Screening of Nutritionally Important Components in Standard and Ancient Cereals
Abstract
1. Introduction
2. Materials and Methods
2.1. Grain Material and Sample Preparation
2.2. Chemicals and Reagents
2.3. Determination of Protein Content
2.4. Determination of the Concentration of Phytic Phosphorus, Inorganic Phosphorus, and Total Glutathione
2.4.1. Determination of Phytic Acid and Inorganic Phosphorus
2.4.2. Determination of Total Glutathione
2.5. Determination of Total Phosphorus
2.6. Determination of Yellow Pigment
2.7. Identification and Determination of Total Phenolic Compounds and Antioxidant Activity
2.8. Determination of Arabinoxylan and β-Glucan
2.9. HPLC Analysis
2.10. Mineral Element Analysis
2.11. Statistical Analysis
3. Results
3.1. The Concentration of Protein, Antioxidants, and Dietary Fiber in the Standard and Ancient Grains
3.2. Essential Elements and Their Ratios with Phytic Acid in the Standard and Ancient Grains
3.3. Phenolic Acids and Antioxidant Status in the Standard and Ancient Grains
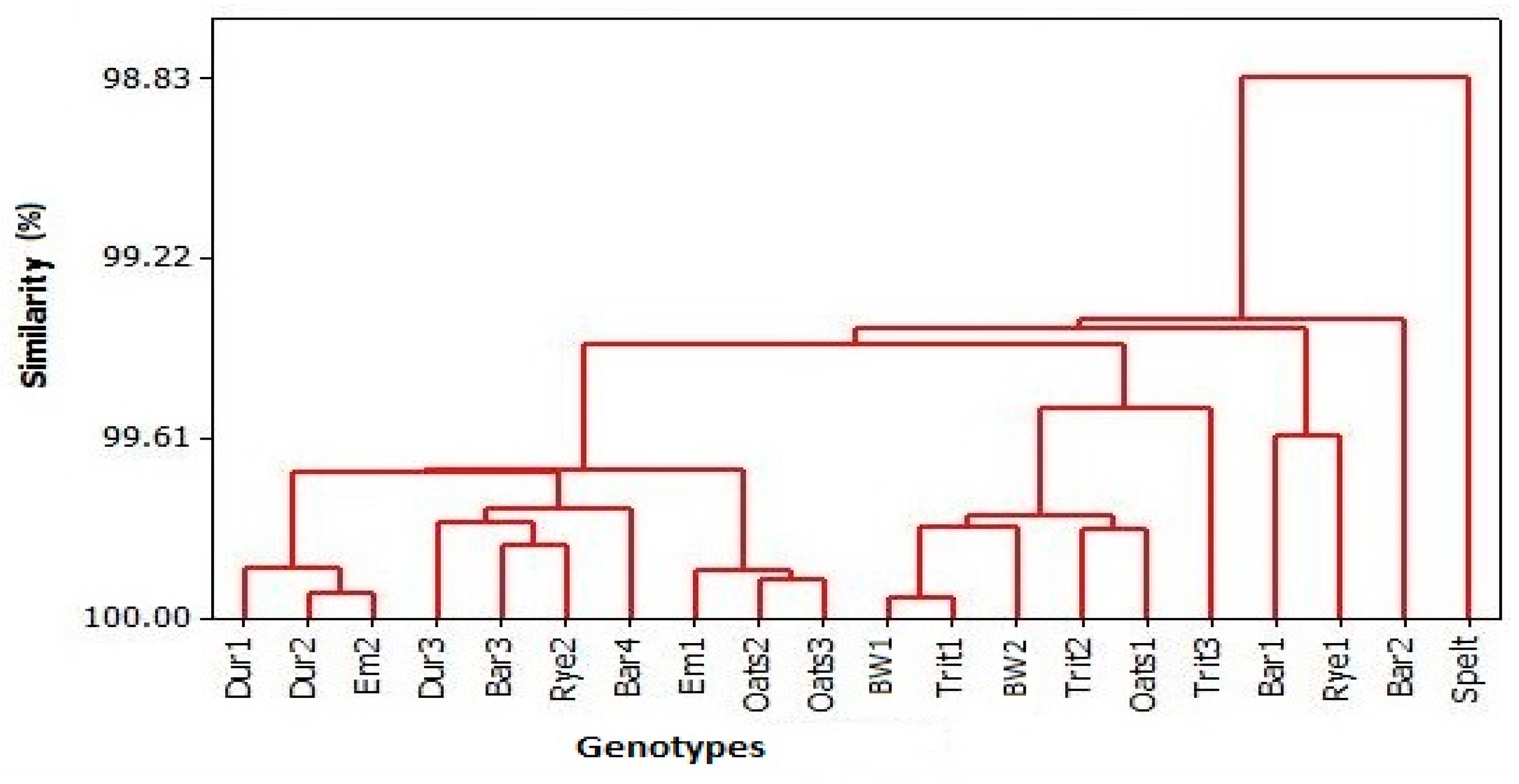
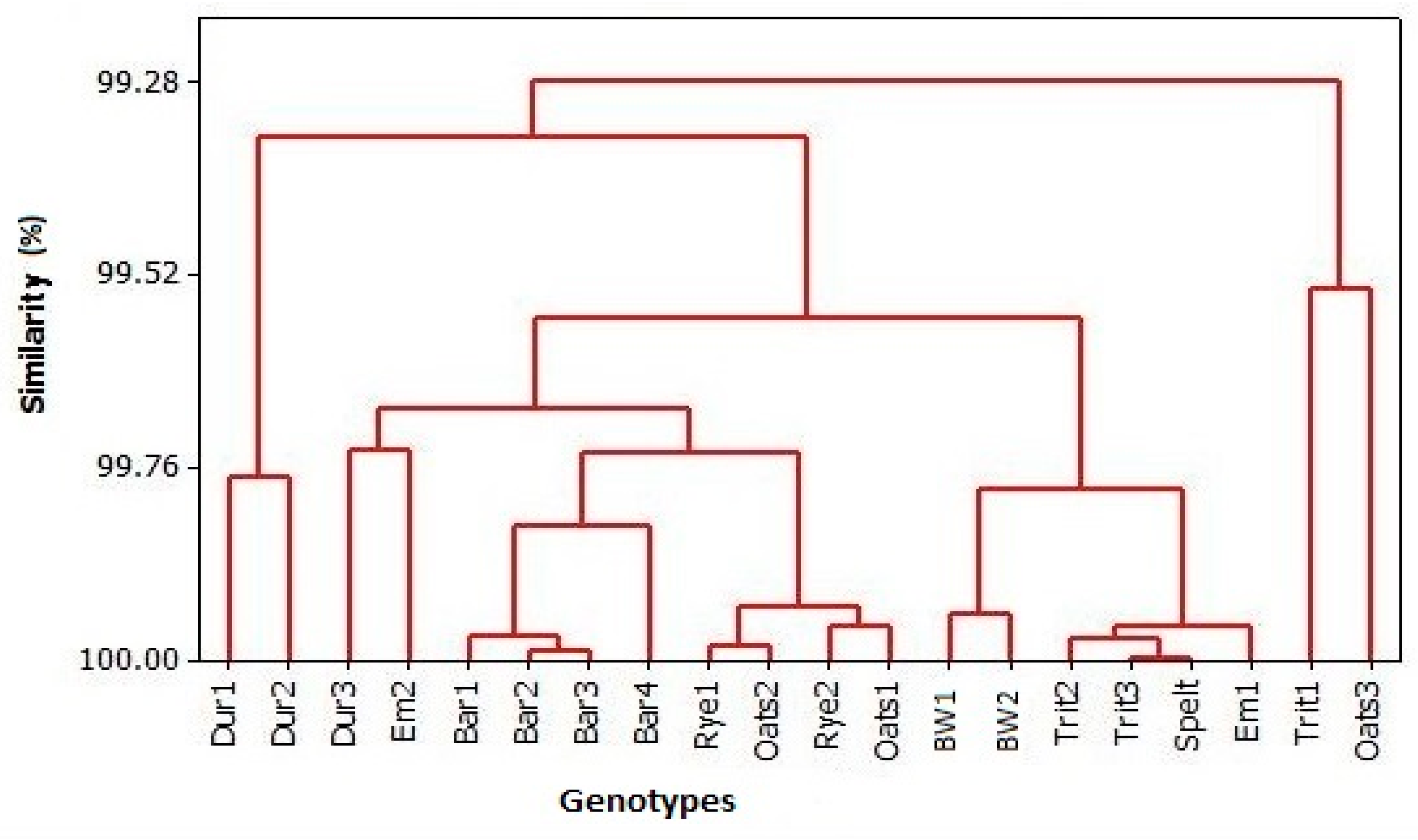
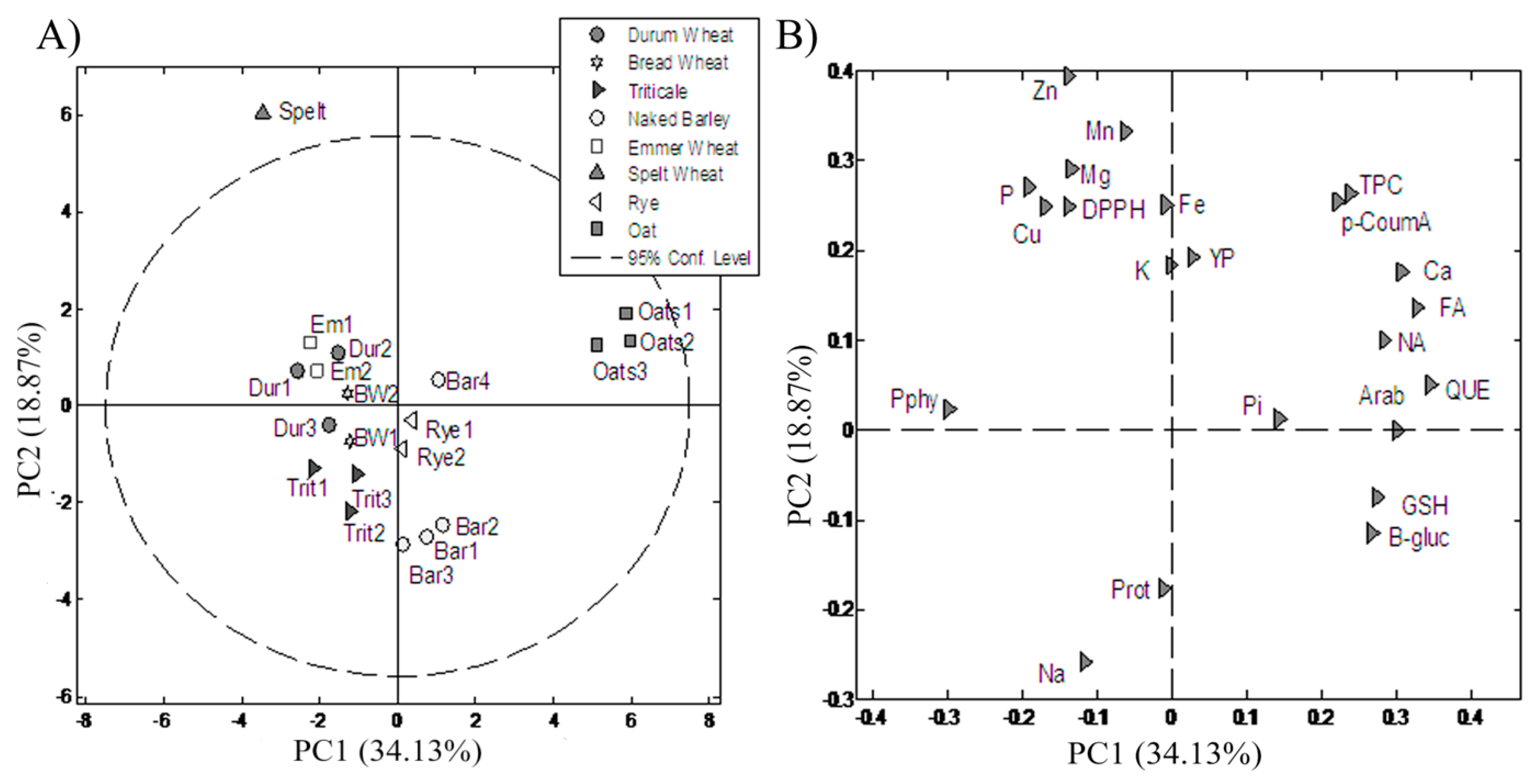
3.4. PC Analysis
4. Discussion
4.1. Concentration of Protein and Dietary Fiber in the Standard and Ancient Grains
4.2. Essential Element Levels in the Standard and Ancient Grains
4.3. Antioxidants and Phenolic Acids in the Standard and Ancient Grains
4.4. PC Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Mitrevski, J.; Pantelic, N.Ð.; Dodevska, M.S.; Kojic, J.S.; Vulic, J.J.; Zlatanovic, S.; Gorjanović, S.; Lalicic-Petronijevic, J.; Marjanovic, S.; Antic, V.V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Jordanovska, S.; Jovović, Z.; Dolijanović, Ž.; Dragičević, V.; Branković, G.; Đekić, V. Nutritional properties of Macedonian landraces of small grain cereals as a source of new genetic variability. Genetika 2018, 50, 863–883. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and Resilient Cereals and Pseudo-Cereals for Marginal Agriculture: Molecular Advances for Improving Nutrient Bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- FAO. Statistical Yearbook 2023 Faostat Analytical Brief 79, Agricultural Production Statistics 2000–2022; FAO: Rome, Italy, 2023; ISSN 2709-006X. [Google Scholar]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; De Maio, A.C.; Catalano, A.; Ceramella, J.; Iacopetta, D.; Bonofiglio, D.; Saturnino, C.; Sinicropi, M.S. Ancient Wheat as Promising Nutraceuticals for the Prevention of Chronic and Degenerative Diseases. Curr. Med. Chem. 2023, 30, 3384–3403. [Google Scholar] [CrossRef]
