Nutrient Stability in NASA Spaceflight Experiment Rodent Food Bars
Abstract
1. Introduction
2. Methods
2.1. NuRFB Composition
2.2. NuRFB Sampling for Biochemical Analysis
2.3. Nutrient and Lipid Oxidation Analysis
- (a)
- Riboflavin (microbiological method): The sample was first hydrolyzed with dilute hydrochloric acid and adjusted for pH. The amount of riboflavin was determined turbidimetrically by comparing the growth response of the sample using the bacteria Lactobacillus rhamnosus with the growth response of the riboflavin standard [20,21].
- (b)
- Thiamine (fluorometric method): The sample was autoclaved under weakly acidic conditions followed by incubation with a buffered enzyme solution in order to complete the release of any bound thiamine. Further, this solution was purified on a cation exchange column. Potassium ferricyanide was added to an aliquot of the purified solution to convert thiamine to thiochrome, which was then extracted, read on a fluorometer, and quantified using an external standard [12,22,23,24].
2.4. Statistical Analysis
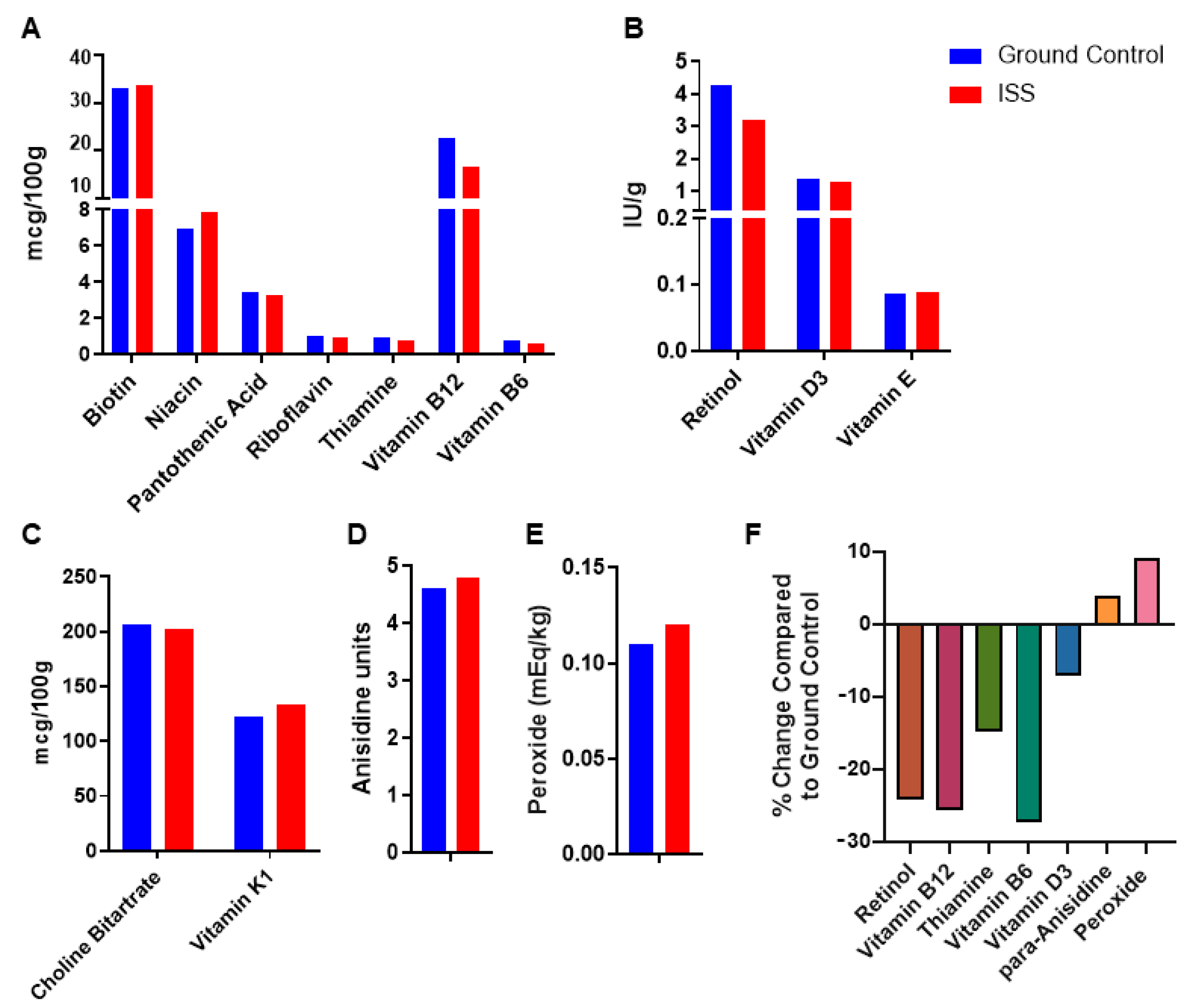
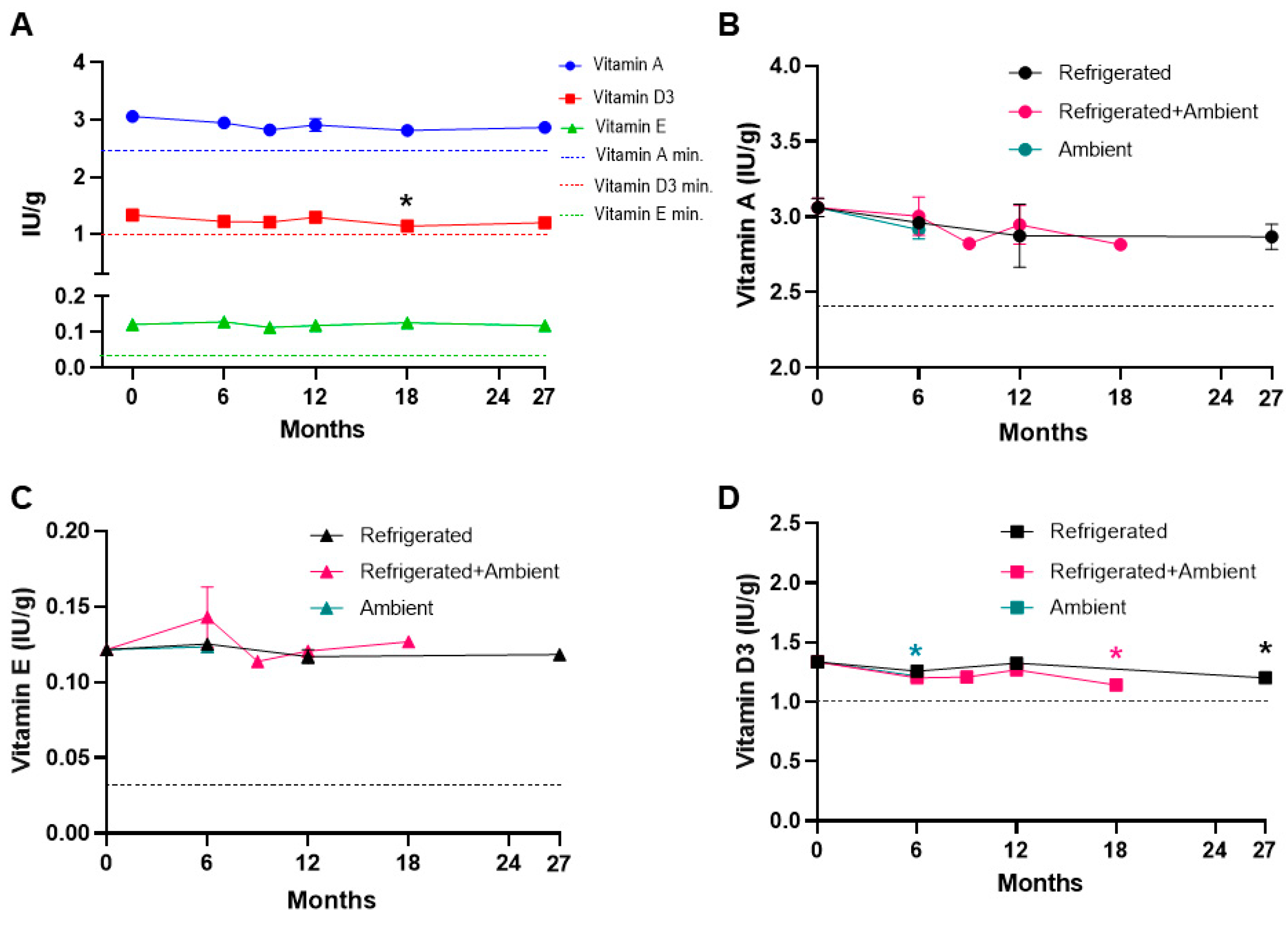
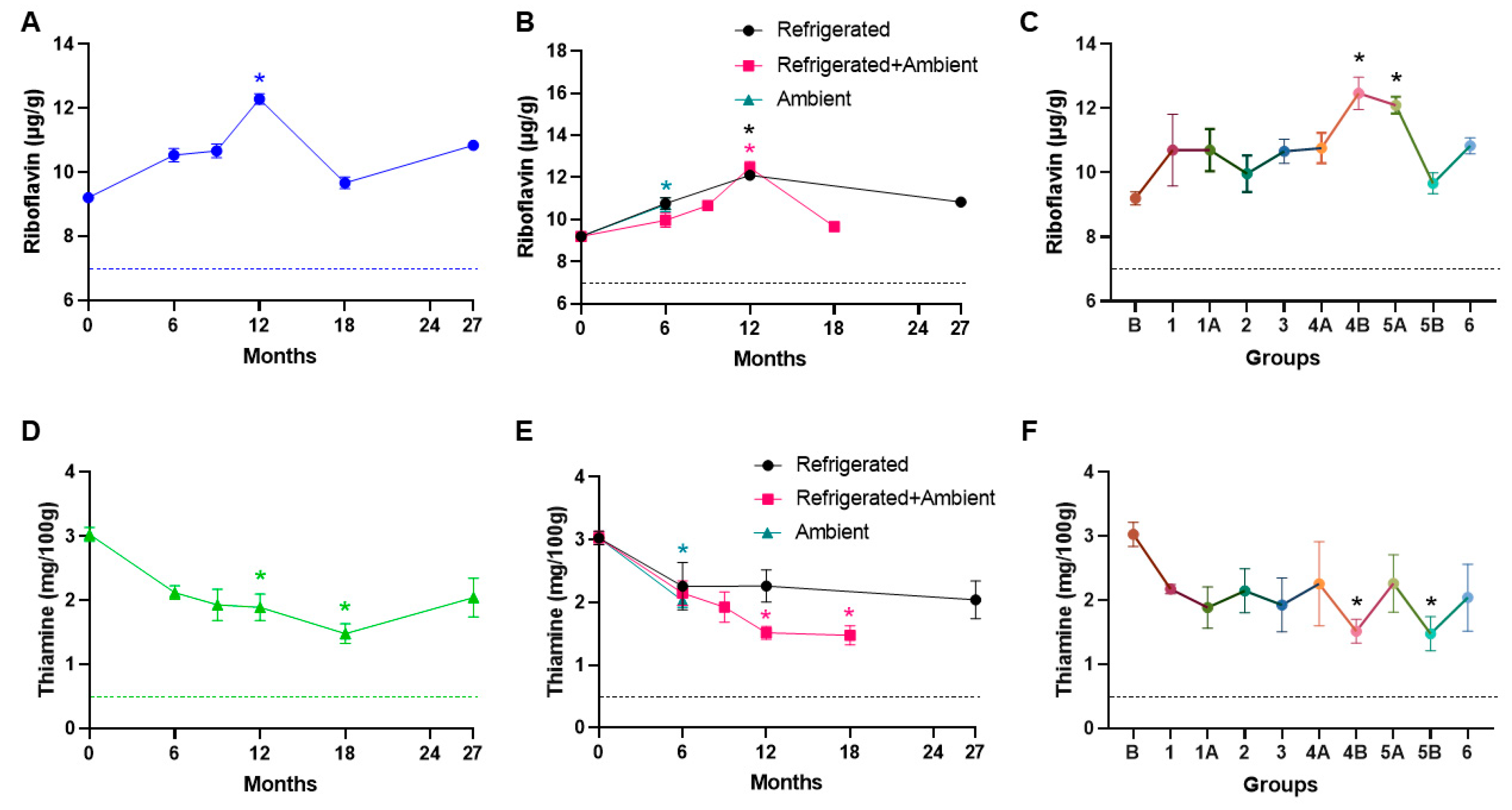
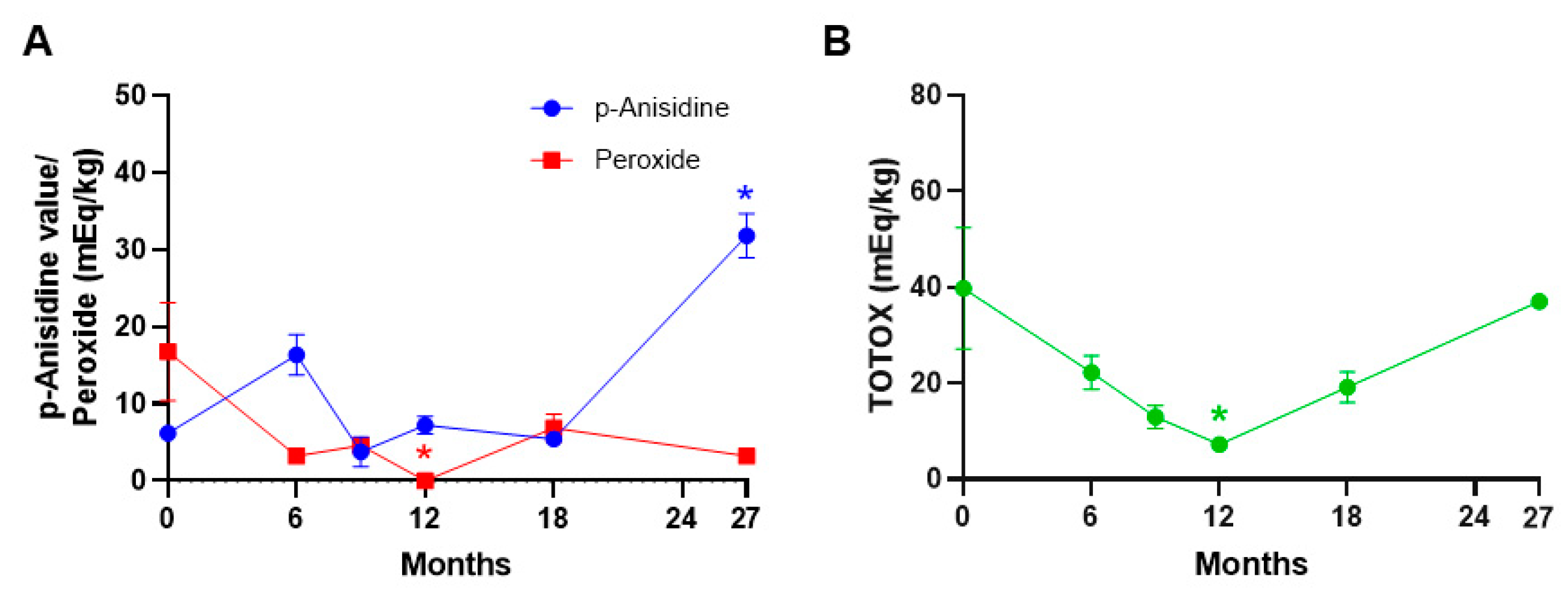
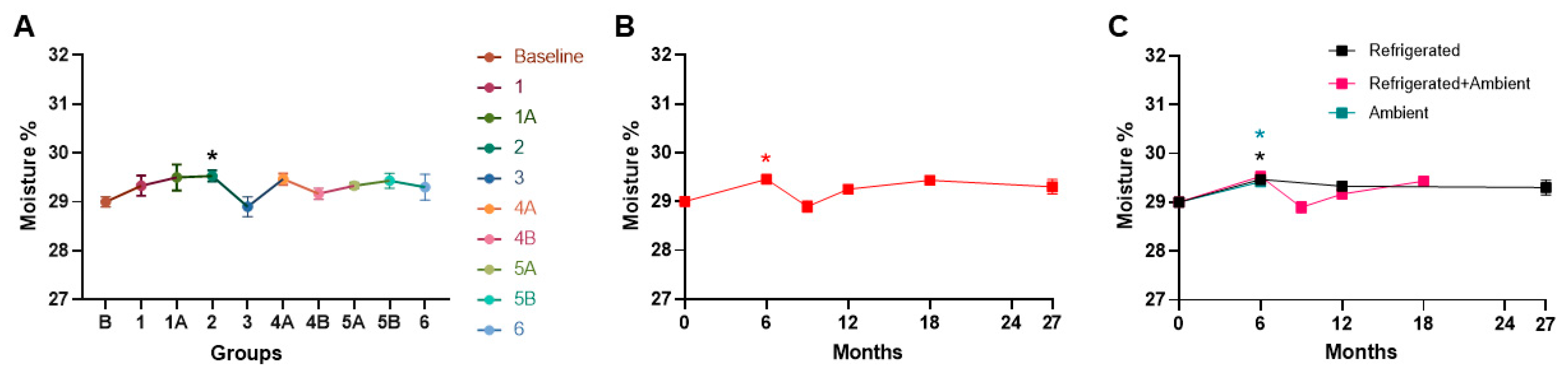
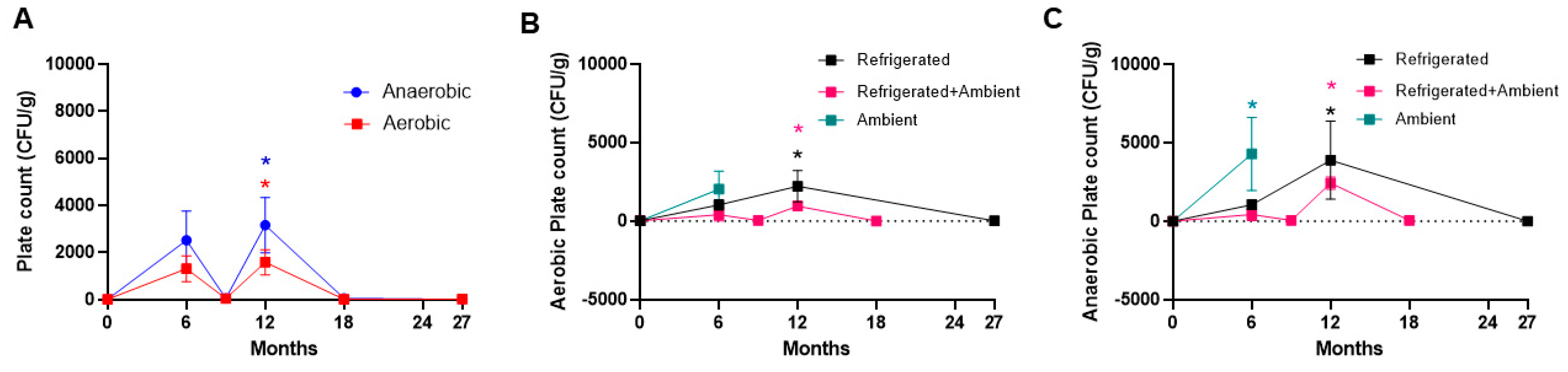
