Anticholinesterase Activity and Bioactive Compound Profiling of Six Hop (Humulus lupulus L.) Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Plants Materials
2.3. Extraction Process
2.4. Chromatographic Analysis
2.5. Analysis of Total Phenolic Content
2.6. Antioxidant Activity
2.7. Chelating Activity
2.8. Anticholinesterase Activity
2.9. Antityrosinase Activity
2.10. Inhibition of Hyaluronidase
2.11. Antimicrobial Activity
2.12. Analysis of the Results
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Simone, N.D.; Russo, P.; Tufariello, M.; Fragasso, M.; Solimando, M.; Capozzi, V.; Grieco, F.; Spano, G. Autochthonous Biological Resources for the Production of Regional Craft Beers: Exploring Possible Contributions of Cereals, Hops, Microbes, and Other Ingredients. Foods 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.; Sánchez, C.; Bravo, R.; Rodriguez, A.; Barriga, C.; Juánez, J.C. The Sedative Effects of Hops (Humulus lupulus), a Component of Beer, on the Activity/Rest Rhythm. Acta Physiol. Hung. 2012, 99, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Przybyś, M.; Skomra, U. Hops as a Source of Biologically Active Compounds. Pol. J. Agron. 2020, 43, 83–102. [Google Scholar] [CrossRef]
- Nagel, J.; Culley, L.K.; Lu, Y.; Liu, E.; Matthews, P.D.; Stevens, J.F.; Page, J.E. EST Analysis of Hop Glandular Trichomes Identifies an O-Methyltransferase That Catalyzes the Biosynthesis of Xanthohumol. Plant Cell 2008, 20, 186–200. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Flythe, M.D.; Kagan, I.A.; Wang, Y.; Narvaez, N. Hops (Humulus lupulus L.) Bitter Acids: Modulation of Rumen Fermentation and Potential As an Alternative Growth Promoter. Front. Vet. Sci. 2017, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Fahle, A.; Bereswill, S.; Heimesaat, M.M. Antibacterial Effects of Biologically Active Ingredients in Hop Provide Promising Options to Fight Infections by Pathogens Including Multi-Drug Resistant Bacteria. Eur. J. Microbiol. Immunol. 2022, 12, 22–30. [Google Scholar] [CrossRef]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Blanco Ayala, T.; Pérez de la Cruz, V.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Moir, M. Hops—A Millennium Review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A Story That Begs to Be Told. A Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [PubMed]
- Oledzka, E. Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery. Int. J. Mol. Sci. 2024, 25, 3398. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.A.; Danenhower, T.M.; Force, L.J.; Petersen, K.J.; Betts, T.A. HPLC Analysis of α- and β-Acids in Hops. J. Chem. Educ. 2008, 85, 954. [Google Scholar] [CrossRef]
- Chrisfield, B.J.; Gugino, B.K.; Hopfer, H.; Elias, R.J. Effect of Copper-Based Fungicide Treatments on the Quality of Hop Produced in the Northeastern United States. J. Am. Soc. Brew. Chem. 2022, 80, 169–179. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Characterization of Key Aroma Compounds in Pellets of Different Hop Varieties (Humulus lupulus L.) by Means of the Sensomics Approach. J. Agric. Food Chem. 2019, 67, 12044–12053. [Google Scholar] [CrossRef]
- Van Opstaele, F.; De Causmaecker, B.; Aerts, G.; De Cooman, L. Characterization of Novel Varietal Floral Hop Aromas by Headspace Solid Phase Microextraction and Gas Chromatography–Mass Spectrometry/Olfactometry. J. Agric. Food Chem. 2012, 60, 12270–12281. [Google Scholar] [CrossRef]
- Betancur, M.; López, J.; Salazar, F. Antimicrobial Activity of Compounds from Hop (Humulus lupulus L.) Following Supercritical Fluid Extraction: An Overview. Chil. J. Agric. Res. 2023, 83, 499–509. [Google Scholar] [CrossRef]
- Bizaj, K.; Škerget, M.; Košir, I.J.; Knez, Ž. Hop (Humulus lupulus L.) Essential Oils and Xanthohumol Derived from Extraction Process Using Solvents of Different Polarity. Horticulturae 2022, 8, 368. [Google Scholar] [CrossRef]