- Majzoobi, M.; Jafarzadeh, S.; Teimouri, S.; Ghasemlou, M.; Hadidi, M.; Brennan, C.S. The Role of Ancient Grains in Alleviating Hunger and Malnutrition. Foods 2023, 12, 2213. [Google Scholar] [CrossRef] [PubMed]
- Montevecchi, G.; Setti, L.; Olmi, L.; Buti, M.; Laviano, L.; Antonelli, A.; Sgarbi, E. Determination of Free Soluble Phenolic Compounds in Grains of Ancient Wheat Varieties (Triticum sp. pl.) by Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Suchowilska, E.; Bieńkowska, T.; Stuper-Szablewska, K.; Wiwart, M. Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture 2020, 10, 591. [Google Scholar] [CrossRef]
- Konvalina, P.; Suchý, K.; Stehno, Z.; Capouchová, I.; Moudrý, J. Drought Tolerance of Different Wheat Species (Triticum L.). In Vulnerability of Agriculture, Water and Fisheries to Climate Change; Behnassi, M., SyomitiMuteng’e, M., Ramachandran, G., Shelat, K., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Dragičević, V.; Brankov, M.; Stoiljković, M.; Tolimir, M.; Kanatas, P.; Travlos, I.; Simić, M. Kernel color and fertilization as factors of enhanced maize quality. Front. Plant Sci. 2022, 13, 1027618. [Google Scholar] [CrossRef]
- Dragicevic, V.; Dolijanović, Ž.; Janosevic, B.; Brankov, M.; Stoiljkovic, M.; Dodevska, M.S.; Simić, M. Enhanced Nutritional Quality of Sweet Maize Kernel in Response to Cover Crops and Bio-Fertilizer. Agronomy 2021, 11, 981. [Google Scholar] [CrossRef]
- Brouns, F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting phytate-element interactions: Implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Crit. Rev. Food Sci. Nutr. 2020, 62, 1696–1712. [Google Scholar] [CrossRef]
- Feizollahi, E.; Mirmahdi, R.S.; Zoghi, A.; Zijlstra, R.T.; Roopesh, M.S.; Vasanthan, T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021, 143, 110284. [Google Scholar] [CrossRef]
- Brankovic, G.; Dragičević, V.; Dodig, D.; Zoric, M.; Knežević, D.; Žilić, S.; Denčić, S.; Šurlan, G. Genotype x Environment interaction for antioxidants and phytic acid contents in bread and durum wheat as influenced by climate. Chil. J. Agric. Res. 2015, 75, 139–146. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Safdar, L.B.; Foulkes, M.J.; Kleiner, F.H.; Bhosale, R.A.; Fisk, I.D.; Boden, S.A. Challenges facing sustainable protein production: Opportunities for cereals. Plant Commun. 2023, 4, 100716. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.M.; Batterham, M.J.; Ray, S.; Beck, E.J. Whole grain, bran and cereal fibre consumption and CVD: A systematic review. Br. J. Nutr. 2019, 121, 914–937. [Google Scholar] [CrossRef]
