Recent Progress of Molecularly Imprinted Technique for the Detection of Mycotoxins in Food
Abstract
:1. Introduction
2. Preparation Strategies of MIPs
2.1. Bulk Polymerization
2.2. Precipitation Polymerization
2.3. In Situ Polymerization
2.4. Emulsion Polymerization
2.5. Suspension Polymerization
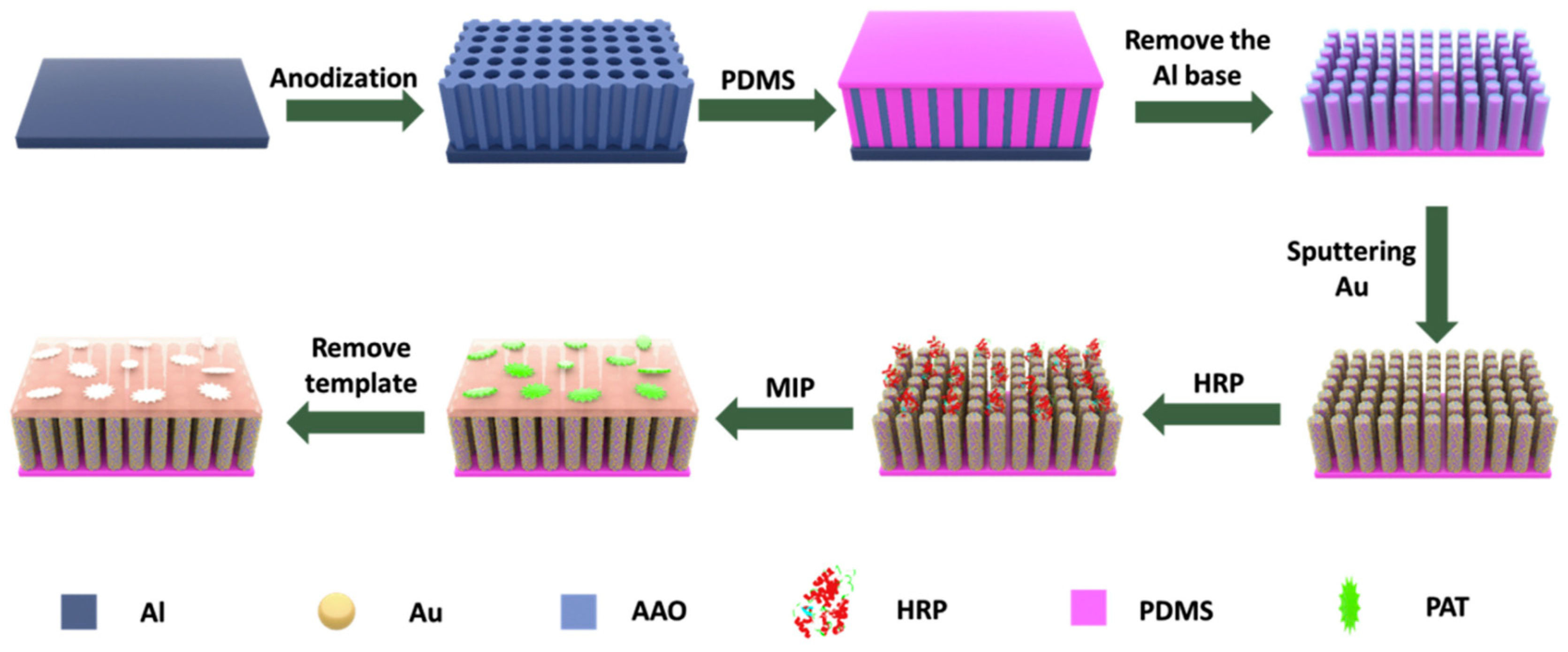
2.6. Surface Imprinting Polymerization
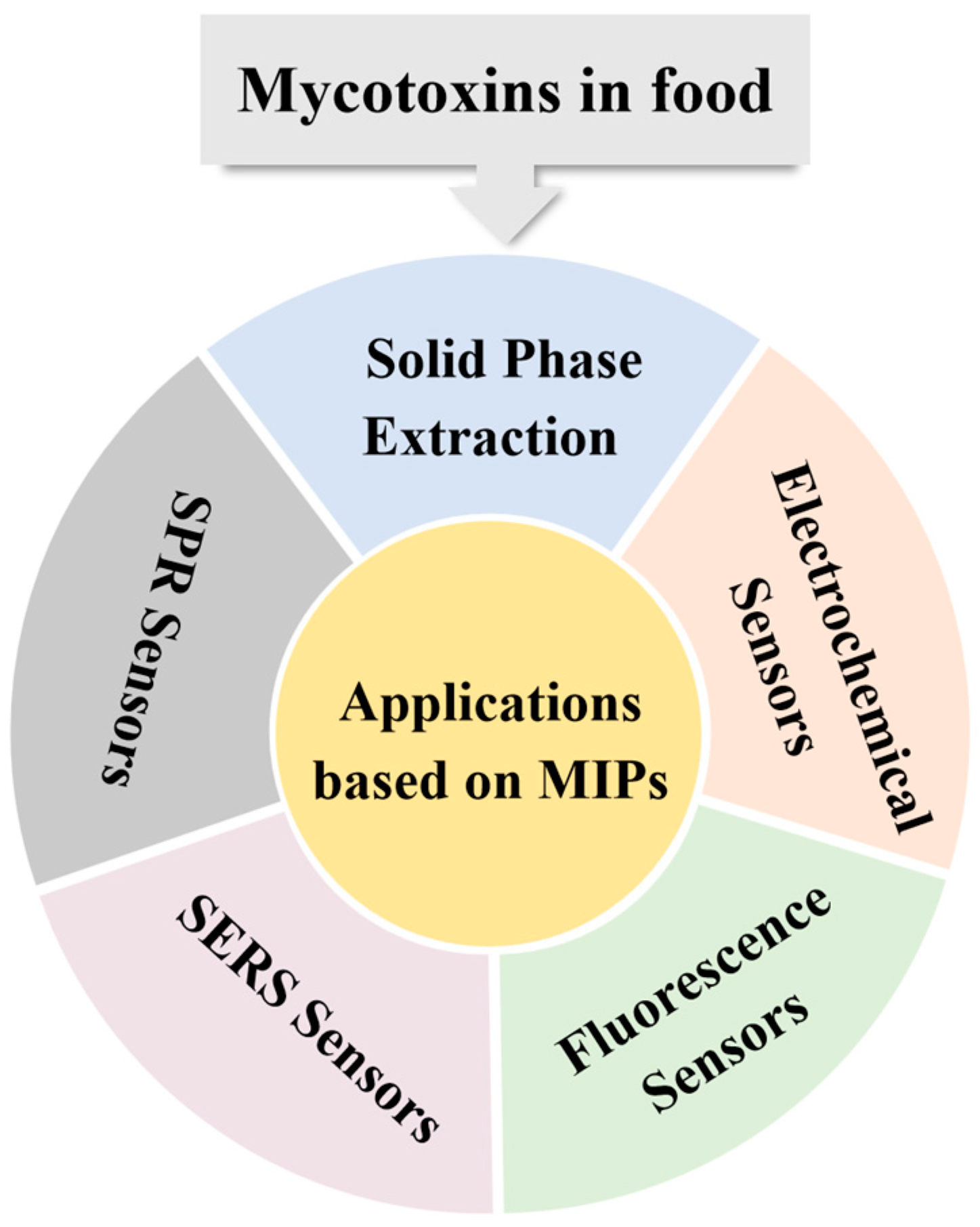
3. Applications Based on MIPs
3.1. Solid-Phase Extraction
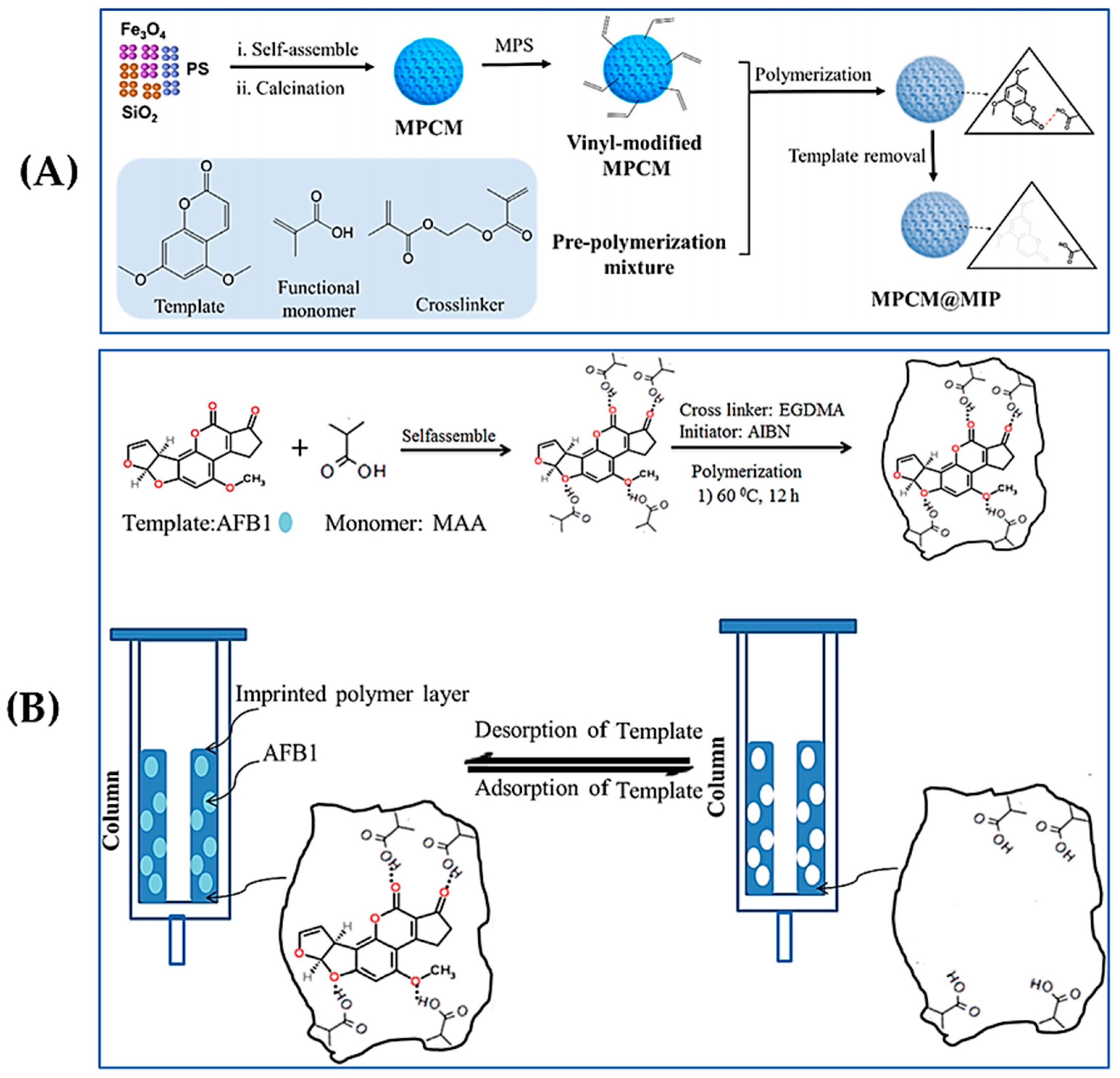
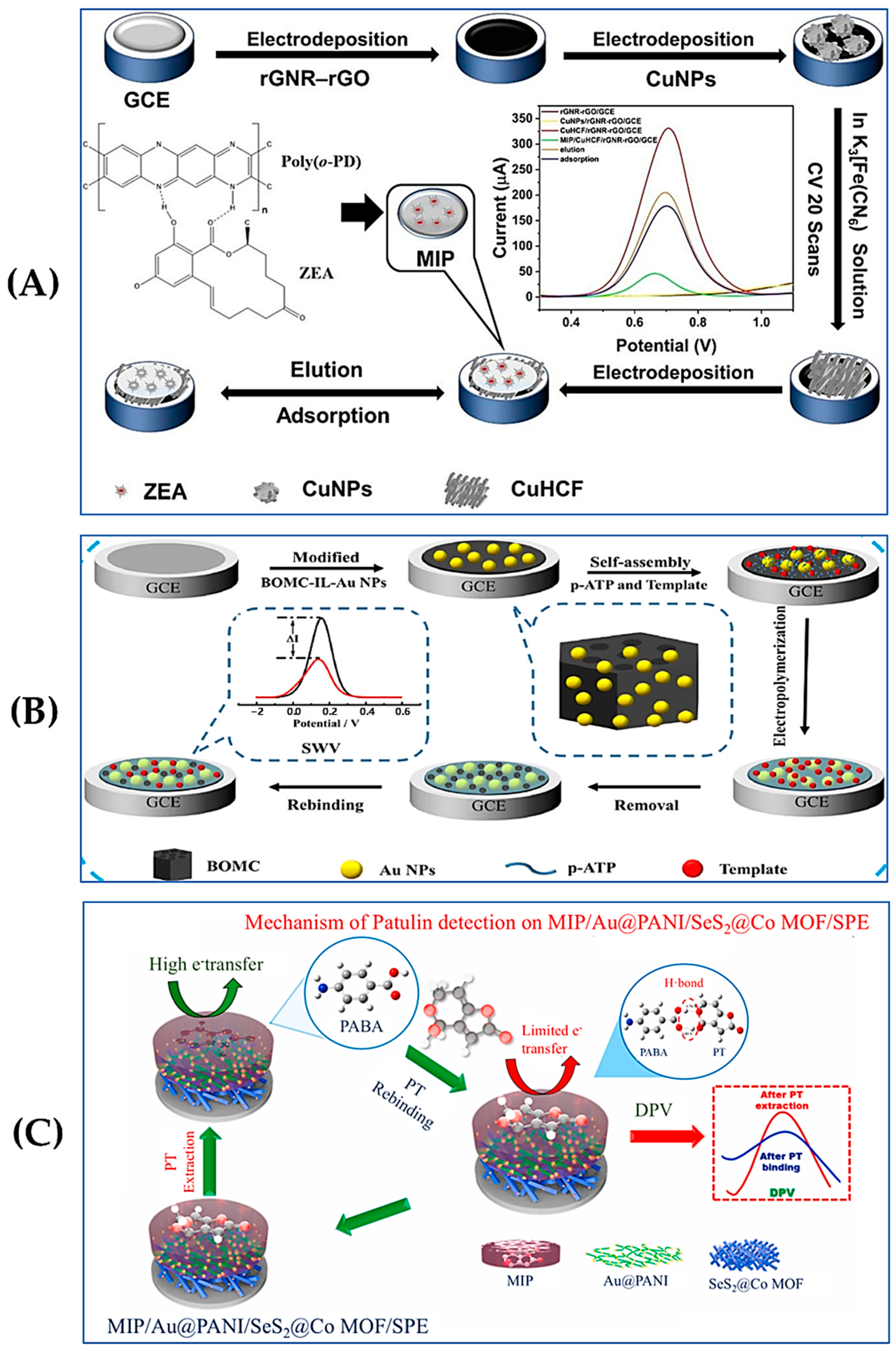
3.2. Electrochemical Sensors
3.3. Fluorescence Sensors
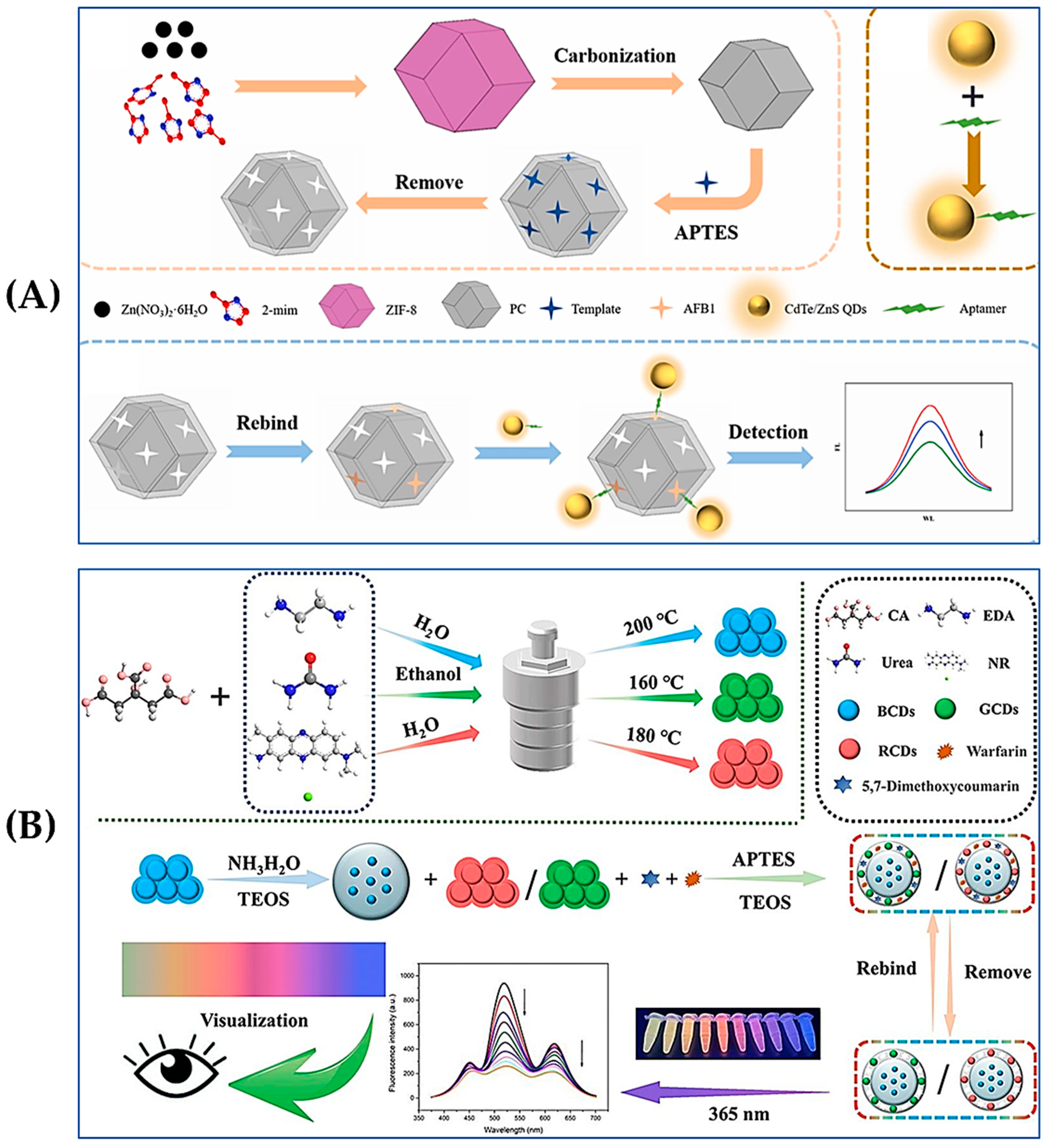
3.4. SERS Sensors
3.5. SPR Sensors
| Areas of Application | Mycotoxins | LODs | Linear Rages | Matrix Samples | Pre-Treatment | % Recovery | Imprinting Factor | Ref. |
|---|---|---|---|---|---|---|---|---|
| SPE | AFB1 | 0.1 μg/L | 0.1~10 μg/L | peanut | extract with methanol/KH2PO4, filtrate | 93~102% | 2.19 | [51] |
| SPE | AFB1 | 0.05 ng/mL | 10~1000 ng/mL | soy sauce | extract with methanol/water, dilute with water | 96% | N/A | [52] |
| SPE | AFs | 0.05 μg/kg (AFG2); 0.06 μg/kg (AFG1); 0.06 μg/kg (AFB2); 0.05 μg/kg (AFB1); | 0.1~50 μg/kg | rice, corn, wheat, peanut and soybean | extract with acetonitrile/water, filtrate, dilute with 1% Tween-20 PBS | 82.6~116.7% | 2.42 | [53] |