- Fischer, B.; Gevinski, E.V.; da Silva, D.M.; Júnior, P.A.L.; Bandiera, V.J.; Lohmann, A.M.; Rigo, D.; Duarte, P.F.; Franceschi, E.; Zandoná, G.P.; et al. Extraction of Hops Pelletized (Humulus lupulus) with Subcritical CO2 and Hydrodistillation: Chemical Composition Identification, Kinetic Model, and Evaluation of Antioxidant and Antimicrobial Activity. Food Res. Int. 2023, 167, 112712. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Heghes, S.C.; Vostinaru, O.; Rus, L.M.; Mogosan, C.; Iuga, C.A.; Filip, L. Antispasmodic Effect of Essential Oils and Their Constituents: A Review. Molecules 2019, 24, 1675. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, S.H.; Bagheri, S.M.; Dashti-R, M.H. Relaxant Effect of Humulus lupulus Extracts on Isotonic Rat’s Ileum Contractions. Avicenna J. Phytomed. 2014, 4, 53–58. [Google Scholar] [PubMed]
- Slatnar, A.; Stampar, F.; Veberic, R.; Jakopic, J. HPLC-MS(n) Identification of Betalain Profile of Different Beetroot (Beta vulgaris L. ssp. vulgaris) Parts and Cultivars. J. Food Sci. 2015, 80, C1952–C1958. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS Profiling of Phenolic Compounds from Mango (Mangifera indica L.) Seed Kernel of Different Cultivars and Maturation Stages as a Preliminary Approach to Determine Functional and Nutraceutical Value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Karadimou, C.; Avgidou, M.S.; Petsa, E.; Papadakis, E.-N.; Theocharis, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Koundouras, S. An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.). Molecules 2022, 27, 7107. [Google Scholar] [CrossRef] [PubMed]
- Soininen, T.H.; Jukarainen, N.; Soininen, P.; Auriola, S.O.K.; Julkunen-Tiitto, R.; Oleszek, W.; Stochmal, A.; Karjalainen, R.O.; Vepsäläinen, J.J. Metabolite Profiling of Leek (Allium porrum L.) Cultivars by (1) H NMR and HPLC-MS. Phytochem. Anal. 2014, 25, 220–228. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.Z.; Ismail, H.; Kayani, W.K.; Bhatti, M.Z.; Ismail, H.; Kayani, W.K. Plant Secondary Metabolites: Therapeutic Potential and Pharmacological Properties. In Secondary Metabolites—Trends and Reviews; IntechOpen: London, UK, 2022; ISBN 978-1-80355-208-8. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Skomra, U. Metodyka integrowanej produkcji chmielu/dr Urszula Skomra. In Instytut Uprawy Nawożenia i Gleboznawstwa Państwowy Instytut Badawczy Puławy; Główny Inspektorat Ochrony Roślin i Nasiennictwa: Warszawa, Poland, 2021. [Google Scholar]
- Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Hop Flower Supercritical Carbon Dioxide Extracts Coupled with Carriers with Solubilizing Properties—Antioxidant Activity and Neuroprotective Potential. Antioxidants 2023, 12, 1722. [Google Scholar] [CrossRef]
- Gościniak, A.; Szulc, P.; Zielewicz, W.; Walkowiak, J.; Cielecka-Piontek, J. Multidirectional Effects of Red Clover (Trifolium pratense L.) in Support of Menopause Therapy. Molecules 2023, 28, 5178. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Muzykiewicz, A.; Florkowska, K.; Nowak, A.; Zielonka-Brzezicka, J.; Klimowicz, A. Antioxidant Activity of St. John’s Wort Extracts Obtained with Ultrasound-Assisted Extraction. Pomeranian J. Life Sci. 2019, 65, 89–93. [Google Scholar] [CrossRef]
- Liao, H.; Dong, W.; Shi, X.; Liu, H.; Yuan, K. Analysis and Comparison of the Active Components and Antioxidant Activities of Extracts from Abelmoschus esculentus L. Pharmacogn. Mag. 2012, 8, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Cielecka-Piontek, J. Determining Antioxidant Activity of Cannabis Leaves Extracts from Different Varieties—Unveiling Nature’s Treasure Trove. Antioxidants 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-Cyclodextrin as an Effective Carrier of Curcumin—Piperine Nutraceutical System with Improved Enzyme Inhibition Properties. J. Enzym. Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia Glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Gajendra, K.; Pratap, G.K.; Poornima, D.V.; Shantaram, M.; Ranjita, G. Natural Acetylcholinesterase Inhibitors: A Multi-Targeted Therapeutic Potential in Alzheimer’s Disease. Eur. J. Med. Chem. Rep. 2024, 11, 100154. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of Acetylcholinesterase and Butyrylcholinesterase Meet Immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Chen, W.-C.; Tseng, T.-S.; Hsiao, N.-W.; Lin, Y.-L.; Wen, Z.-H.; Tsai, C.-C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.-C. Discovery of Highly Potent Tyrosinase Inhibitor, T1, with Significant Anti-Melanogenesis Ability by Zebrafish In Vivo Assay and Computational Molecular Modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Walkowiak, J.; Cielecka-Piontek, J. The Antioxidant and Neuroprotective Potential of Leaves and Inflorescences Extracts of Selected Hemp Varieties Obtained with scCO2. Antioxidants 2023, 12, 1827. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Ahumada-Castro, U.; Paris, I. Dopamine and L-Dopa as Selective Endogenous Neurotoxins. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 255–289. ISBN 978-3-031-15080-7. [Google Scholar]
- Grabowska, K.; Podolak, I.; Galanty, A.; Załuski, D.; Makowska-Wąs, J.; Sobolewska, D.; Janeczko, Z.; Żmudzki, P. In Vitro Anti-Denaturation and Anti-Hyaluronidase Activities of Extracts and Galactolipids from Leaves of Impatiens parviflora DC. Nat. Prod. Res. 2016, 30, 1219–1223. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M. Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens. Appl. Sci. 2024, 14, 6340. [Google Scholar] [CrossRef]
- Korbecka-Paczkowska, M.; Karpiński, T.M. In Vitro Assessment of Antifungal and Antibiofilm Efficacy of Commercial Mouthwashes against Candida Albicans. Antibiotics 2024, 13, 117. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.; Seo, K.-S.; Kang, K.-Y.; Park, S.H.; Ha, T.H.; Ahn, J.-W.; Kang, S.-Y. Comparative Study on Phenolic Compounds and Antioxidant Activities of Hop (Humulus lupulus L.) Strobile Extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef]
- Albani, C.M.; Iglesias, A.; Albanese, A.; Fuentes, G.; Orallo, D.; Maggi, M.; Elissondo, M.C. Evaluation of the Protoscolicidal Activity of Humulus lupulus Methanolic Extracts on Echinococcus Granulosus Sensu Stricto. Evid.-Based Complement. Altern. Med. 2024, 2024, 6251666. [Google Scholar] [CrossRef]
- Bilska, A.; Kobus-Cisowska, J.; Wojtczak, J.; Kowalski, R.; Kaczmarek, E. Antioxidant Activity of Humulus lupulus Phenolic Hop Extracts in Creating a New Pâté: An Element Affecting Fat Stability and Microbiological Quality during Storage. Molecules 2024, 29, 1561. [Google Scholar] [CrossRef] [PubMed]
- Tatasciore, S.; Santarelli, V.; Neri, L.; González Ortega, R.; Faieta, M.; Di Mattia, C.D.; Di Michele, A.; Pittia, P. Freeze-Drying Microencapsulation of Hop Extract: Effect of Carrier Composition on Physical, Techno-Functional, and Stability Properties. Antioxidants 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Önder, F.C.; Ay, M.; Sarker, S.D. Comparative Study of Antioxidant Properties and Total Phenolic Content of the Extracts of Humulus lupulus L. and Quantification of Bioactive Components by LC–MS/MS and GC–MS. J. Agric. Food Chem. 2013, 61, 10498–10506. [Google Scholar] [CrossRef]
- Paniagua-García, A.I.; Ruano-Rosa, D.; Díez-Antolínez, R. Fractionation of High-Value Compounds from Hops Using an Optimised Sequential Extraction Procedure. Antioxidants 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; Penna, G.L.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Swieca, M.; Cichocka, J.; Gawlik-Dziki, U. The Phenolic Content and Antioxidant Activity of the Aqueous and Hydroalcoholic Extracts of Hops and Their Pellets. J. Inst. Brew. 2013, 119, 103–110. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological Activity of Humulus lupulus (L.) Essential Oil and Its Main Components against Sitophilus granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef]