- Prasadi, N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Dodevska, M.S.; Sobajic, S.S.; Dragicevic, V.D.; Stankovic, I.; Ivanovic, N.D.; Djordjevic, B.I. The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults. Nutrients 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Schupfer, E.; Pak, S.C.; Wang, S.; Micalos, P.S.; Jeffries, T.; Ooi, S.L.; Golombick, T.; Harris, G.; El-Omar, E. The effects and benefits of arabinoxylans on human gut microbiota–A narrative review. Food Biosci. 2021, 43, 101267. [Google Scholar] [CrossRef]
- Mio, K.; Ogawa, R.; Tadenuma, N.; Aoe, S. Arabinoxylan as well as β-glucan in barley promotes GLP-1 secretion by increasing short-chain fatty acids production. Biochem. Biophys. Rep. 2022, 32, 101343. [Google Scholar] [CrossRef]
- Prins, A.; Kosik, O. Genetic Approaches to Increase Arabinoxylan and β-Glucan Content in Wheat. Plants 2023, 12, 3216. [Google Scholar] [CrossRef]
- Syeunda, C.; Awika, J.M. Mechanisms of flavonoid inhibition of Maillard reaction product formation in relation to whole grains processing. Food Chem. 2024, 449, 139237. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Danielski, R.; Ikeda, C. Phenolic compounds in cereal grains and effects of processing on their composition and bioactivities: A review. J. Food Bioact. 2021, 15, 15281. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Araújo Sugizaki, C.S.; Lima, G.C.; Veloso Naves, M.M. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Dragičević, V.; Sredojević, S.; Perić, V.; Nišavić, A.; Srebrić, M. Validation study of a rapid colorimetric method for the determination of phytic acid and norganic phosphorus from grains. Acta Period. Technol. 2011, 42, 11–21. [Google Scholar] [CrossRef]
- Sari-Gorla, M.; Ferrario, S.; Rossini, L.; Frova, C.; Villa, M. Developmental expression of glutathione-S-transferase in maize and its possible connection with herbicide tolerance. Euphytica 1993, 67, 221–230. [Google Scholar] [CrossRef]
- ISO13730; Meat and Meat Products—Determination of Total Phosphorus Content—Spectrometric Method. Institute for Standardization of Serbia: Belgrade, Serbia, 1996. Available online: https://iss.rs/en/project/show/iso:proj:22789 (accessed on 16 December 2024).