| SPE | AFB1 | 0.4 ng/mL | 5~1000 ng/mL | soy sauce, vinegar | extract with methanol, centrifuge | 73~92% | 1.5 | [55] |
| SPE | AFB1; AFB2 | 0.0024 ng/mL (AFB1); 0.0004 ng/mL (AFB2) | 0.005~0.5 ng/mL (AFB1); 0.001~0.1 ng/mL (AFB2) | corn, peanut, edible oil | extract with methanol/water, centrifuge | 89~105% | N/A | [56] |
| SPE | AFB1; AFB2; AFG1; AFG2 | 0.23~0.33 μg/kg | 0.1~400 μg/kg | wheat, rice, corn | extract with methanol/water, centrifuge, filtrate, dilute with phosphate buffer, refiltrate | 95.3~98.5% | 3.29 (AFB1); 2.81 (AFB2); 3.22 (AFG1); 3.00 (AFG2) | [62] |
| SPE | AFs | 0.003~0.09 ng/mL | 0.02~200 ng/mL (AFG2); 0.3~200 ng/mL (AFG1); 0.01~200 ng/mL (AFB2); 0.2~200 ng/mL (AFB1); | rice, corn, wheat and peanut | extract with ACN/water, centrifuge, concentrate with nitrogen stream, redissolve with ACN | 85.4~105.4% | 2.98 | [64] |
| Electrochemical sensors | AFB1 | 0.52 pg/mL | 1.56~31.23 pg/mL | cinnamon | N/A | 98.21% | N/A | [72] |
| Electrochemical sensors | ZEN | 0.09 ng/mL | 0.25~500 ng/mL | corn meal | N/A | 98.59% (50 ng/mL); 102.18% (100 ng/mL); 97.30% (250 ng/mL) | N/A | [74] |
| Electrochemical sensors | ZEN | 0.25 ng/L | 1~10 ng/L | rice | extract with EtOH/ACN, centrifuge, dilute with PBS | 100% | N/A | [76] |
| Electrochemical sensors | ZEN | 1 × 10−4 ng/mL | 0.005~1 ng/mL | corn, rice, beer | corn and rice: extract with ACN/water, centrifuge, dilute with PBS beer: degas, dilute with PBS | 96~110% | N/A | [75] |