- do Nascimento, F.M.G.; Marques, S.P.D.; Trevisan, M.T.S.; Owen, R.W.; Pereira, L.R.; Lima, T.C.; de Sousa, A.F.; Maia, C.E.G. Inhibitory Capacity of Extracts and Main Constituents of Hop Flowers. Future J. Pharm. Sci. 2023, 9, 111. [Google Scholar] [CrossRef]
- Paventi, G.; Rotundo, G.; Pistillo, M.; D’Isita, I.; Germinara, G.S. Bioactivity of Wild Hop Extracts against the Granary Weevil, Sitophilus granarius (L.). Insects 2021, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Woo, H.S.; Kim, J.Y.; Ryuk, J.A.; Park, K.H.; Ko, B.S. Phenols Displaying Tyrosinase Inhibition from Humulus lupulus. J. Enzym. Inhib. Med. Chem. 2016, 31, 742–747. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Zhang, X.; Zheng, J.; Hu, W.; Teng, B. Hop Tannins as Multifunctional Tyrosinase Inhibitor: Structure Characterization, Inhibition Activity, and Mechanism. Antioxidants 2022, 11, 772. [Google Scholar] [CrossRef]
- Yang, H.; Oh, K.-E.; Jo, Y.; Ahn, J.; Liu, Q.; Turk, A.; Jang, J.; Hwang, B.Y.; Kiyong, L.; Lee, M.K. Characterization of Tyrosinase Inhibitory Constituents from the Aerial Parts of Humulus Japonicus Using LC-MS/MS Coupled Online Assay. Bioorg. Med. Chem. 2017, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Dong, J.; Xiao, L.; Liu, G.; Qian, Z.; Miao, J. Inhibition and Interaction with Hyaluronidase by Compounds from Hop (Humulus lupulus L.) Flowers. Asian J. Chem. 2013, 25, 10262–10266. [Google Scholar] [CrossRef]
- Hall, A.J.; Babish, J.G.; Darland, G.K.; Carroll, B.J.; Konda, V.R.; Lerman, R.H.; Bland, J.S.; Tripp, M.L. Safety, Efficacy and Anti-Inflammatory Activity of Rho Iso-Alpha-Acids from Hops. Phytochemistry 2008, 69, 1534–1547. [Google Scholar] [CrossRef]
- Hurth, Z.; Faber, M.-L.; Gendrisch, F.; Holzer, M.; Haarhaus, B.; Cawelius, A.; Schwabe, K.; Schempp, C.M.; Wölfle, U. The Anti-Inflammatory Effect of Humulus lupulus Extract In Vivo Depends on the Galenic System of the Topical Formulation. Pharmaceuticals 2022, 15, 350. [Google Scholar] [CrossRef]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium Acnes and Staphylococcus Aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Santagostini, L.; Forino, M.; Piazza, S.; Colombo, E.; Taglialatela-Scafati, O.; Fico, G.; Dell’Agli, M. A Bio-Guided Assessment of the Anti-Inflammatory Activity of Hop Extracts (Humulus lupulus L. Cv. Cascade) in Human Gastric Epithelial Cells. J. Funct. Foods 2019, 57, 95–102. [Google Scholar] [CrossRef]
- Niederau, C.; Bhargava, S.; Schneider-Kramman, R.; Jankowski, J.; Craveiro, R.B.; Wolf, M. Xanthohumol Exerts Anti-Inflammatory Effects in an in Vitro Model of Mechanically Stimulated Cementoblasts. Sci. Rep. 2022, 12, 14970. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, E.; Sabry, A.; Khalil, W.K.B. Neuroprotective Effects of Onion and Garlic Root Extracts against Alzheimer’s Disease in Rats: Antimicrobial, Histopathological, and Molecular Studies. BioTechnologia 2022, 103, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Prasansuklab, A.; Theerasri, A.; Payne, M.; Ung, A.T.; Tencomnao, T. Acid-Base Fractions Separated from Streblus Asper Leaf Ethanolic Extract Exhibited Antibacterial, Antioxidant, Anti-Acetylcholinesterase, and Neuroprotective Activities. BMC Complement. Altern. Med. 2018, 18, 223. [Google Scholar] [CrossRef]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 6: Correlation and Linear Regression. Indian J. Dermatol. 2016, 61, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Czigle, S.; Nagy, M.; Mladěnka, P.; Tóth, J. Pharmacokinetic and Pharmacodynamic Herb-Drug Interactions—Part I. Herbal Medicines of the Central Nervous System. PeerJ 2023, 11, e16149. [Google Scholar] [CrossRef] [PubMed]
- Knöss, W.; Chinou, I. Regulation of Medicinal Plants for Public Health--European Community Monographs on Herbal Substances. Planta Med. 2012, 78, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Lupuli Flos—Herbal Medicinal Product | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/lupuli-flos (accessed on 13 December 2024).
