deren Beitrag zum Bieraroma. Monatschr. Brau. 1979, 32, 249–254. [Google Scholar]
- Tressl, R.; Bahri, D.; Kossa, M. Formation of off-flavour components in beer. In The Analysis and Control of Less Desirable Flavors in Foods and Beverages; Charalambous, G., Ed.; Academic Press: New York, NY, USA, 1980; pp. 293–318. [Google Scholar]
- Kerler, J.; Grosch, W. Character impact odorants of boiled chicken: Changes during refrigerated storage and reheating. Z. Für Leb. Und -Forsch. A 1997, 205, 232–238. [Google Scholar] [CrossRef]
- Kerscher, R.; Grosch, W. Comparison of the aroma of cooked beef, pork and chicken. In Frontiers of Flavour Science; Schieberle, P., Engel, K.-H., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2000; pp. 17–20. [Google Scholar]
- Labbe, D.; Rytz, A.; Morgenegg, C.; Ali, S.; Martin, N. Subthreshold olfactory stimulation can enhance sweetness. Chem. Senses. 2007, 32, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Dietz, F.; Traud, J. Geruchs-und Geschmacks-Schwellen-Konzentrationen von Phenolkörpern. Gwf Wasser Abwasser. 1978, 119, 318–325. [Google Scholar]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography–olfactometry (gc-o), odor activity value (oav), gas chromatography-mass spectrometry (gc–ms) and flame photometric detection (fpd). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. J. Agric. Food. Chem. 2011, 59, 8347–8357. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the key aroma volatile compounds in cranberry (vaccinium macrocarpon ait.) Using gas chromatography–olfactometry (gc-o) and odor activity value (oav). J. Agric. Food. Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Sendra, E.; Carbonell-Barrachina, Á.A.; Martínez, J.J.; Hernández, F. Fatty acids composition of Spanish black (Morus nigra L.) and white (morus alba L.) mulberries. Food Chem. 2016, 190, 566–571. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, M.; Zhang, Y.; Zhang, Y.; Guo, X.; Huo, R. Effects of Pretreatment on the Volatile Composition, Amino Acid, and Fatty Acid Content of Oat Bran. Foods 2022, 11, 3070. [Google Scholar] [CrossRef]

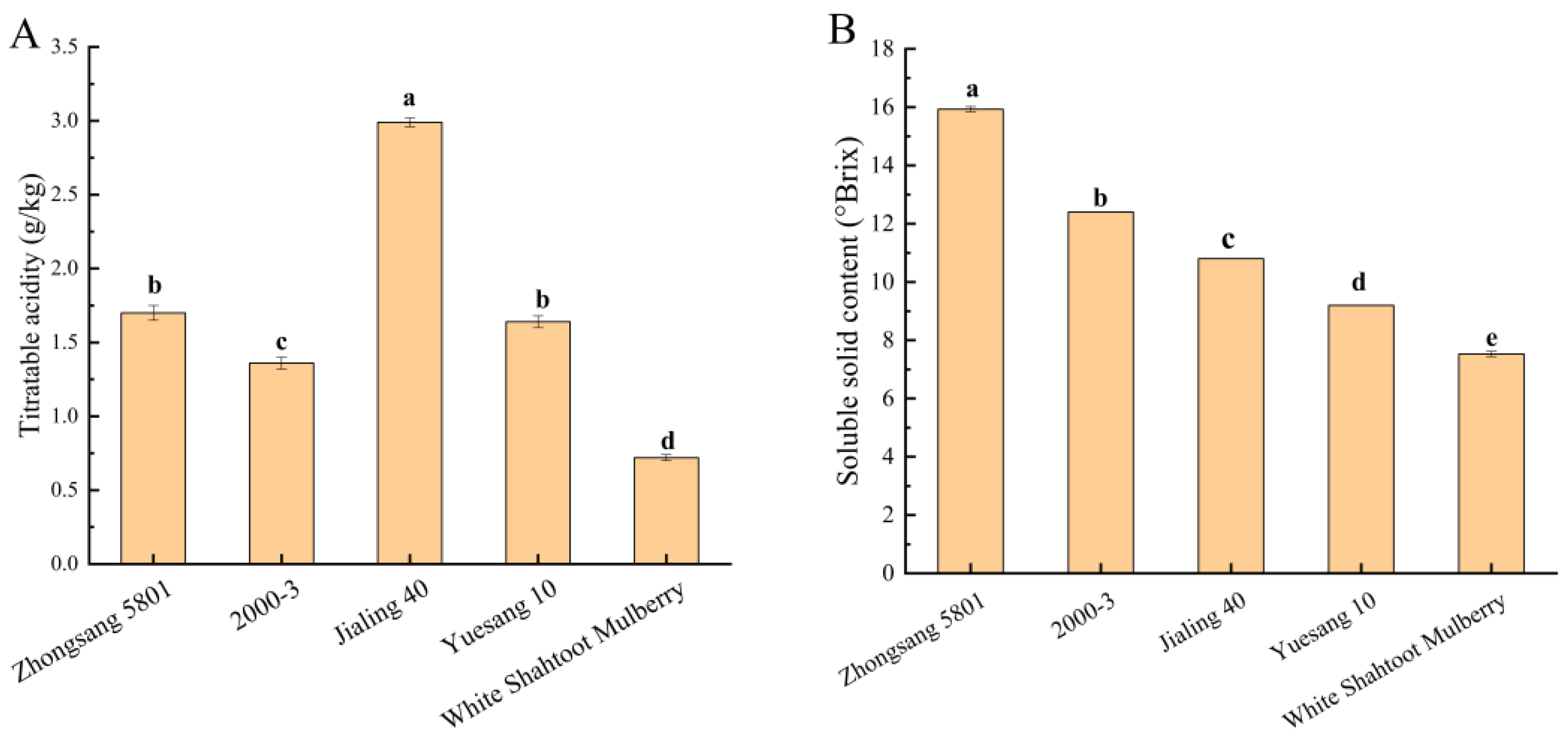
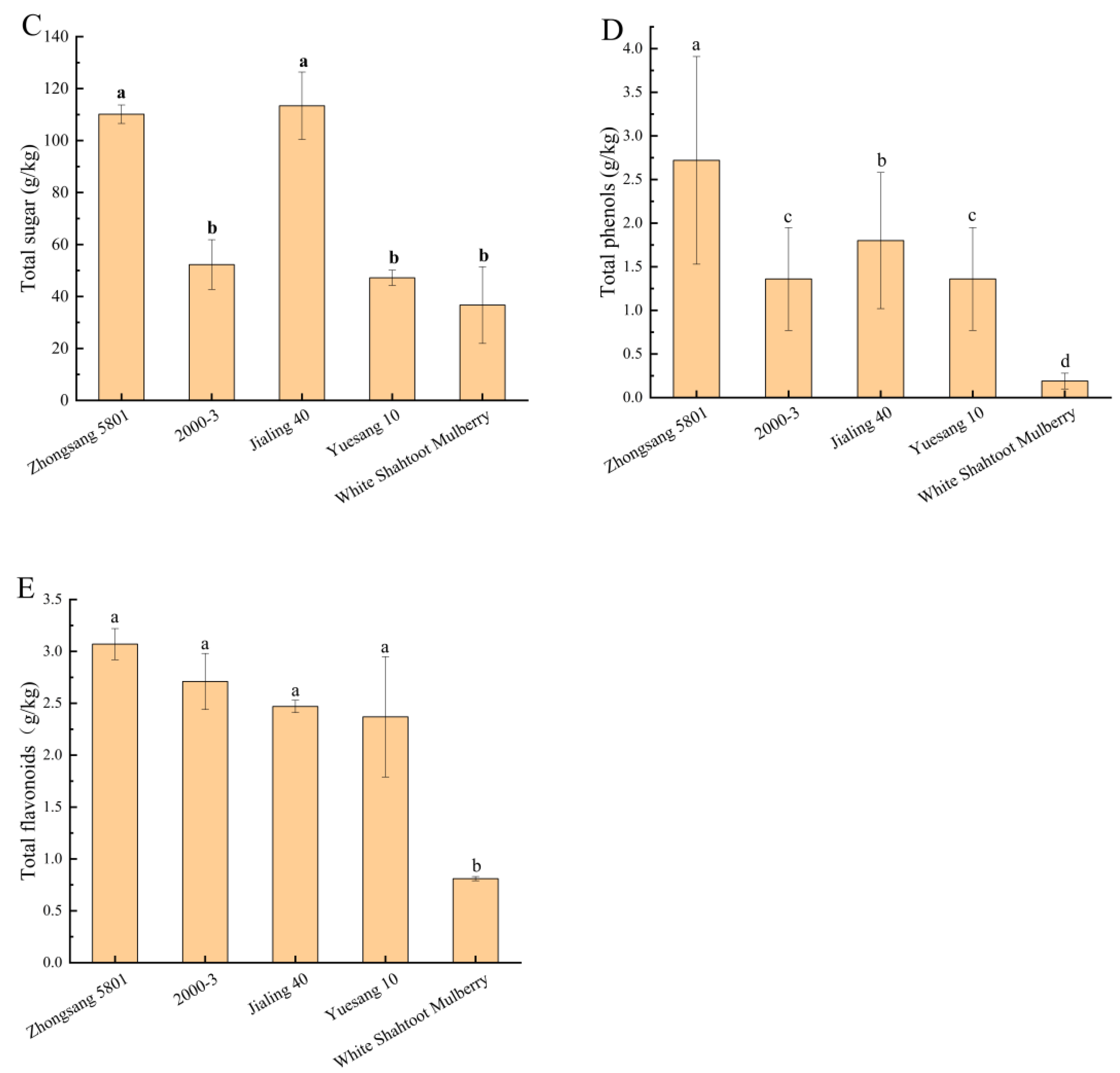
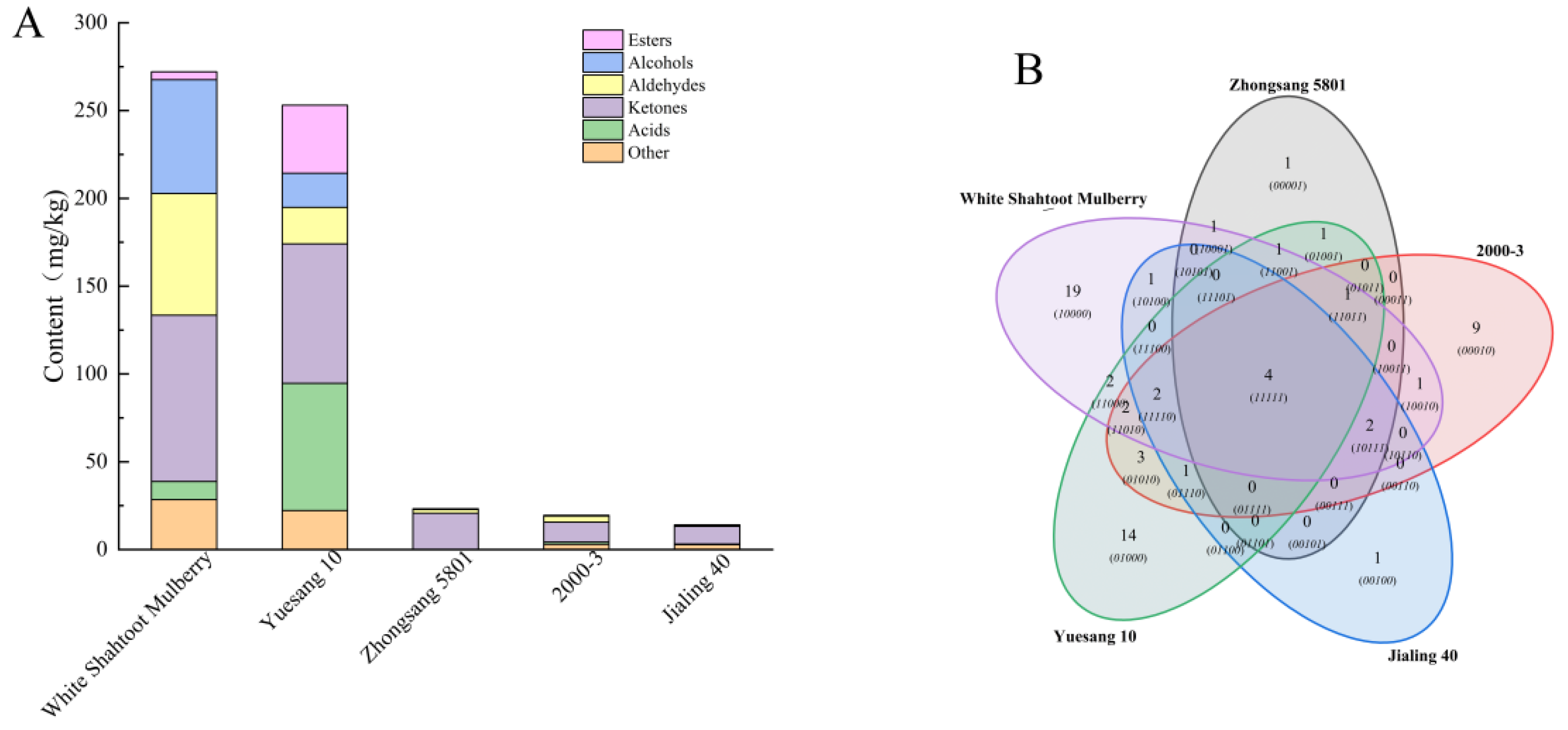
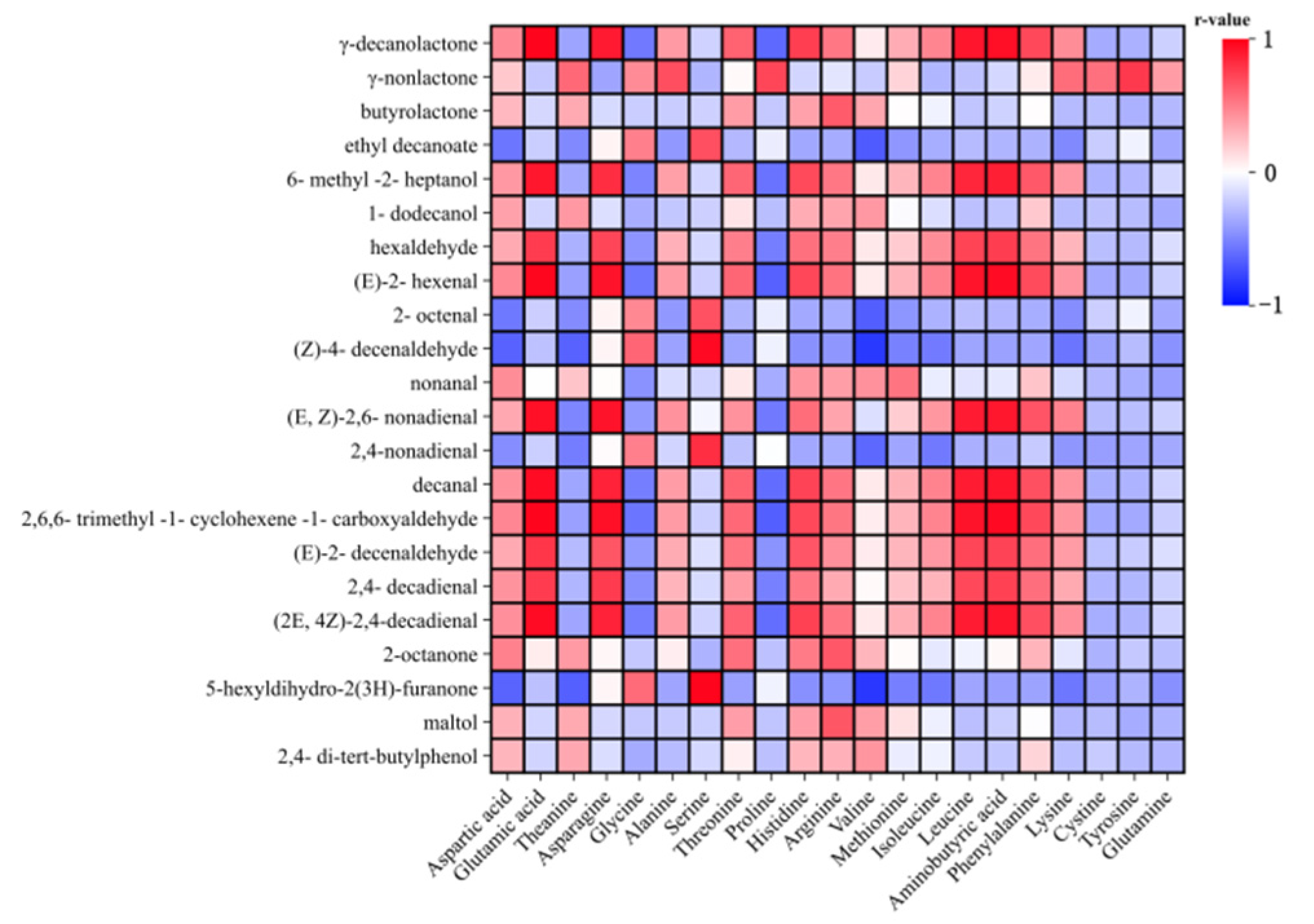
| Cultivar | Zhongsang 5801 | 2000-3 | Jialing 40 | Yuesang 10 | White Shahtoot Mulberry | |
|---|---|---|---|---|---|---|
| Index | ||||||
| Soluble sugar | Fructose | 38.89 ± 6.55 a | 39.66 ± 11.31 a | 22.51 ± 2.90 ab | 19.75 ± 1.04 b | 31.19 ± 7.82 a |