| Electrochemical sensors | PT | 0.66 pM | 0.001~100 nm | apple juice | dilute with PBS | 94.5~106.4% | 15.4 | [77] |
| Fluorescence Sensors | AFs | 0.016 mg/L | 4~15 μg/kg | non-dairy beverages (four almond based-, three soy based-, and three rice based-beverages) | centrifuge | 99 ± 4~107 ± 5% | 30.6 | [80] |
| Fluorescence Sensors | AFB1 | 4 pg/mL | 0.01~20 ng/mL | edible oil (peanut, corn, and olive) | extract with methanol/water, filtrate | 91.9~102.6% | 4.77 | [81] |
| Fluorescence Sensors | AFB1; ZEN | 3.2 Pg/mL (AFB1); 18 Pg/mL (ZEN) | 0.01~100 ng/mL (AFB1); 0.03~100 ng/mL (ZEN) | corn and peanut oil | extract with methanol/water, redissolve in PBS | 96.3~103.7% (AFB1); 99.1~102.6% (ZEN) | 21.89(AFB1); 21.95(ZEN) | [84] |
| SERS Sensors | PAT | 5.37 × 10−12 M | 7 × 10−12~5 × 10−8 M | blueberry sauce, grapefruit sauce, and orange juice | extract withethyl acetate/n-hexane solution, desiccation with sodium sulfate, solvent evaporation, dilute with water | 96~101% | N/A | [92] |
| SERS Sensors | PAT | 8.5 × 10−11 M | 5 × 10−10~10−6 M | blueberry jam, grapefruit jam and orange juice | N/A | 96.43~112.83% | N/A | [93] |
| SPR Sensors | OTA | 0.028 ng/mL | 0.1~20 ng/mL | dried fig | extract with acetonitrile/water, filtrate, dilute with PBS | 98 ± 2.43~100 ± 8.3% | 2.85 | [98] |
| SPR Sensors | AFB1 | 1.04 pg/mL | 0.0001~10 pg/mL | ground corn, peanut | extract with methanol/water, centrifuge, dilute with PBS | 96.63~105.94% | 5.91 | [99] |
| SPR Sensors | ZEN | 0.33 ng/L | 1~10 ng/L | rice grain | extract with ethanol/acetonitrile solution, centrifuge, dilute with PBS | N/A | N/A | [101] |
4. Conclusions
- The preparation conditions of MIPs play a major role in the performance of the final products. Therefore, further optimization could help to improve the performance of current MIPs. The template molecules should possess the right size, shape, and functional groups. Moreover, the functional groups should not hinder the polymerization reaction. Functional monomers should be selected as inert as possible to avoid excessive non-specific adsorption. Preparation conditions should be improved to ensure optimal adsorption performance of MIPs [102].
- MIPs need to be evaluated for imprinting efficiency by adsorption and selectivity experiments, and performance differences need to be compared with non-imprinted polymers (NIPs).
- The selectivity and interference resistance of MIPs in complex food matrices still need to be improved.
- The reusability of MIPs should be further considered to address the demands of practical applications.
- During the polymerization process, appropriate safety measures should be taken.