- Combination: Valerianae Radix and Lupuli Flos—Herbal Medicinal Product | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/valerianae-radix-and-lupuli-flos (accessed on 13 December 2024).
- Sekiguchi, F.; Fujita, T.; Deguchi, T.; Yamaoka, S.; Tomochika, K.; Tsubota, M.; Ono, S.; Horaguchi, Y.; Ichii, M.; Ichikawa, M.; et al. Blockade of T-Type Calcium Channels by 6-Prenylnaringenin, a Hop Component, Alleviates Neuropathic and Visceral Pain in Mice. Neuropharmacology 2018, 138, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Zamzow, D.R.; Elias, V.; Legette, L.L.; Choi, J.; Stevens, J.F.; Magnusson, K.R. Xanthohumol Improved Cognitive Flexibility in Young Mice. Behav. Brain Res. 2014, 275, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-L.; Hsu, C.-K.; Lu, W.-J.; Hsieh, C.-Y.; Hsiao, G.; Chou, D.-S.; Wu, G.-J.; Sheu, J.-R. Neuroprotective Effects of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), in Ischemic Stroke of Rats. J. Agric. Food Chem. 2012, 60, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes To Confer Protection against Oxidative Damage in PC12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef]
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an Active Constituent from Hope, Affords Protection against Kainic Acid-Induced Excitotoxicity in Rats. Neurochem. Int. 2020, 133, 104629. [Google Scholar] [CrossRef]
- Rancán, L.; Paredes, S.D.; García, I.; Muñoz, P.; García, C.; López de Hontanar, G.; de la Fuente, M.; Vara, E.; Tresguerres, J.A.F. Protective Effect of Xanthohumol against Age-Related Brain Damage. J. Nutr. Biochem. 