| Glucose | 26.19 ± 6.29 a | 18.63 ± 3.72 a | 6.43 ± 4.59 b | 16.25 ± 2.20 ab | 16.83 ± 4.44 a | |
| Sucrose | - | - | - | - | - | |
| Maltose | - | - | - | - | - | |
| Lactose | - | - | - | - | - | |
| Organic acid | Oxalic acid | 0.07 ± 0.00 c | 0.10 ± 0.01 a | 0.08 ± 0.00 b | 0.07 ± 0.00 c | 0.03 ± 0.00 d |
| Tartaric acid | 0.11 ± 0.01 b | 0.08 ± 0.00 c | 0.08 ± 0.00 c | 0.09 ± 0.00 bc | 0.16 ± 0.02 a | |
| Malic acid | 1.33 ± 0.01 d | 1.85 ± 0.05 a | 1.26 ± 0.07 d | 1.44 ± 0.01 c | 1.54 ± 0.07 b | |
| Lactic acid | - | - | - | - | - | |
| Ascorbic acid | - | - | - | - | - | |
| Acetic acid | - | 0.04 ± 0.01 a | - | 0.02 ± 0.00 a | - | |
| Citric acid | - | - | - | - | - | |
| Succinic acid | - | - | - | - | - |
| Cultivar | Zhongsang 5801 | 2000-3 | Jialing 40 | Yuesang 10 | White Shahtoot Mulberry | |
|---|---|---|---|---|---|---|
| Index | ||||||
| Umami amino acid | Aspartic acid | 126.93 ± 10.80 b | 31.58 ± 13.13 c | 46.09 ± 3.92 c | 159.31 ± 5.49 a | 158.29 ± 9.19 a |
| Glutamic acid | 23.17 ± 16.59 b | 6.13 ± 1.89 b | 2.30 ± 0.49 b | 28.01 ± 19.83 b | 811.48 ± 40.87 a | |
| Sweet amino acid | Theanine | 37.63 ± 3.16 a | 2.79 ± 1.52 c | 21.68 ± 6.30 b | 34.25 ± 2.31 a | 10.31 ± 1.27 c |