- Vancetovic, J.; Zilic, S.; Bozinovic, S.; Ignjatovic-Micic, D. Simulating of Top-Cross system for enhancement of antioxidants in maize grain. Span. J. Agric. Res. 2014, 12, 467–476. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Szőllősi, R.; Varga, I.S.I. Total antioxidant power in some species of Labiatae: Adaptation of FRAP method. Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- McCleary, B.V.; Codd, R. Measurement of (1 → 3),(1 → 4)-β-D-glucan in barley and oats: A streamlined enzymic pro-cedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Miljin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crops Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Helrich, K. AOAC Official Methods of Analysis of AOAC International, 15th ed.; USA Association of Analytical Communities/AOAC Inc.: Washington, DC, USA, 1990. [Google Scholar]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic Compositions and Antioxidant Activities Differ Significantly among Sorghum Grains with Different Applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef] [PubMed]
- Torbica, A.; Radosavljević, M.; Belović, M.; Djukić, N.; Marković, S. Overview of nature, frequency and technological role of dietary fibre from cereals and pseudocereals from grain to bread. Carbohydr. Polym. 2022, 290, 119470. [Google Scholar] [CrossRef]
- Nie, C.; Yan, X.; Xie, X.; Zhang, Z.; Zhu, J.; Wang, Y.; Wang, X.; Xu, N.; Luo, Y.; Sa, Z.; et al. Structure of β-glucan from Tibetan hull-less barley and its in vitro fermentation by human gut microbiota. Chem. Biol. Technol. Agric. 2021, 8, 12. [Google Scholar] [CrossRef]
- Bender, D.; Regner, M.; D’Amico, S.; Jäger, H.; Tömösközi, S.; Schoenlechne, R. Effect of arabinoxylan extraction on gluten-freesourdough-breadproperties. J. Food Qual. 2018, 2018, 5719681. [Google Scholar] [CrossRef]
- Johnson, C.R.; Thavarajah, D.; Thavarajah, P. The influence of phenolic and phytic acid food matrix factors on iron bioavailability potential in 10 commercial lentil genotypes (Lens culinaris L.). J. Food Compos. Anal. 2013, 3, 82–86. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Pan, F.; Yuan, L.; Yin, X. Effects of increasing rates of zinc fertilization on phytic acid and phytic acid/zinc molar ratio in zinc bio-fortified wheat. Field Crops Res. 2015, 184, 58–64. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.Z.; Feng, M.L.; Hao, X.L.; Zhong, Q.M.; Tong, L.G.; Wang, Z.H. Rutin, quercetin, and free amino acid analysis in buckwheat (Fagopyrum) seeds from different locations. Genet. Mol. Res. 2015, 14, 19040–19048. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; San Martín-Martínez, E.; Valverde, M.E.; Rodríguez-Aza, Y.; Berríos, J.D.J.; Mora-Escobedo, R. Profile analysis and correlation across phenolic compounds, isoflavones and antioxidant capacity during germination of soybeans (Glycine max L.). CyTA-J. Food 2017, 15, 516–524. [Google Scholar] [CrossRef]
- Kim, M.Y.; Jang, G.Y.; Lee YLee, M.; Ji, Y.M.; Yoon, N.; Lee, S.H.; Kim, K.M.; Lee, J.; Jeong, H.S. Free and bound form bioactive compound profiles in germinated black soybean (Glycine max L.). Food Sci. Biotechnol. 2016, 25, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2023, 12, 38. [Google Scholar] [CrossRef]
- Shelke, V.; Kale, A.; Kulkarni, Y.A.; Bhanudas Gaikwad, A. Phloretin: A comprehensive review of its potential against diabetes and associated complications. J. Pharm. Pharmacol. 2024, 76, 201–212. [Google Scholar] [CrossRef]
- Sun, B.; Huo, H.-Z.; Cai, A.-H.; Xie, Y.-C.; Li, H.-Y.; Li, D.-P. Determination of contents of eight phenolic acids in Malus doumeri fruit by HPLC. Guangxi Zhiwu/Guihaia 2021, 41, 1135–1144. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xiao, J.; Xu, B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Camerlengo, F.; Kiszonas, A.M. Genetic factors influencing triticale quality for food. J. Cereal Sci. 2023, 113, 103744. [Google Scholar] [CrossRef]