- The large-scale production and commercial application of MIPs encounter cost and technical challenges.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhao, C.; Picchetti, P.; Zheng, K.; Zhang, X.; Wu, Y.; Shen, Y.; De Cola, L.; Shi, J.; Guo, Z.; et al. Quantitative SERS sensor for mycotoxins with extraction and identification function. Food Chem. 2024, 456, 140040. [Google Scholar] [CrossRef]
- Mwabulili, F.; Li, P.; Shi, J.; Zhang, H.; Xie, Y.; Ma, W.; Sun, S.; Yang, Y.; Li, Q.; Li, X.; et al. Research diversity and advances in simultaneous removal of multi-mycotoxin. Toxicon 2024, 250, 108106. [Google Scholar] [CrossRef] [PubMed]
- Pokoo-Aikins, A.; McDonough, C.M.; Mitchell, T.R.; Hawkins, J.A.; Adams, L.F.; Read, Q.D.; Li, X.; Shanmugasundaram, R.; Rodewald, E.; Acharya, P.; et al. Mycotoxin contamination and the nutritional content of corn targeted for animal feed. Poult. Sci. 2024, 103, 104303. [Google Scholar] [CrossRef]
- Zhai, W.; Wei, D.; Cao, M.; Wang, Z.; Wang, M. Biosensors based on core-shell nanoparticles for detecting mycotoxins in food: A review. Food Chem. 2023, 429, 136944. [Google Scholar] [CrossRef]
- Hua, M.; Ahmad, W.; Li, S.; Zhang, X.; Chen, X.; Chen, Q. Recent advances in electrochemiluminescence sensors for monitoring mycotoxins in food. Trends Food Sci. Technol. 2024, 153, 104706. [Google Scholar] [CrossRef]
- Castell, A.; Arroyo-Manzanares, N.; Viñas, P.; López-García, I.; Campillo, N. Advanced materials for magnetic solid-phase extraction of mycotoxins: A review. Trends Analyt. Chem. 2024, 178, 117826. [Google Scholar] [CrossRef]
- Kerstner, F.; Garda-Buffon, J. Mycotoxins in plant-based beverages: An updated occurrence. Food Res. Int. 2024, 194, 114863. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.C.; Kong, Z.L.; Wu, Y.S.; Huang, C.N.; Ogawa, T.; Lin, J.T.; Yang, D.J. Establishment of appropriate conditions for the efficient determination of multiple mycotoxins in tea samples and assessment of their drinking risks. Food Chem. 2024, 463, 141438. [Google Scholar] [CrossRef]
- Khan, R. Mycotoxins in food: Occurrence, health implications, and control strategies-A comprehensive review. Toxicon 2024, 248, 108038. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ji, J.; Wang, J.-S.; Sun, X. Co-contamination and interaction of fungal toxins and other environmental toxins. Trends Food Sci. Technol. 2020, 103, 162–178. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Zeng, H.; Fu, W.; Wang, J.; Tan, Y.; Zheng, C.; Qiu, Z.; Luo, J.; Lv, C.; et al. Low-dose microcystin-LR antagonizes aflatoxin B1 induced hepatocarcinogenesis through decreasing cytochrome P450 1A2 expression and aflatoxin B1-DNA adduct generation. Chemosphere 2020, 248, 126036. [Google Scholar] [CrossRef] [PubMed]
- Frangiamone, M.; Lazaro, A.; Cimbalo, A.; Font, G.; Manyes, L. In vitro and in vivo assessment of AFB1 and OTA toxic effects and the beneficial role of bioactive compounds. A systematic review. Food Chem. 2024, 447, 138909. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kumari, A.; Kumar, A.; Arya, V.; Chauhan, A.; Upadhyay, N.K.; Guleria, I.; Amarowicz, R.; Kumar, D.; Kuca, K. Biosensors for detection of pesticide residue, mycotoxins and heavy metals in fruits and vegetables: A concise review. Microchem. J. 2024, 205, 111292. [Google Scholar] [CrossRef]
- Suo, Z.; Niu, X.; Wei, M.; Jin, H.; He, B. Latest strategies for rapid and point of care detection of mycotoxins in food: A review. Anal. Chim. Acta 2023, 1246, 340888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, X.; Song, S.; Sun, M.; Kuang, H.; Xu, C.; Guo, L. Rapid and simultaneous detection of five mycotoxins and their analogs with a gold nanoparticle-based multiplex immuno-strip sensor. Food Microbiol. 2024, 121, 104510. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, L.; Li, T.; Li, C.; Zhang, W.; Sun, X.; Yue, X.; Xu, W. In silico simulation-guided engineering of multifunctional bivalent light up aptamer for sensitive and on-site detection of AFB1 using label-free ratiometric fluorescent aptasensor. Sens. Actuators B Chem. 2024, 420, 136425. [Google Scholar] [CrossRef]
- Appell, M.; Mueller, A. Mycotoxin Analysis Using Imprinted Materials Technology: Recent Developments. J. AOAC Int. 2016, 99, 861–864. [Google Scholar] [CrossRef]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J. Sep. Sci. 2021, 44, 1727–1751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Zhai, W.; Li, X.; Li, D.; Lin, H.; Han, S. Electrokinetic Preseparation and Molecularly Imprinted Trapping for Highly Selective SERS Detection of Charged Phthalate Plasticizers. Anal. Chem. 2020, 93, 946–955. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Cetinkaya, A.; Bellur Atici, E.; Ozkan, S.A. Detection of the antipsychotic drug cariprazine using a low-cost MIP-based electrochemical sensor and its electrooxidation behaviour. Microchem. J. 2024, 207, 112105. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H. Mesoporous structured MIPs@CDs fluorescence sensor for highly sensitive detection of TNT. Biosens. Bioelectron. 2016, 85, 950–956. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Lu, Y.; Feng, Z.; Zhang, S.; Sun, T. Prepared Sandwich structure WS(2)/ag@MIP composite for ultrasensitive SERS detection of trace 17beta-estradiol in food. Food Chem. 2024, 460, 140731. [Google Scholar] [CrossRef]
- Cavalera, S.; Anfossi, L.; Di Nardo, F.; Baggiani, C. Mycotoxins-Imprinted Polymers: A State-of-the-Art Review. Toxins 2024, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent progress of the research of metal-organic frameworks-molecularly imprinted polymers (MOFs-MIPs) in food safety detection field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Sonne, C.; Kim, K.H. Progress and challenges in sensing of mycotoxins using molecularly imprinted polymers. Environ. Pollut. 2022, 305, 119218. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Erdem, O.; Es, I.; Saylan, Y.; Atabay, M.; Gungen, M.A.; Olmez, K.; Denizli, A.; Inci, F. In situ synthesis and dynamic simulation of molecularly imprinted polymeric nanoparticles on a micro-reactor system. Nat. Commun. 2023, 14, 4840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. Trends Analyt. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Włoch, M.; Datta, J. Synthesis and polymerisation techniques of molecularly imprinted polymers. In Mip Synthesis, Characteristics and Analytical Application; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–40. [Google Scholar] [CrossRef]