| Asparagine | 12.75 ± 4.59 d | 466.16 ± 34.85 b | 4.01 ± 0.29 d | 236.77 ± 68.77 c | 1406.01 ± 87.42 a | |
| Glycine | 134.05 ± 30.81 a | 146.09 ± 20.78 a | 76.5 ± 2.22 b | 27.36 ± 14.04 c | 13.14 ± 3.68 c | |
| Alanine | 116.81 ± 15.09 a | 37.97 ± 17.78 b | 31.85 ± 5.65 b | 44.09 ± 3.15 b | 93.19 ± 3.24 a | |
| Serine | - | 211.3 ± 23.67 a | 26.57 ± 6.00 b | 17.31 ± 0.52 b | 19.8 ± 6.80 b | |
| Threonine | 60.12 ± 23.46 ab | 35.84 ± 6.97 b | 35.37 ± 5.97 b | 59.18 ± 23.35 ab | 83.49 ± 7.40 a | |
| Proline | 88.06 ± 6.09 a | 44.54 ± 3.76 c | 64.27 ± 0.89 b | 29.06 ± 1.58 d | 11.45 ± 5.70 e | |
| Bitter amino acid | Histidine | 34.5 ± 8.24 b | - | - | 110.88 ± 22.02 a | 155.38 ± 23.58 a |
| Arginine | 70.33 ± 38.74 a | - | - | 183.42 ± 110.67 a | 199.58 ± 34.04 a | |
| Valine | 109.01 ± 21.08 d | 20.45 ± 8.25 c | 195.54 ± 4.79 a | 213.55 ± 4.16 a | 147.58 ± 9.18 b | |
| Methionine | 88.32 ± 40.00 a | 31.25 ± 5.69 b | 43.10 ± 2.71 ab | 90.45 ± 31.89 a | 90.90 ± 8.77 a | |
| Isoleucine | 103.68 ± 29.68 b | 71.28 ± 31.96 b | 249.78 ± 25.33 a | 126.97 ± 15.12 b | 227.02 ± 22.01 a | |
| Leucine | 32.44 ± 9.78 c | 21.66 ± 9.01 c | 73.81 ± 9.65 b | 24.78 ± 4.84 c | 171.22 ± 3.86 a | |
| Aminobutyric acid | 74.29 ± 32.23 b | 7.85 ± 4.06 c | 90.74 ± 3.34 b | 28.59 ± 6.19 c | 368.86 ± 5.81 a | |