| Protein | Pi | Pphy | YP | TPCs | GSH | β-glucan | Arabinoxylan | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | mg·g−1 | μg·g−1 | nmol·g−1 | g 100·g−1 | ||||||||||||||||||||
| Dur1 | 15.03 a | ± | 0.32 * | 0.37 k | ± | 0.019 | 4.80 bc | ± | 0.02 | 2.75 hi | ± | 0.06 | 1402. 7 h | ± | 3.51 | 77.86 k | ± | 2.80 | 0.11 h | ± | 0.01 | 5.55 i | ± | 0.21 |
| Dur2 | 11.93 ef | ± | 0.24 | 0.50 i | ± | 0.005 | 4.54 defg | ± | 0.02 | 4.70 b | ± | 0.15 | 1240.9 i | ± | 9.23 | 85.05 jk | ± | 5.99 | 0.13 h | ± | 0.02 | 5.39 j | ± | 0.18 |
| Dur3 | 12.60 bcd | ± | 0.48 | 0.48 i | ± | 0.003 | 4.50 fg | ± | 0.06 | 4.39 c | ± | 0.04 | 926.0 k | ± | 14.69 | 96.86 jk | ± | 5.36 | 0.14 h | ± | 0.01 | 4.81 j | ± | 0.31 |
| BW1 | 10.50 ij | ± | 0.22 | 0.39 jk | ± | 0.013 | 4.48 fg | ± | 0.00 | 3.40 f | ± | 0.09 | 735.4 l | ± | 7.00 | 130.57 i | ± | 5.99 | 0.17 h | ± | 0.02 | 6.19 h | ± | 0.26 |
| BW2 | 10.53 hij | ± | 0.26 | 0.37 k | ± | 0.005 | 4.73 cde | ± | 0.06 | 3.05 g | ± | 0.10 | 1550.1 f | ± | 1.48 | 107.41 ij | ± | 9.98 | 0.19 h | ± | 0.04 | 6.74 f | ± | 0.35 |
| Trit1 | 12.53 cde | ± | 0.26 | 0.41 j | ± | 0.012 | 5.02 ab | ± | 0.06 | 2.54 j | ± | 0.06 | 789.0 l | ± | 8.90 | 226.82 f | ± | 2.38 | 0.18 h | ± | 0.01 | 6.50 fg | ± | 0.30 |
| Trit2 | 12.03 de | ± | 0.36 | 0.55 h | ± | 0.003 | 4.67 cdefg | ± | 0.12 | 2.66 ij | ± | 0.06 | 1115.9 j | ± | 8.08 | 179.30 g | ± | 2.78 | 0.23 h | ± | 0.03 | 6.47 g | ± | 0.25 |
| Trit3 | 13.00 bc | ± | 0.83 | 0.49 i | ± | 0.005 | 4.68 cdef | ± | 0.09 | 2.90 gh | ± | 0.02 | 925.8 k | ± | 5.45 | 188.87 g | ± | 5.99 | 0.13 h | ± | 0.01 | 6.32 gh | ± | 0.21 |
| Bar1 | 11.13 g | ± | 0.54 | 0.87 b | ± | 0.010 | 4.46 g | ± | 0.00 | 3.77 e | ± | 0.11 | 1115.1 j | ± | 11.18 | 607.34 a | ± | 21.16 | 4.15 b | ± | 0.28 | 4.25 l | ± | 0.34 |
| Bar2 | 14.50 a | ± | 0.24 | 0.91 a | ± | 0.015 | 4.50 fg | ± | 0.01 | 3.67 e | ± | 0.04 | 1784.5 d | ± | 6.19 | 399.74 d | ± | 4.36 | 4.59 a | ± | 0.38 | 3.86 m | ± | 0.37 |
| Bar3 | 11.23 g | ± | 0.27 | 0.72 e | ± | 0.019 | 4.70 cdef | ± | 0.17 | 3.61 e | ± | 0.13 | 924.1 k | ± | 8.88 | 364.56 e | ± | 7.59 | 3.78 c | ± | 0.40 | 4.54 k | ± | 0.30 |
| Bar4 | 13.17 b | ± | 0.53 | 0.60 g | ± | 0.010 | 4.19 h | ± | 0.05 | 4.16 d | ± | 0.02 | 2175.2 b | ± | 4.18 | 498.73 b | ± | 2.80 | 3.44 cd | ± | 0.18 | 6.26 gh | ± | 0.33 |
| Em1 | 10.12 jk | ± | 0.18 | 0.54 h | ± | 0.011 | 4.53 efg | ± | 0.07 | 2.63 ij | ± | 0.15 | 1486.6 g | ± | 1.61 | 155.36 h | ± | 2.76 | 0.13 h | ± | 0.03 | 3.84 m | ± | 0.32 |
| Em2 | 10.10 jk | ± | 0.30 | 0.69 ef | ± | 0.011 | 4.76 cd | ± | 0.04 | 3.65 e | ± | 0.02 | 1631.4 e | ± | 11.87 | 170.73 gh | ± | 3.37 | 0.17 h | ± | 0.04 | 4.81 j | ± | 0.30 |
| Spelt | 9.85 k | ± | 0.23 | 0.82 c | ± | 0.013 | 5.22 a | ± | 0.02 | 5.01 a | ± | 0.06 | 1735.5 d | ± | 4.66 | 217.64 f | ± | 4.37 | 0.39 gh | ± | 0.08 | 0.51 n | ± | 0.17 |
| Rye1 | 11.03 ghi | ± | 0.61 | 0.72 e | ± | 0.005 | 4.13 h | ± | 0.01 | 4.19 d | ± | 0.15 | 1261.2 i | ± | 7.46 | 397.13 d | ± | 0.57 | 0.62 fg | ± | 0.04 | 7.25 e | ± | 0.33 |
| Rye2 | 11.07 ghi | ± | 0.51 | 0.56 h | ± | 0.007 | 4.04 h | ± | 0.10 | 4.04 d | ± | 0.02 | 1082.8 j | ± | 11.87 | 370.17 e | ± | 8.37 | 0.85 f | ± | 0.13 | 7.62 d | ± | 0.28 |
| Oats1 | 11.40 fg | ± | 0.32 | 0.63 g | ± | 0.008 | 4.06 h | ± | 0.09 | 4.73 b | ± | 0.10 | 2359.1 a | ± | 3.98 | 473.78 c | ± | 9.37 | 3.05 e | ± | 0.17 | 12.69 a | ± | 0.33 |
| Oats2 | 11.10 gh | ± | 0.29 | 0.76 d | ± | 0.000 | 4.00 h | ± | 0.11 | 3.26 f | ± | 0.15 | 2404.6 a | ± | 10.25 | 481.18 bc | ± | 8.37 | 3.70 c | ± | 0.39 | 12.04 b | ± | 0.25 |
| Oats3 | 11.13 g | ± | 0.84 | 0.67 f | ± | 0.022 | 3.49 i | ± | 0.07 | 2.81 hi | ± | 0.02 | 1981.7 c | ± | 1.18 | 461.39 c | ± | 6.94 | 3.31 de | ± | 0.48 | 10.65 c | ± | 0.73 |
| Tukey HSD (p < 0.05) | 0.11 | 0.006 | 0.041 | 0.036 | 9.934 | 4.246 | 0.066 | 0.048 | ||||||||||||||||