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.D.; Iyer, J.; Petereit, J.; Dolling-Boreham, R.M.; Tyryshkina, A.; Paul, A.M.; Gilbert, R.; Jensen, M.; Woolsey, R.J.; Anand, S.; et al. Artificial gravity partially protects space-induced neurological deficits in Drosophila melanogaster. Cell Rep. 2022, 40, 111279. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.; Grindeland, R.; Barrett, J.; Dalton, B.; Mandel, A.; Wade, C. Evaluation of NASA Foodbars as a standard diet for use in Short-Term rodent space flight studies. Nutrition 2003, 19, 947–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, G.-S.; Tou, J.C.; Yu, D.; Girten, B.E.; Cohen, J. The past, present, and future of National Aeronautics and Space Administration spaceflight diet in support of microgravity rodent experiments. Nutrition 2014, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.L.; Ericsson, A.C. Microbiota and reproducibility of rodent models. Lab Anim. 2017, 46, 114–122. [Google Scholar] [CrossRef]
- Tuck, C.J.; De Palma, G.; Takami, K.; Brant, B.; Caminero, A.; Reed, D.E.; Muir, J.G.; Gibson, P.R.; Winterborn, A.; Verdu, E.F.; et al. Nutritional profile of rodent diets impacts experimental reproducibility in microbiome preclinical research. Sci. Rep. 2020, 10, 17784. [Google Scholar] [CrossRef] [PubMed]
- A Pellizzon, M.; Ricci, M.R. Choice of Laboratory Rodent Diet May Confound Data Interpretation and Reproducibility. Curr. Dev. Nutr. 2020, 4, nzaa031. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US). Subcommitee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals, 4th ed.; Nutrient Requirements of the Mouse; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-S.; Tou, J.C.; Reiss-Bubenheim, D.A.; Hill, E.L.; Liittschwager, K.W.; Girten, B.E.; Pena-Yewkukhiw, E. Oxidative and nutrient stability of a standard rodent spaceflight diet during long-term storage. Lab Anim. 2012, 41, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tao, L. Oxidation of Polyunsaturated Fatty Acids and its Impact on Food Quality and Human Health. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- AOAC. Vitamin A—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 992.06; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Vitamin A—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 992.04; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Cort, W.M.; Vicente, T.S.; Waysek, E.H.; Williams, B.D. Vitamin E content of feedstuffs determined by high-performance liquid chromatographic fluorescence. J. Agric. Food Chem. 1983, 31, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Speek, A.J.; Schrijver, J.; Schreurs, W.H.P. Vitamin E Composition of Some Seed Oils as Determined by High-Performance Liquid Chromatography with Fluorometric Detection. J. Food Sci. 2006, 50, 121–124. [Google Scholar] [CrossRef]
- McMurray, C.H.; Blanchflower, W.J.; A Rice, D. Influence of Extraction Techniques on Determination of α-Tocopherol in Animal Feedstuffs. J. AOAC Int. 1980, 63, 1258–1261. [Google Scholar] [CrossRef]