| Phenylalanine | 48.34 ± 23.60 b | 15.58 ± 11.37 b | - | 59.44 ± 18.64 ab | 99.07 ± 3.31 a | |
| Odorless amino acid | Lysine | 91.69 ± 30.7 a | 33.63 ± 5.22 b | 64.59 ± 4.26 ab | 45.54 ± 5.06 b | 88.71 ± 7.64 a |
| Cystine | 3233.59 ± 844.51 a | 1352.87 ± 379.63 b | 3229.53 ± 204.24 a | 1505.03 ± 183.31 b | 1418.01 ± 71.89 b | |
| Tyrosine | 118.52 ± 9.52 a | 61.68 ± 18.31 c | 92.43 ± 2.78 b | 58.46 ± 7.12 c | 59.72 ± 5.09 c | |
| Glutamine | 195.09 ± 12.42 b | - | 265.65 ± 54.93 a | - | 55.82 ± 12.46 b | |
| Essential amino acid | 533.6 ± 97.7 c | 229.69 ± 14.73 d | 662.2 ± 35.12 b | 619.92 ± 20.81 bc | 908.01 ± 43.7 a | |
| Non-essential amino acid | 4153.81 ± 831.44 a | 2147.01 ± 398.82 b | 3812.62 ± 155.19 a | 2382.38 ± 140.27 b | 4382.07 ± 86.63 a | |
| Total amino acids | 4799.34 ± 935.85 a | 2598.28 ± 186.24 b | 4604.63 ± 393.43 a | 3075.90 ± 140.8 b | 5689.03 ± 134.6 a | |
| Components | Odor Description a | Odor Threshold b (mg/kg) | OAV | ||||
|---|---|---|---|---|---|---|---|
| Zhongsang 5801 | 2000-3 | Jialing-40 | Yuesang10 | White Shahtoot Mulberry | |||
| γ-Decanolactone | Fat, fruit, lactone, peach | 0.0011 e | - d | - | - | - | 709.09 |
| γ-Nonlactone | Apricot, fruit | 0.0021 e | 71.43 | - | - | - | - |