| P | K | Ca | Mg | Na | Fe | Mn | Zn | Cu | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g·kg−1 | mg·kg−1 | ||||||||||||||||||||||||||
| Dur1 | 6.12 de | ± | 0.05 * | 2.99 h | ± | 0.02 | 397.0 k | ± | 4.94 | 1274.0 b | ± | 8.82 | 73.50 c | ± | 3.45 | 51.65 d | ± | 1.76 | 30.40 f | ± | 0.98 | 38.20 b | ± | 1.63 | 7.10 b | ± | 0.24 |
| Dur2 | 6.25 cd | ± | 0.04 | 3.22 e | ± | 0.03 | 486.5 fg | ± | 7.86 | 1183.5 g | ± | 9.37 | 56.95 gh | ± | 3.74 | 44.85 ef | ± | 1.27 | 28.80 g | ± | 1.63 | 32.30 efg | ± | 1.80 | 6.20 de | ± | 0.33 |
| Dur3 | 5.41 gh | ± | 0.06 | 2.68 kl | ± | 0.03 | 377.0 l | ± | 8.08 | 1097.5 k | ± | 9.74 | 54.50 hi | ± | 1.86 | 40.60 g | ± | 1.71 | 22.65 i | ± | 1.10 | 33.30 de | ± | 1.39 | 6.60 c | ± | 0.33 |
| BW1 | 4.94 k | ± | 0.00 | 3.02 fg | ± | 0.02 | 491.5 ef | ± | 8.31 | 1090.5 k | ± | 7.90 | 62.65 fg | ± | 0.50 | 38.65 g | ± | 1.59 | 32.60 cde | ± | 1.47 | 32.40 efg | ± | 1.31 | 5.30 f | ± | 0.33 |
| BW2 | 5.25 hi | ± | 0.05 | 3.03 f | ± | 0.02 | 478.0 g | ± | 7.63 | 1256.5 c | ± | 9.37 | 66.90 def | ± | 1.54 | 39.90 g | ± | 1.47 | 34.00 bc | ± | 1.39 | 30.05 h | ± | 0.94 | 6.45 cd | ± | 0.29 |
| Trit1 | 5.73 f | ± | 0.04 | 2.73 ij | ± | 0.02 | 336.5 m | ± | 7.41 | 1202.5 f | ± | 9.74 | 81.35 b | ± | 2.57 | 36.50 h | ± | 1.55 | 31.85 e | ± | 1.59 | 32.65 ef | ± | 1.43 | 7.05 b | ± | 0.37 |
| Trit2 | 5.69 f | ± | 0.04 | 3.39 c | ± | 0.03 | 442.0 i | ± | 4.94 | 1238.5 d | ± | 12.31 | 92.95 a | ± | 1.88 | 23.50 k | ± | 0.82 | 26.30 h | ± | 1.14 | 23.85 k | ± | 1.02 | 3.60 ij | ± | 0.33 |
| Trit3 | 5.76 f | ± | 0.05 | 2.99 gh | ± | 0.02 | 462.5 h | ± | 10.10 | 1148.0 i | ± | 9.55 | 62.00 fg | ± | 1.59 | 28.40 j | ± | 1.14 | 32.10 e | ± | 1.14 | 34.95 c | ± | 1.67 | 4.85 g | ± | 0.45 |
| Bar1 | 5.71 f | ± | 0.02 | 2.52 m | ± | 0.03 | 401.5 k | ± | 5.61 | 1171.5 gh | ± | 8.27 | 88.6 a | ± | 1.72 | 55.70 c | ± | 1.14 | 13.40 l | ± | 0.57 | 19.30 l | ± | 1.14 | 5.55 f | ± | 0.45 |
| Bar2 | 5.80 f | ± | 0.05 | 2.67 kl | ± | 0.02 | 497.5 e | ± | 6.96 | 1092.0 k | ± | 7.72 | 81.85 b | ± | 1.19 | 34.85 hi | ± | 1.67 | 13.60 l | ± | 0.65 | 31.15 gh | ± | 1.43 | 3.80 i | ± | 0.33 |
| Bar3 | 5.74 f | ± | 0.06 | 3.23 e | ± | 0.02 | 455.0 h | ± | 9.43 | 1134.0 j | ± | 8.45 | 65.00 ef | ± | 2.12 | 24.40 k | ± | 0.49 | 10.70 m | ± | 0.73 | 18.70 l | ± | 0.98 | 3.35 j | ± | 0.29 |
| Bar4 | 6.07 e | ± | 0.01 | 3.33 d | ± | 0.03 | 547.0 d | ± | 9.88 | 1174.5 g | ± | 9.37 | 57.00 gh | ± | 1.59 | 45.05 ef | ± | 0.94 | 15.75 k | ± | 0.86 | 27.25 i | ± | 1.18 | 7.35 ab | ± | 0.37 |
| Em1 | 6.56 b | ± | 0.03 | 2.70 jk | ± | 0.02 | 368.5 l | ± | 7.86 | 1220.0 e | ± | 8.08 | 61.95 fg | ± | 0.82 | 59.65 b | ± | 2.16 | 34.20 b | ± | 1.63 | 35.00 c | ± | 1.55 | 7.55 a | ± | 0.45 |
| Em2 | 6.41 bc | ± | 0.07 | 2.24 n | ± | 0.02 | 258.5 n | ± | 8.31 | 1039.0 l | ± | 7.35 | 68.20 cdef | ± | 2.65 | 49.85 f | ± | 1.92 | 32.50 de | ± | 1.39 | 38.00 b | ± | 1.22 | 7.70 a | ± | 0.41 |
| Spelt | 7.94 a | ± | 0.08 | 3.76 a | ± | 0.03 | 538.0 d | ± | 7.63 | 1568.5 a | ± | 11.57 | 63.10 fg | ± | 1.91 | 57.50 c | ± | 2.37 | 39.20 a | ± | 1.88 | 70.25 a | ± | 2.33 | 7.15 b | ± | 0.53 |
| Rye1 | 5.10 ijk | ± | 0.01 | 2.67 l | ± | 0.02 | 414.0 j | ± | 6.29 | 1040.5 l | ± | 7.53 | 72.00 cd | ± | 1.59 | 62.70 a | ± | 1.47 | 23.60 i | ± | 1.22 | 31.35 fgh | ± | 0.94 | 6.20 de | ± | 0.24 |
| Rye2 | 5.08 ijk | ± | 0.05 | 2.76 i | ± | 0.03 | 464.0 h | ± | 6.29 | 1040.5 l | ± | 7.90 | 73.85 c | ± | 1.41 | 45.55 e | ± | 1.02 | 21.05 j | ± | 1.18 | 32.55 ef | ± | 1.59 | 6.15 de | ± | 0.45 |
| Oats1 | 5.47 g | ± | 0.08 | 3.02 fg | ± | 0.02 | 816.5 a | ± | 7.41 | 1161.5 h | ± | 11.94 | 50.00 i | ± | 2.12 | 34.00 i | ± | 1.14 | 33.60 bcd | ± | 0.73 | 34.40 cd | ± | 0.98 | 4.55 gh | ± | 0.37 |
| Oats2 | 5.15 ij | ± | 0.06 | 3.48 b | ± | 0.01 | 761.5 b | ± | 7.86 | 1140.0 ij | ± | 7.35 | 42.00 j | ± | 1.59 | 43.00 f | ± | 1.63 | 26.85 h | ± | 1.10 | 25.45 j | ± | 1.02 | 4.35 h | ± | 0.12 |