2017, 49, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Chen, X.; Liu, P.; Wang, S.; Song, F.; Zhang, Z.; Zhu, F.; Huang, X.; Liu, J.; et al. The Prenylflavonoid Xanthohumol Reduces Alzheimer-Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ohya, R.; Kondo, K.; Nakayama, H. Iso-α-Acids, Hop-Derived Bitter Components of Beer, Attenuate Age-Related Inflammation and Cognitive Decline. Front. Aging Neurosci. 2019, 11, 16. [Google Scholar] [CrossRef]
- Ano, Y.; Yoshikawa, M.; Takaichi, Y.; Michikawa, M.; Uchida, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, Bitter Components in Beer, Suppress Inflammatory Responses and Attenuate Neural Hyperactivation in the Hippocampus. Front. Pharmacol. 2019, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Chen, Y.-J.; Yu, J.; Du, Z.-Y.; Yuan, Q.; Sun, Y.-R.; Wu, X.; Li, Z.-Q.; Wu, X.-H.; Hu, J.; et al. ISO-Alpha-Acids Improve the Hematoma Resolution and Prevent Peri-Hematoma Inflammations by Transforming Microglia via PPARgamma-CD36 Axis in ICH Rats. Int. Immunopharmacol. 2020, 83, 106396. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Obara, K.; Saito, J.; Umeda, S.; Ano, Y. Effects of Hop Bitter Acids, Bitter Components in Beer, on Cognition in Healthy Adults: A Randomized Controlled Trial. J. Agric. Food Chem. 2020, 68, 206–212. [Google Scholar] [CrossRef]

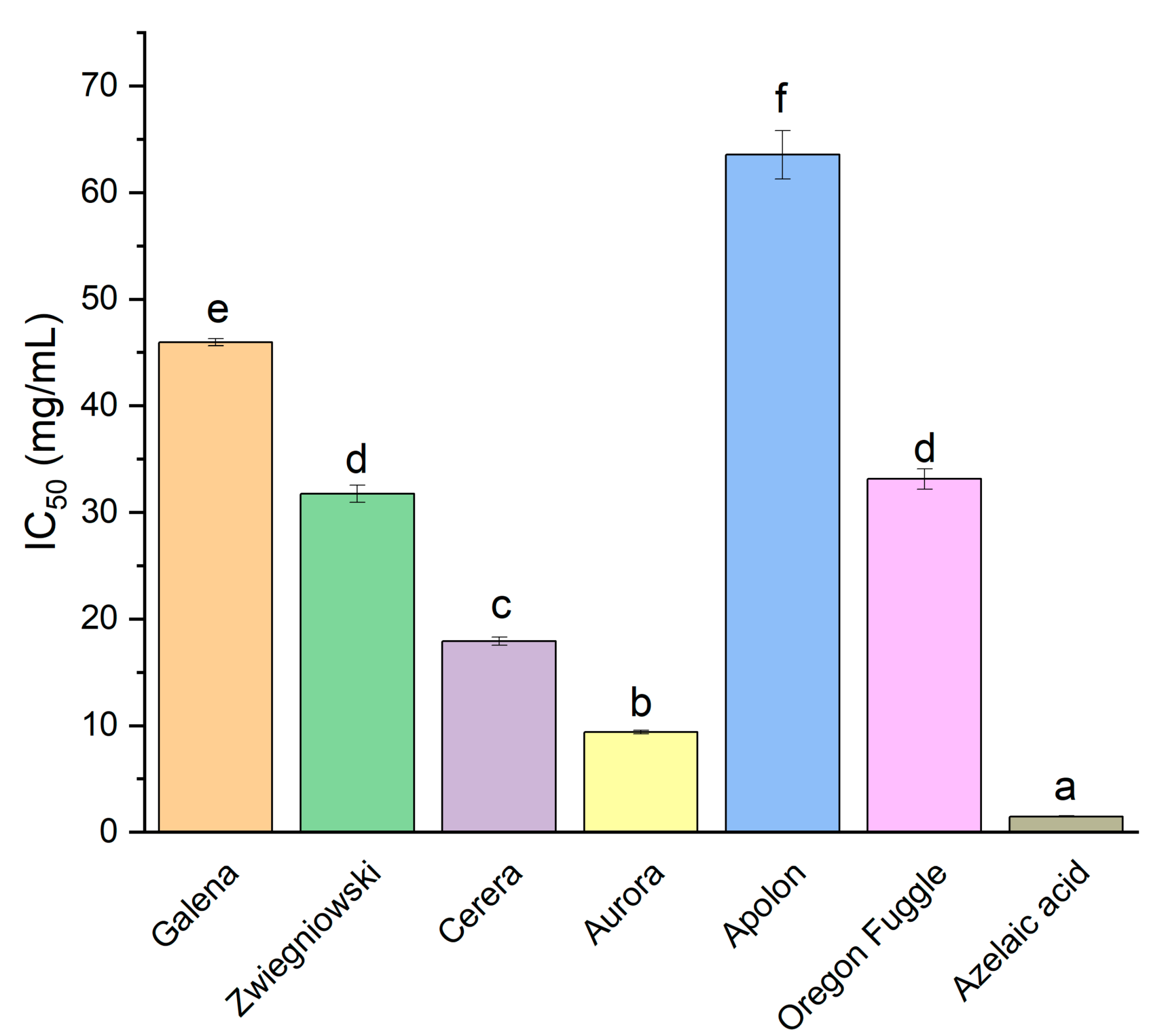

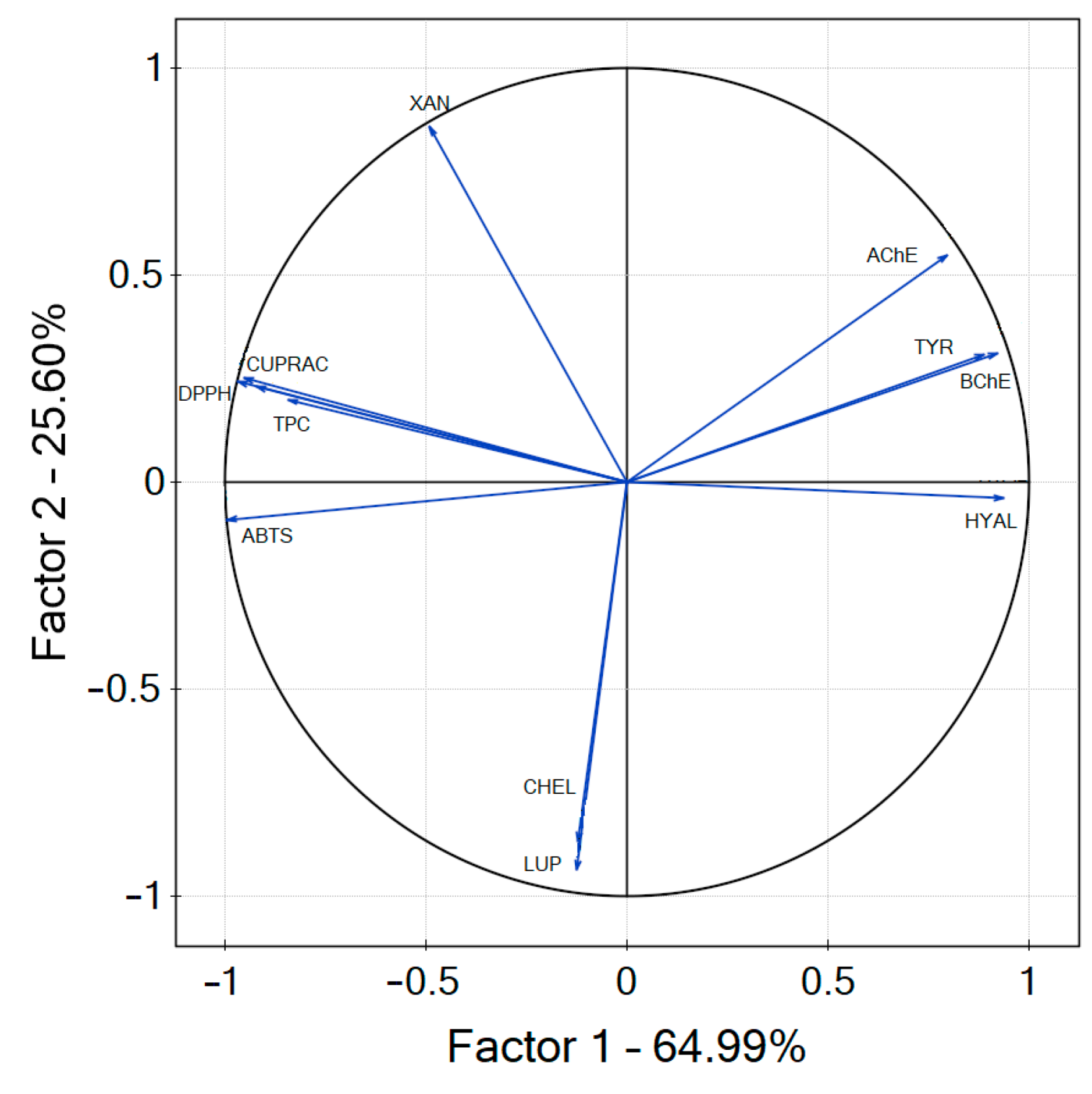
| Cultivar | Assignment | Origin |
|---|---|---|
| Apolon | bitter | Slovenia |
| Aurora | aroma | Slovenia |
| Cerera | aroma | Slovenia |
| Galena | bitter | USA |
| Oregon Fuggle | aroma | USA |
| Zwienigowski | aroma | Russia |
| Variety | Xanthohumol | Lupulone |
|---|---|---|
| Amount of Active Compound (mg)/Dry Plant Material (g) | ||