- Mpupa, A.; Selahle, S.K.; Mizaikoff, B.; Nomngongo, P.N. Recent Advances in Solid-Phase Extraction (SPE) Based on Molecularly Imprinted Polymers (MIPs) for Analysis of Hormones. Chemosensors 2021, 9, 151. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Song, Z.; Li, J.; Lu, W.; Li, B.; Yang, G.; Bi, Y.; Arabi, M.; Wang, X.; Ma, J.; Chen, L. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. Trends Analyt. Chem. 2022, 146, 116504. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Zeng, J.; Hu, X.; Wang, X.; Yu, L.; Wang, D.; Cheng, L.; Ahmed, R.; Romanovski, V.; et al. Multi-templates molecularly imprinted polymers for simultaneous recognition of multiple targets: From academy to application. Trends Analyt. Chem. 2023, 166, 117173. [Google Scholar] [CrossRef]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Manopriya, S.; Hareesh, K. The prospects and challenges of solar electrochemical capacitors. J. Energy Storage 2021, 35, 102294. [Google Scholar] [CrossRef]
- Masoumi, S.; O’Shaughnessy, S.; Pakdel, A. Organic-based flexible thermoelectric generators: From materials to devices. Nano Energy 2022, 92, 106774. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cardenas, S. Advanced polymeric solids containing nano- and micro-particles prepared via emulsion-based polymerization approaches. A review. Anal. Chim. Acta 2022, 1208, 339669. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Pathak, P.K. Development of surface imprinted nanospheres using the inverse suspension polymerization method for electrochemical ultra sensing of dacarbazine. Anal. Chim. Acta 2017, 974, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Z.; Zhang, Z.Y.; Jiao, J.; Gai, Q.Y.; Liu, Y.; Wang, Y.; Qu, D.; Fu, Y.J. A novel surface molecularly imprinted polymer based on the natural biological macromolecule sporopollenin for the specific and efficient adsorption of resveratrol from Polygonum cuspidatum extracts. Int. J. Biol. Macromol. 2024, 280, 136168. [Google Scholar] [CrossRef] [PubMed]
- Hasaneen, N.; Akhtarian, S.; Pulicharla, R.; Brar, S.K.; Rezai, P. Surface molecularly imprinted polymer-based sensors for antibiotic detection. Trends Analyt Chem. 2024, 170, 117389. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, H.; Deng, Z.; Bu, J.; Li, T.; Yang, Y.; Zhong, S. A critical review of molecularly imprinted solid phase extraction technology. J. Polym. Res. 2021, 28, 401. [Google Scholar] [CrossRef]
- Nazim, T.; Lusina, A.; Ceglowski, M. Recent Developments in the Detection of Organic Contaminants Using Molecularly Imprinted Polymers Combined with Various Analytical Techniques. Polymers 2023, 15, 3868. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Q.; Wang, F.; Yang, H. Dummy molecularly imprinted solid-phase extraction-SERS determination of AFB1 in peanut. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 288, 122130. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; He, J.; Chen, N.; Huang, Z. Combined biocompatible medium with molecularly imprinted polymers for determination of aflatoxins B1 in real sample. J. Sep. Sci. 2019, 42, 3679–3687. [Google Scholar] [CrossRef]
- Rui, C.; He, J.; Li, Y.; Liang, Y.; You, L.; He, L.; Li, K.; Zhang, S. Selective extraction and enrichment of aflatoxins from food samples by mesoporous silica FDU-12 supported aflatoxins imprinted polymers based on surface molecularly imprinting technique. Talanta 2019, 201, 342–349. [Google Scholar] [CrossRef]
- Zamruddin, N.M.; Herman, H.; Rijai, L.; Hasanah, A.N. Factors Affecting the Analytical Performance of Magnetic Molecularly Imprinted Polymers. Polymers 2022, 14, 3008. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, R.; Li, W.; Li, X.; Sun, J.; Jiao, S.; Dai, S.; Dou, M.; Xu, R.; Li, Q.; et al. Three-Dimensional Ordered Macroporous Magnetic Inverse Photonic Crystal Microsphere-Based Molecularly Imprinted Polymer for Selective Capture of Aflatoxin B(1). ACS Appl. Mater. 2022, 14, 18845–18853. [Google Scholar] [CrossRef]
- Suo, L.; Hu, M. One-Step Synthesis of Polydopamine-Coated Dummy Molecularly Imprinted Polymers on the Surface of Fe3O4 for Selective Extraction and Enrichment of Aflatoxin B in Foods Prior to High-Performance Liquid Chromatography Detection. J. Anal. Chem. 2023, 78, 213–221. [Google Scholar] [CrossRef]
- Song, Y.P.; Li, N.; Zhang, H.C.; Wang, G.N.; Liu, J.X.; Liu, J.; Wang, J.P. Dummy template molecularly imprinted polymer for solid phase extraction of phenothiazines in meat based on computational simulation. Food Chem. 2017, 233, 422–428. [Google Scholar] [CrossRef]
- Guo, W.; Jing, Z.; Du, Q. Research progress of metal–organic frameworks-molecularly imprinted polymers for specific recognition. Microchem. J. 2023, 191, 108908. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Surya, S.G.; Beduk, T.; Vijjapu, M.T.; Lamaoui, A.; Durmus, C.; Timur, S.; Shekhah, O.; Mani, V.; Amine, A.; et al. Metal-Organic Frameworks Meet Molecularly Imprinted Polymers: Insights and Prospects for Sensor Applications. ACS Appl. Mater. 2022, 14, 49399–49424. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Sun, D.; Li, J.; Ostovan, A.; Wang, X.; Ma, J.; You, J.; Muhammad, T.; Chen, L.; Arabi, M. The metal- and covalent-organic frameworks-based molecularly imprinted polymer composites for sample pretreatment. Trends Analyt. Chem. 2024, 178, 117830. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Qi, Y.; Zhang, Z.; Cong, S.; She, Y.; Cao, X. Synthesis of metal-organic framework @molecularly imprinted polymer adsorbents for solid phase extraction of organophosphorus pesticides from agricultural products. J. Chromatogr. B 2022, 1188, 123081. [Google Scholar] [CrossRef]