- AOAC. Vitamin D3—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 2011.11; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Riboflavin—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 940.33; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Riboflavin—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 960.46; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Thiamin—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 942.33; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Thiamin—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 953.17; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Thiamin—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 957.17; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Peroxide—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 965.33; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- United States Pharmacopeia (USP). 36th Rev, ‘Peroxide Value’. In Fats & Fixed Oils; USP Conv.: Rockville, MD, USA, 2013; p. 173. [Google Scholar]
- AOAC. p-Anisidine—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method Cd 18-90; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- United States Pharmacopeia (USP). 38th Rev, “p-Anisidine Value”. In Fats & Fixed Oils; USP Conv.: Rockville, MD, USA, 2015; p. 285. [Google Scholar]
- AOAC. Moisture—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 925.09; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Moisture—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 926.08; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Aerobic bacteria—Association of Official Analytical Chemists. In Official Methods Anal. AOAC International 18th Ed. Method 966.23; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Maturin, L.; Peeler, J. Chapter 3. Aerobic Plate Count. In Food and Drug Administration (FDA), Bacteriological Analytical Manual Online; Silver Spring: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Beetner, G.; Tsao, T.; Frey, A.; Harper, J. Degradation of Thiamine and Riboflavin during extrusion processing. J. Food Sci. 1974, 39, 207–208. [Google Scholar] [CrossRef]
- Connell, J. Control of Fish Quality; Fishing News (Books) Ltd.: Surrey, UK, 1975. [Google Scholar]
- Tang, H.; Rising, H.H.; Majji, M.; Brown, R.D. Long-Term Space Nutrition: A Scoping Review. Nutrients 2021, 14, 194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mironeasa, S.; Coţovanu, I.; Mironeasa, C.; Ungureanu-Iuga, M. A Review of the Changes Produced by Extrusion Cooking on the Bioactive Compounds from Vegetal Sources. Antioxidants 2023, 12, 1453. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.N.; Asif, M.; Ali, R. Stability of Vitamins during Extrusion. Crit. Rev. Food Sci. Nutr. 2009, 49, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P. Advances in instrumental methods to determine food quality deterioration. In Food and Beverage Stability and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2011; pp. 381–404. [Google Scholar] [CrossRef]