| Galena | 0.554 ± 0.008 b | 4.124 ± 0.025 c |
| Zwiegniowski | 0.494 ± 0.006 a | 9.228 ± 0.073 e |
| Cerera | 0.656 ± 0.010 c | 6.248 ± 0.029 d |
| Aurora | 0.665 ± 0.009 c | 3.182 ± 0.026 b |
| Apolon | 0.654 ± 0.014 c | 0.702 ± 0.006 a |
| Oregon Fuggle | 0.651 ± 0.008 c | 3.969 ± 0.028 c |
| Variety | Total Phenolic Content |
|---|---|
| mg GAE/g | |
| Galena | 9.92 ± 0.38 a |
| Zwiegniowski | 10.99 ± 0.49 a,b |
| Cerera | 20.26 ± 0.88 d |
| Aurora | 22.47 ± 1.15 d |
| Apolon | 12.91 ± 0.41 b |
| Oregon Fuggle | 17.24 ± 0.67 c |
| Variety | DPPH | ABTS | CUPRAC | FRAP |
|---|---|---|---|---|
| mg Trolox/g Plant Material | ||||
| Galena | 28.49 ± 0.30 a | 27.02 ± 0.62 a | 33.48 ± 0.55 a | 22.79 ± 0.25 a |
| Zwiegniowski | 35.46 ± 0.26 b | 31.37 ± 0.64 b | 38.53 ± 0.62 b | 26.33 ± 1.20 b |
| Cerera | 45.62 ± 0.22 c | 37.14 ± 0.96 c | 52.09 ± 0.62 c | 35.76 ± 0.18 c |
| Aurora | 57.66 ± 0.57 d | 41.36 ± 1.08 d | 66.44 ± 0.73 e | 44.34 ± 0.88 e |
| Apolon | 35.19 ± 0.62 b | 24.48 ± 0.45 a | 38.83 ± 0.42 b | 26.20 ± 0.46 b |
| Oregon Fuggle | 59.94 ± 0.40 e | 36.63 ± 0.48 c | 63.04 ± 0.54 d | 39.66 ± 0.49 d |
| Variety | AChE | BChE |
|---|---|---|
| IC 50 (mg/mL) | ||
| Galena | 79.50 ± 0.69 e | 36.84 ± 2.07 d |
| Zwiegniowski | 46.56 ± 0.56 c | 42.62 ± 1.62 e |
| Cerera | 46.56 ± 0.97 c | 29.40 ± 0.28 c |
| Aurora | 24.39 ± 1.27 b | 20.38 ± 0.61 b |
| Apolon | 143.82 ± 4.59 f | 62.09 ± 3.36 f |
| Oregon Fuggle | 56.02 ± 1.99 d | 25.03 ± 1.17 c |
| Galantamine | 0.024 ± 0.001 a | 0.163 ± 0.004 a |
| Extract or Compound | MIC [mg/mL] | ||
|---|---|---|---|
| Staphylococcus aureus | Pseudomonas aeruginosa | Candida albicans | |
| Galena | 150 | 150/>150 | 75 |