- Kardani, F.; Mirzajani, R.; Tamsilian, Y.; Kiasat, A.; Bakhshandeh Farajpour, F. A novel immunoaffinity column based metal–organic framework deep eutectic solvents @ molecularly imprinted polymers as a sorbent for the solid phase extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from cereals samples. Microchem. J. 2023, 187, 108366. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, C.-J.; Tian, Y.; Weng, J.-J.; Wang, M.-Y.; Du, X.; He, J.; Li, H.-Y.; Li, Y.-Y.; Niu, H.-Y. COF-COOH@MIP as solid-phase extraction adsorbent for selective and sensitive detection of metamitron in environmental water. Microchem. J. 2024, 206, 111648. [Google Scholar] [CrossRef]
- Su, L.H.; Qian, H.L.; Yang, C.; Wang, C.; Wang, Z.; Yan, X.P. Integrating molecular imprinting into flexible covalent organic frameworks for selective recognition and efficient extraction of aflatoxins. J. Hazard. Mater. 2024, 467, 133755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liao, J.; Liu, C.; Liu, J.; Chen, P.; Zhou, J.; Du, Z.; Liu, Y.; Luo, Y.; Liu, Y.; et al. Metal-organic frameworks/metal nanoparticles as smart nanosensing interfaces for electrochemical sensors applications: A mini-review. Front. Bioeng. Biotechnol. 2023, 11, 1251713. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (bio) sensors go green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, W.; Li, X.; Yang, Y.; Zhou, B. Recent advances in ratiometric electrochemical sensors for food analysis. Food Chem. X 2024, 23, 101681. [Google Scholar] [CrossRef]
- Mutlu, E.; Senocak, A.; Demirbas, E.; Koca, A.; Akyuz, D. Selective and sensitive molecularly imprinted polymer-based electrochemical sensor for detection of deltamethrin. Food Chem. 2024, 463, 141121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Al Faysal, A.; Cetinkaya, A.; Erdogan, T.; Ozkan, S.A.; Golcu, A. Comparative study of two MIP-based electrochemical sensors for selective detection and quantification of the antiretroviral drug lopinavir in human serum. Talanta 2025, 281, 126791. [Google Scholar] [CrossRef]
- Chen, L.; Mamat, X.; Aisa, H.A. Determination of aflatoxin B1 by biomass derived porous carbon modified electrode with molecularly imprinted polymer. Electroanalysis 2023, 35, e202200371. [Google Scholar] [CrossRef]
- Elfadil, D.; Lamaoui, A.; Della Pelle, F.; Amine, A.; Compagnone, D. Molecularly Imprinted Polymers Combined with Electrochemical Sensors for Food Contaminants Analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xie, H.; Li, X.; Zhu, Y.; Huang, L.; Zhong, M.; Chen, L. Construction of a self-reporting molecularly-imprinted electrochemical sensor based on CuHCF modified by rGNR-rGO for the detection of zearalenone. Food Chem. 2024, 448, 139154. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Zhang, M.; Zhao, F.; Zeng, B. Ionic liquid assisted molecular self-assemble and molecular imprinting on gold nanoparticles decorated boron-doped ordered mesoporous carbon for the detection of zearalenone. Talanta 2020, 217, 121032. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.Ç.; Özdemir, N.; Boyacıoğlu, H.; Yola, M.L. A novel molecularly imprinted electrochemical sensor based on graphitic carbon nitride nanosheets decorated bovine serum albumin@MnO2 nanocomposite for zearalenone detection. J. Food Compost. Anal. 2024, 125, 105857. [Google Scholar] [CrossRef]
- Selvam, S.P.; Kadam, A.N.; Maiyelvaganan, K.R.; Prakash, M.; Cho, S. Novel SeS2-loaded Co MOF with Au@PANI comprised electroanalytical molecularly imprinted polymer-based disposable sensor for patulin mycotoxin. Biosens. Bioelectron. 2021, 187, 113302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Li, Z.; Wang, T.; Sun, Q.; Le, T.; Jirimutu. A novel fluorescent sensing platform based on nitrogen-doped carbon quantum dots for rapid and sensitive detection of aflatoxin B1 in corn flour. Lwt 2023, 185, 115130. [Google Scholar] [CrossRef]
- Diaz-Alvarez, M.; Martin-Esteban, A. Molecularly Imprinted Polymer-Quantum Dot Materials in Optical Sensors: An Overview of Their Synthesis and Applications. Biosensors 2021, 11, 79. [Google Scholar] [CrossRef]
- Chmangui, A.; Driss, M.R.; Touil, S.; Bermejo-Barrera, P.; Bouabdallah, S.; Moreda-Piñeiro, A. Aflatoxins screening in non-dairy beverages by Mn-doped ZnS quantum dots—Molecularly imprinted polymer fluorescent probe. Talanta 2019, 199, 65–71. [Google Scholar] [CrossRef]
- Chi, H.; Liu, G. A fluorometric sandwich biosensor based on molecular imprinted polymer and aptamer modified CdTe/ZnS for detection of aflatoxin B1 in edible oil. Lwt 2023, 180, 114726. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Cao, W.; Mao, B.; Liu, Y.; Kang, Z. Advances in carbon dots: From the perspective of traditional quantum dots. Mater. Chem. Front. 2020, 4, 1586–1613. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Hou, Y.; Jia, B.; Fu, L.; Jia, M.; Zhou, L.; Lu, J.; Kong, W. Recent advances in synthesis and modification of carbon dots for optical sensing of pesticides. J. Hazard. Mater. 2022, 422, 126881. [Google Scholar] [CrossRef]