| Oats3 | 5.01 jk | ± | 0.04 | 2.51 m | ± | 0.02 | 729.0 c | ± | 8.98 | 1097.0 k | ± | 9.55 | 71.00 cde | ± | 1.06 | 61.00 ab | ± | 2.86 | 33.90 bcd | ± | 0.82 | 32.10 efg | ± | 1.63 | 6.00 e | ± | 0.41 |
| Tukey HSD (p < 0.05) | 0.032 | 0.006 | 1.87 | 2.32 | 1.17 | 0.39 | 0.26 | 0.25 | 0.07 | ||||||||||||||||||
| GA | CAT | EPI | DiCA | di-p-CoumA | NA | NG | DA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dur1 | n.d. | n.d. | n.d. | 74.60 | ± | 0.93 | 36.77 | ± | 0.54 | 38.53 | ± | 0.48 | n.d. | n.d. | ||||||||||
| Dur2 | n.d. | n.d. | n.d. | 59.03 | ± | 0.85 | n.d. | 37.04 | ± | 0.53 | n.d. | n.d. | ||||||||||||
| Dur3 | n.d. | n.d. | 38.51 | ± | 0.06 | 54.19 | ± | 0.20 | 43.35 | ± | 0.61 | 40.92 | ± | 0.51 | n.d. | n.d. | ||||||||
| BW1 | n.d. | n.d. | n.d. | 65.68 | ± | 0.84 | 45.19 | ± | 0.25 | 38.05 | ± | 0.22 | n.d. | 25.48 | ± | 0.34 | ||||||||
| BW2 | n.d. | 31.07 | ± | 0.70 | 38.72 | ± | 0.32 | 83.28 | ± | 0.56 | 61.72 | ± | 0.78 | 40.33 | ± | 0.50 | 46.42 | ± | 0.58 | n.d. | ||||
| Trit1 | n.d. | n.d. | n.d. | 87.32 | ± | 1.10 | 32.08 | ± | 0.17 | 39.96 | ± | 0.51 | n.d. | 26.15 | ± | 0.38 | ||||||||
| Trit2 | n.d. | n.d. | n.d. | 113.7 | ± | 1.47 | 31.79 | ± | 0.17 | 39.31 | ± | 0.50 | n.d. | 25.57 | ± | 0.37 | ||||||||
| Trit3 | n.d. | n.d. | 39.30 | ± | 0.03 | 87.92 | ± | 0.72 | 36.82 | ± | 0.17 | 38.01 | ± | 0.48 | n.d. | 25.63 | ± | 0.37 | ||||||
| Bar1 | 33.36 | ± | 5.88 * | 443.29 | ± | 5.80 | 44.61 | ± | 0.12 | 38.89 | ± | 0.12 | 48.18 | ± | 0.25 | 38.31 | ± | 0.21 | n.d. | 28.54 | ± | 0.37 | ||
| Bar2 | 35.13 | ± | 6.09 | 459.19 | ± | 6.04 | 41.86 | ± | 0.06 | 39.81 | ± | 0.04 | 42.65 | ± | 0.20 | 38.30 | ± | 0.49 | 46.66 | ± | 0.56 | 26.89 | ± | 0.59 |
| Bar3 | 31.52 | ± | 4.71 | 360.52 | ± | 4.65 | 39.13 | ± | 0.10 | 37.45 | ± | 0.06 | 42.13 | ± | 0.18 | 34.02 | ± | 0.45 | n.d. | 27.68 | ± | 0.40 | ||
| Bar4 | 31.16 | ± | 5.57 | 426.00 | ± | 5.46 | 45.31 | ± | 0.33 | 50.07 | ± | 0.22 | 62.54 | ± | 0.45 | 36.69 | ± | 0.48 | n.d. | 27.43 | ± | 0.40 | ||
| Em1 | n.d. | n.d. | n.d. | 105.9 | ± | 1.36 | 30.03 | ± | 0.13 | 38.52 | ± | 0.50 | 49.51 | ± | 0.71 | 28.77 | ± | 0.62 | ||||||
| Em2 | n.d. | n.d. | n.d. | 97.42 | ± | 1.25 | 28.45 | ± | 0.13 | 36.24 | ± | 0.47 | 52.33 | ± | 0.76 | 26.45 | ± | 0.65 | ||||||
| Spelt | n.d. | n.d. | 42.34 | ± | 0.25 | 64.28 | ± | 0.52 | 22.45 | ± | 0.45 | 35.22 | ± | 0.51 | n.d. | n.d. | ||||||||
| Rye1 | n.d. | 35.57 | ± | 0.99 | n.d. | 79.45 | ± | 1.15 | n.d. | 36.44 | ± | 0.53 | n.d. | n.d. | ||||||||||
| Rye2 | n.d. | 33.84 | ± | 1.17 | n.d. | 92.59 | ± | 1.34 | n.d. | 37.78 | ± | 0.55 | n.d. | n.d. | ||||||||||
| Oats1 | 31.48 | ± | 0.45 | n.d. | 121.69 | ± | 2.28 | n.d. | 231.6 | ± | 2.71 | 50.68 | ± | 0.66 | 47.10 | ± | 0.64 | 38.30 | ± | 0.63 | ||||
| Oats2 | 32.90 | ± | 0.47 | n.d. | 100.26 | ± | 3.05 | n.d. | 280.42 | ± | 3.47 | 48.22 | ± | 0.61 | 42.35 | ± | 0.64 | 32.48 | ± | 0.55 | ||||
| Oats3 | 34.23 | ± | 0.49 | n.d. | 114.25 | ± | 2.06 | n.d. | 210.2 | ± | 2.45 | 45.84 | ± | 0.60 | 45.22 | ± | 0.61 | 35.45 | ± | 0.59 | ||||
| ChlA | CA | p-CoumA | FA | CIN | IFA | QUE | ||||||||||||||||||
| Dur1 | n.d. | n.d. | 30.59 | ± | 0.03 | 28.34 | ± | 0.28 | 21.44 | ± | 0.31 | 28.74 | ± | 0.35 | 8.87 | ± | 24.44 | |||||||
| Dur2 | n.d. | n.d. | 30.81 | ± | 0.03 | 29.23 | ± | 0.27 | n.d. | 28.60 | ± | 0.32 | 10.38 | ± | 21.89 | |||||||||
| Dur3 | n.d. | n.d. | 27.62 | ± | 0.02 | 29.34 | ± | 0.33 | 21.55 | ± | 0.31 | 29.18 | ± | 0.40 | 6.63 | ± | 15.97 | |||||||
| BW1 | 27.95 | ± | 0.02 | 28.58 | ± | 0.05 | 27.26 | ± | 0.05 | 30.90 | ± | 0.28 | 21.48 | ± | 0.37 | 28.02 | ± | 0.31 | 10.23 | ± | 12.75 | |||
| BW2 | 27.77 | ± | 0.42 | n.d. | 29.93 | ± | 0.02 | 28.72 | ± | 0.31 | 21.21 | ± | 0.33 | 28.31 | ± | 0.38 | 6.83 | ± | 26.98 | |||||
| Trit1 | n.d. | n.d. | 29.20 | ± | 0.03 | 30.18 | ± | 0.33 | n.d. | 31.40 | ± | 0.42 | 7.64 | ± | 13.88 | |||||||||
| Trit2 | n.d. | n.d. | 27.60 | ± | 0.03 | 29.57 | ± | 0.30 | n.d. | 29.00 | ± | 0.37 | 8.29 | ± | 19.79 | |||||||||