| Zwiegniowski | 150 | 150/>150 | 75 |
| Cerera | 150 | 150 | 75 |
| Aurora | 150 | 150/>150 | 75 |
| Apolon | 150 | 150/>150 | 75/150 |
| Oregon | 150 | 150/>150 | 75 |
| Octenidine dihydrochloride | 0.4–1.6 µg/mL | 0.4–3.1 µg/mL | 0.2–1.6 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagan, B.; Czerny, B.; Stasiłowicz-Krzemień, A.; Szulc, P.; Skomra, U.; Karpiński, T.M.; Lisiecka, J.; Kamiński, A.; Kryszak, A.; Zimak-Krótkopad, O.; et al. Anticholinesterase Activity and Bioactive Compound Profiling of Six Hop (Humulus lupulus L.) Varieties. Foods 2024, 13, 4155. https://doi.org/10.3390/foods13244155
Sagan B, Czerny B, Stasiłowicz-Krzemień A, Szulc P, Skomra U, Karpiński TM, Lisiecka J, Kamiński A, Kryszak A, Zimak-Krótkopad O, et al. Anticholinesterase Activity and Bioactive Compound Profiling of Six Hop (Humulus lupulus L.) Varieties. Foods. 2024; 13(24):4155. https://doi.org/10.3390/foods13244155
Chicago/Turabian StyleSagan, Bartłomiej, Bogusław Czerny, Anna Stasiłowicz-Krzemień, Piotr Szulc, Urszula Skomra, Tomasz M. Karpiński, Jolanta Lisiecka, Adam Kamiński, Aleksandra Kryszak, Oskar Zimak-Krótkopad, and et al. 2024. "Anticholinesterase Activity and Bioactive Compound Profiling of Six Hop (Humulus lupulus L.) Varieties" Foods 13, no. 24: 4155. https://doi.org/10.3390/foods13244155
APA StyleSagan, B., Czerny, B., Stasiłowicz-Krzemień, A., Szulc, P., Skomra, U., Karpiński, T. M., Lisiecka, J., Kamiński, A., Kryszak, A., Zimak-Krótkopad, O., & Cielecka-Piontek, J. (2024). Anticholinesterase Activity and Bioactive Compound Profiling of Six Hop (Humulus lupulus L.) Varieties. Foods, 13(24), 4155. https://doi.org/10.3390/foods13244155