- Irwin, J.W.; Hedges, N. Measuring lipid oxidation. In Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Sawston, UK, 2004; pp. 289–316. [Google Scholar] [CrossRef]
- Salehi Pourbavarsad, M.; Jalili Jalalieh Behnaz Owuor, J.; Jackson, W.A.; Muirhead, D. Humidity Condensate Stabilization Using an Engineered Biologically Active Storage Tank. In Proceedings of the 50th International Conference on Environmental System, Virtual, 12–14 July 2021. [Google Scholar]
- Cooper, M.; Perchonok, M.; Douglas, G.L. Initial assessment of the nutritional quality of the space food system over three years of ambient storage. NPJ Microgravity 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | Quantity (g/kg) |
|---|---|
| Casein | 100 |
| DL-Methionine | 3 |
| Wheat Gluten | 120 |
| Wheat Flour, Durum 2nd clear | 225 |
| Corn Starch | 198.889 |
| Corn Syrup | 100 |
| Sucrose | 100 |
| Soybean Oil | 40 |
| Cellulose | 50 |
| Mineral Mix, AIN-93G-MX | 35 |
| Calcium Carbonate | 5 |
| Vitamin Mix, AIN-93-VX | 20 |
| Choline Bitartrate | 2.5 |
| Vitamin B12 | 0.23 |
| Thiamin (81%) | 0.36 |
| Folic Acid | 0.012 |
| Vitamin K1, phylloquinone | 0.001 |
| TBHQ, antioxidant | 0.008 |
| Sample Group | Time Under Refrigeration (Months) | Time at Ambient Temperature (Months) | Rationale/Objective |
|---|---|---|---|
| Baseline | 0 | 0 | Baseline value before storage |
| 1 | 0 | 6 | Bag prepared using nitrogen gas |