| Trit3 | n.d. | n.d. | 28.72 | ± | 0.06 | 30.46 | ± | 0.33 | n.d. | 33.43 | ± | 0.43 | 9.39 | ± | 15.60 | |||||||||
| Bar1 | 96.68 | ± | 0.98 | 29.85 | ± | 0.08 | 27.44 | ± | 0.45 | 33.80 | ± | 0.42 | 21.21 | ± | 1.27 | n.d. | 14.59 | ± | 19.55 | |||||
| Bar2 | 73.30 | ± | 0.63 | 29.07 | ± | 0.04 | 29.95 | ± | 0.45 | 32.26 | ± | 0.40 | n.d. | n.d. | 15.40 | ± | 31.26 | |||||||
| Bar3 | 89.14 | ± | 0.87 | 29.08 | ± | 0.03 | 28.31 | ± | 0.42 | 30.39 | ± | 0.38 | n.d. | n.d. | 14.84 | ± | 16.11 | |||||||
| Bar4 | 100.6 | ± | 1.13 | 31.13 | ± | 0.03 | 32.04 | ± | 0.47 | 33.13 | ± | 0.42 | n.d. | n.d. | 13.94 | ± | 37.96 | |||||||
| Em1 | n.d. | n.d. | 28.45 | ± | 0.42 | 30.11 | ± | 0.39 | n.d. | n.d. | 8.47 | ± | 26.17 | |||||||||||
| Em2 | n.d. | n.d. | 31.45 | ± | 0.48 | 34.22 | ± | 0.45 | n.d. | n.d. | 7.25 | ± | 28.54 | |||||||||||
| Spelt | n.d. | n.d. | 34.55 | ± | 0.09 | 28.56 | ± | 0.31 | n.d. | 27.89 | ± | 0.38 | 6.45 | ± | 30.69 | |||||||||
| Rye1 | n.d. | n.d. | 36.85 | ± | 0.11 | 30.45 | ± | 0.31 | n.d. | 28.85 | ± | 0.36 | 8.45 | ± | 21.86 | |||||||||
| Rye2 | n.d. | n.d. | 32.83 | ± | 0.06 | 28.35 | ± | 0.29 | n.d. | 30.53 | ± | 0.37 | 9.51 | ± | 18.75 | |||||||||
| Oats1 | n.d. | 41.55 | ± | 0.56 | 34.26 | ± | 0.66 | 76.28 | ± | 0.68 | n.d. | 27.48 | ± | 0.07 | 31.39 | ± | 41.30 | |||||||
| Oats2 | n.d. | 45.88 | ± | 0.55 | 36.23 | ± | 0.69 | 78.42 | ± | 0.76 | n.d. | 26.56 | ± | 0.02 | 25.45 | ± | 42.18 | |||||||
| Oats3 | n.d. | 38.22 | ± | 0.29 | 42.56 | ± | 0.41 | 60.22 | ± | 0.47 | n.d. | 27.45 | ± | 0.02 | 28.56 | ± | 35.44 | |||||||
| Genotypes | DPPH | FRAP | ||||
|---|---|---|---|---|---|---|
| µmol TE·g−1 | µmol Fe2+ eq·g−1 | |||||
| Dur1 | 201.1 a | ± | 1.88 * | 20.81 m | ± | 0.29 |
| Dur2 | 183.7 cd | ± | 4.95 | 29.87 g | ± | 0.47 |
| Dur3 | 192.8 b | ± | 7.87 | 25.03 j | ± | 0.47 |
| BW1 | 173.6 e | ± | 3.75 | 26.97 i | ± | 0.41 |
| BW2 | 155.4 fgh | ± | 0.79 | 30.45 fg | ± | 0.38 |
| Trit1 | 135.4 k | ± | 4.77 | 36.45 de | ± | 0.55 |
| Trit2 | 149.3 ghi | ± | 4.33 | 23.40 k | ± | 0.38 |
| Trit3 | 111.8 l | ± | 2.92 | 18.78 n | ± | 0.28 |
| Bar1 | 117.8 l | ± | 5.99 | 36.12 e | ± | 0.57 |
| Bar2 | 141.7 jk | ± | 3.32 | 37.39 cd | ± | 0.52 |
| Bar3 | 150.2 gh | ± | 4.76 | 28.79 h | ± | 0.45 |
| Bar4 | 183.5 cd | ± | 2.24 | 31.31 f | ± | 0.42 |
| Em1 | 177.1 de | ± | 0.86 | 22.22 l | ± | 0.29 |
| Em2 | 180.5 cde | ± | 6.36 | 25.61 j | ± | 0.45 |
| Spelt | 187.3 bc | ± | 2.50 | 28.32 h | ± | 0.40 |
| Rye1 | 159.4 f | ± | 4.00 | 23.09 kl | ± | 0.36 |
| Rye2 | 177.7 de | ± | 6.36 | 20.01 m | ± | 0.38 |
| Oats1 | 142.4 ijk | ± | 2.13 | 42.15 b | ± | 0.56 |
| Oats2 | 148.2 hij | ± | 5.49 | 45.32 a | ± | 0.67 |
| Oats3 | 156.1 fg | ± | 0.63 | 37.99 c | ± | 0.48 |
| Tukey HSD (p < 0.05) | 1.33 | 0.198 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragičević, V.; Simić, M.; Kandić Raftery, V.; Vukadinović, J.; Dodevska, M.; Đurović, S.; Brankov, M. Screening of Nutritionally Important Components in Standard and Ancient Cereals. Foods 2024, 13, 4116. https://doi.org/10.3390/foods13244116
Dragičević V, Simić M, Kandić Raftery V, Vukadinović J, Dodevska M, Đurović S, Brankov M. Screening of Nutritionally Important Components in Standard and Ancient Cereals. Foods. 2024; 13(24):4116. https://doi.org/10.3390/foods13244116
Chicago/Turabian StyleDragičević, Vesna, Milena Simić, Vesna Kandić Raftery, Jelena Vukadinović, Margarita Dodevska, Sanja Đurović, and Milan Brankov. 2024. "Screening of Nutritionally Important Components in Standard and Ancient Cereals" Foods 13, no. 24: 4116. https://doi.org/10.3390/foods13244116
APA StyleDragičević, V., Simić, M., Kandić Raftery, V., Vukadinović, J., Dodevska, M., Đurović, S., & Brankov, M. (2024). Screening of Nutritionally Important Components in Standard and Ancient Cereals. Foods, 13(24), 4116. https://doi.org/10.3390/foods13244116