- Chi, H.; Liu, G. A novel dual-template molecularly imprinted polymer ratiometric fluorescence sensor based on three-emission carbon quantum dots for accurate naked-eye detection of aflatoxin B1 and zearalenone in vegetable oil. Microchem. J. 2023, 192, 108961. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Xu, Y.; Li, H.; Chen, Q. SERS based sensor for mycotoxins detection: Challenges and improvements. Food Chem. 2021, 344, 128652. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Wang, J.; Wang, Y.; Zhang, Y.; Chen, P. Trace analysis of food by surface-enhanced Raman spectroscopy combined with molecular imprinting technology: Principle, application, challenges, and prospects. Food Chem. 2023, 429, 136883. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Cao, M.; Xiao, Z.; Li, D.; Wang, M. Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate. Foods 2022, 11, 3597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, M.; Wei, D.; Li, X.; Wang, M.; Zhai, W. Constructing graphene oxide/Au nanoparticle cellulose membranes for SERS detection of mixed pesticide residues in edible chrysanthemum. Analyst 2024, 149, 1151–1159. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, Y.; Li, X.; Gao, H.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. A molecularly-imprinted SERS sensor based on a TiO(2)@Ag substrate for the selective capture and sensitive detection of tryptamine in foods. Food Chem. 2022, 394, 133536. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, Q.; Yao, Y.; Zhang, Q.; Huang, J.; Zhang, H.; Qu, L. Thin layer chromatography coupled with surface enhanced Raman scattering for rapid separation and on-site detection of multi-components. J. Chromatogr. A 2023, 1706, 464217. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Li, G.; Xu, X.; Zhu, L.; Chen, X.; Wang, J. Surface-Imprinted Gold Nanoparticle-Based Surface-Enhanced Raman Scattering for Sensitive and Specific Detection of Patulin in Food Samples. Food Anal. Methods 2019, 12, 1648–1657. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Yan, H.; Lu, Z.; Yin, W.; Han, H. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal. Chim. Acta 2020, 1101, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Ezzati Nazhad Dolatabadi, J.; Torbati, M.; Pirpour Tazehkand, A.; Homayouni-Rad, A.; de la Guardia, M. Nanomaterials and new biorecognition molecules based surface plasmon resonance biosensors for mycotoxin detection. Biosens. Bioelectron. 2019, 143, 111603. [Google Scholar] [CrossRef]
- Rebelo, P.; Seguro, I.; Nouws, H.P.A.; Delerue-Matos, C.; Pacheco, J.G. Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review. Chemosensors 2023, 11, 318. [Google Scholar] [CrossRef]
- Mohammadzadeh-Asl, S.; Keshtkar, A.; Ezzati Nazhad Dolatabadi, J.; de la Guardia, M. Nanomaterials and phase sensitive based signal enhancment in surface plasmon resonance. Biosens. Bioelectron. 2018, 110, 118–131. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Armutcu, C.; Denizli, A. Molecularly imprinted polymer film based plasmonic sensors for detection of ochratoxin A in dried fig. Polym. Bull. 2021, 79, 4049–4067. [Google Scholar] [CrossRef]
- Akgonullu, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef]
- Britto, J.S.J.; Guan, X.; Tran, T.K.A.; Lei, Z.; Bahadur, R.; Patel, V.; Zhang, X.; Wong, S.L.; Vinu, A. Emerging Multifunctional Carbon-Nanomaterial-Based Biosensors for Cancer Diagnosis. Small Sci. 2024, 4, 2300221. [Google Scholar] [CrossRef]
- Çapar, N.; Yola, B.B.; Polat, İ.; Bekerecioğlu, S.; Atar, N.; Yola, M.L. A zearalenone detection based on molecularly imprinted surface plasmon resonance sensor including sulfur-doped g-C3N4/Bi2S3 nanocomposite. Microchem. J. 2023, 193, 109141. [Google Scholar] [CrossRef]
- Niu, J.; Du, M.; Wu, W.; Yang, J.; Chen, Q. Advances in the selection of functional monomers for molecularly imprinted polymers: A review. J. Sep. Sci. 2024, 47, e2400353. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Mishra, P.; Mizaikoff, B. Rational In Silico Design of Molecularly Imprinted Polymers: Current Challenges and Future Potential. Int. J. Mol. Sci. 2023, 24, 6785. [Google Scholar] [CrossRef]
- Wisnuwardhani, H.A.; Ibrahim, S.; Mukti, R.R.; Damayanti, S. Computational and experimental investigation on functional monomer selection for a novel molecularly imprinting polymer of remdesivir. J. Mol. Liq. 2024, 414, 126023. [Google Scholar] [CrossRef]
- Donato, L.; Nasser, I.I.; Majdoub, M.; Drioli, E. Green Chemistry and Molecularly Imprinted Membranes. Membranes 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
| Preparation Method | Advantages | Shortcomings | Application Platforms |
|---|---|---|---|
| Bulk polymerization | easy-to-operate, synthetic systems conducive to the generation of more blotting sites | cumbersome post-processing steps, difficulty on removing the template | SPE, chemical sensors, drug delivery systems |
| Precipitation polymerization | adjustable microspheres size and shape | time-consuming, requires large amount of solvent | SPE, environmental monitoring |
| In-situ polymerization | preparation of imprinted materials with specific forms and functions | strict reaction conditions | biosensors, drug analysis, material preparation |
| Emulsion polymerization | MIP microspheres can be prepared with high specific surface area and reusability | the use of surfactants may block the binding sites | drug delivery, catalyst carriers |
| Suspension polymerization | MIP microspheres can be prepared in large sizes, suitable for large-scale preparation | the use of surfactants may contaminate MIPs | industrial separation, environmental pollutant treatment |
| Surface imprinting polymerization | most of the recognition sites are located on the outer layer of the polymer, fast mass transfer rate | strict reaction conditions | biosensors, food safety monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, D.; Wang, Y.; Wang, M.; Zhai, W. Recent Progress of Molecularly Imprinted Technique for the Detection of Mycotoxins in Food. Foods 2024, 13, 4125. https://doi.org/10.3390/foods13244125
Wang Y, Wei D, Wang Y, Wang M, Zhai W. Recent Progress of Molecularly Imprinted Technique for the Detection of Mycotoxins in Food. Foods. 2024; 13(24):4125. https://doi.org/10.3390/foods13244125
Chicago/Turabian StyleWang, Yuan, Dizhe Wei, Yu Wang, Meng Wang, and Wenlei Zhai. 2024. "Recent Progress of Molecularly Imprinted Technique for the Detection of Mycotoxins in Food" Foods 13, no. 24: 4125. https://doi.org/10.3390/foods13244125
APA StyleWang, Y., Wei, D., Wang, Y., Wang, M., & Zhai, W. (2024). Recent Progress of Molecularly Imprinted Technique for the Detection of Mycotoxins in Food. Foods, 13(24), 4125. https://doi.org/10.3390/foods13244125