| Butyrolactone | Caramel, cheese, roasted nut | >1 f | - | - | - | >1.65 | - |
| Ethyl decanoate | Brandy, grape, pear | 0.005 g | - | 12 | - | - | - |
| 6-Methyl-2-heptanol | n.f c | 0.039∽0.40 h | - | - | - | - | 49.58~508.46 |
| 1-Dodecanol | n.f | 0.016 i | - | - | - | 42.5 | - |
| Hexaldehyde | Apple, fat, fresh, green, oil | 0.005 g | - | - | - | - | 316 |
| (E)-2-hexenal | n.f | 0.0887 j | - | - | - | - | 130.78 |
| 2-Octenal | Dandelion, fat, fruit, grass, green, spice | 0.003 k | - | 36.67 | - | - | - |
| (Z)-4-decenaldehyde | Fruit | 0.000004 l | - | 20,000 | - | - | - |
| Nonanal | Fat, floral, green, lemon | 0.0011 g | 127.27 | 163.64 | 100 | 1936.36 | 754.55 |
| (E, Z)-2,6-nonadienal | Cucumber, green, wax | 0.0008 g | 487.5 | 462.5 | 212.5 | - | 1837.5 |
| 2,4-Nonadienal | Cereal, deep fried, fat, watermelon, wet wool | 0.0001 g | - | 1200 | - | - | - |
| Decanal | Floral, fried, orange peel, penetrating, tallow | 0.003 m | - | - | - | - | 30 |
| 2,6,6-Trimethyl-1-cyclohexene-1-carboxyaldehyde | n.f | 0.003 n | - | - | - | - | 30 |
| (E)-2-decenaldehyde | Fat, fish, orange | 0.017~0.25 o | - | - | - | - | 0.5~5.88 |
| 2,4-Decadienal | Coriander, deep fried, fat, oil, oxidized | 0.0003 p | - | - | - | - | 266.67 |