| 1A | 0 | 6 | Bag prepared using ethylene oxide |
| 2 | 3 | 3 | Flight scenario replication |
| 3 | 3 | 6 | Flight scenario replication |
| 4A | 6 | 0 | Extended storage at 4 °C with transfer to AT |
| 4B | 6 | 6 | |
| 5A | 12 | 0 | Longer term storage at 4 °C with transfer to AT |
| 5B | 12 | 6 | |
| 6 | 27 | 0 | Refrigerated control group (4 °C) |
| Vitamins | Lipid Oxidation Markers | Microbiology |
|---|---|---|
| Vitamin A as Retinol (>2.4 IU/g) | Peroxide value | Mold |
| Vitamin D3 (>1 IU/g) | p-Anisidine | Yeast |
| Vitamin E (>0.0320 IU/g) | TOTOX | Total Aerobic Bacteria |
| Thiamine (>0.50 mg/100 mg) | Total Anerobic Bacteria | |
| Riboflavin (>7 mg/g) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, J.; Marsh, T.S.; Fisher, R.J.; Verma, V. Nutrient Stability in NASA Spaceflight Experiment Rodent Food Bars. Foods 2024, 13, 4093. https://doi.org/10.3390/foods13244093
Iyer J, Marsh TS, Fisher RJ, Verma V. Nutrient Stability in NASA Spaceflight Experiment Rodent Food Bars. Foods. 2024; 13(24):4093. https://doi.org/10.3390/foods13244093
Chicago/Turabian StyleIyer, Janani, Tyler S Marsh, Ryan J Fisher, and Vandana Verma. 2024. "Nutrient Stability in NASA Spaceflight Experiment Rodent Food Bars" Foods 13, no. 24: 4093. https://doi.org/10.3390/foods13244093
APA StyleIyer, J., Marsh, T. S., Fisher, R. J., & Verma, V. (2024). Nutrient Stability in NASA Spaceflight Experiment Rodent Food Bars. Foods, 13(24), 4093. https://doi.org/10.3390/foods13244093