| (2E, 4Z)-2,4-decadienal | n.f | 0.00004 q | - | - | - | - | 1250 |
| 2-Octanone | Fat, fragrant, mold | 0.0502 g | 409.36 | 213.35 | 198.21 | 1046.81 | 430.48 |
| 5-Hexyldihydro-2(3H)-furanone | n.f | 0.0097 e | - | 24.74 | - | - | - |
| maltol | n.f | 1.24 r | - | - | - | 2.95 | - |
| 2,4-Di-tert-butylphenol | n.f | 0.5 s | 1.92 | 2.46 | 3.7 | 23.74 | 2.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Lian, Y.; Li, J.; Suo, H.; Song, J.; Wang, M.; Zhang, Y. Quality Characteristics and Flavor Analysis of Five Mulberry Varieties. Foods 2024, 13, 4088. https://doi.org/10.3390/foods13244088
Meng Y, Lian Y, Li J, Suo H, Song J, Wang M, Zhang Y. Quality Characteristics and Flavor Analysis of Five Mulberry Varieties. Foods. 2024; 13(24):4088. https://doi.org/10.3390/foods13244088
Chicago/Turabian StyleMeng, Yingmei, Yinyin Lian, Jiaxin Li, Huayi Suo, Jiajia Song, Mei Wang, and Yu Zhang. 2024. "Quality Characteristics and Flavor Analysis of Five Mulberry Varieties" Foods 13, no. 24: 4088. https://doi.org/10.3390/foods13244088
APA StyleMeng, Y., Lian, Y., Li, J., Suo, H., Song, J., Wang, M., & Zhang, Y. (2024). Quality Characteristics and Flavor Analysis of Five Mulberry Varieties. Foods, 13(24), 4088. https://doi.org/10.3390/foods13244088






