Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Yeast Strains
2.3. Preparation of YPH as an Organic Nitrogen Source
2.4. Fermentation Conditions
2.5. Analysis of Alcohols, Sugars, and Organic Acids
2.6. Analysis of the Aromatic Profile
2.7. Calculation of the Odor Activity Value (OAV)
2.8. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Li, J.; Yuan, M.; Meng, N.; Li, H.; Sun, J.; Sun, B. Influence of nitrogen status on fermentation performances of non-Saccharomyces yeasts: A review. Food Sci. Hum. Wellness 2024, 13, 556–567. [Google Scholar] [CrossRef]
- Alexandre, H.; Charpentier, C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar] [CrossRef]
- Ferreira Mendes, A.; Barbosa, C.; Lage, P.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Ciência Téc. Vitiv. 2011, 26, 17–32. [Google Scholar]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass Content Governs Fermentation Rate in Nitrogen-Deficient Wine Musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.; Cacho, J.; Ferreira, V. Addition of amino acids to grape juice of the Merlot variety: Effect on amino acid uptake and aroma generation during alcoholic fermentation. Food Chem. 2006, 98, 300–310. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Rozes, N. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]
- Su, Y.; Heras, J.M.; Gamero, A.; Querol, A.; Guillamón, J.M. Impact of Nitrogen Addition on Wine Fermentation by S. cerevisiae Strains with Different Nitrogen Requirements. J. Agric. Food Chem. 2021, 69, 6022–6031. [Google Scholar] [CrossRef]
- Petrovic, G.; Kidd, M.; Buica, A. A statistical exploration of data to identify the role of cultivar and origin in the concentration and composition of yeast assimilable nitrogen. Food Chem. 2019, 276, 528–537. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.; Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. Effects of temperature, ammonium and glucose concentrations on yeast growth in a model wine system. Int. J. Food Sci. Technol. 2006, 41, 1152–1157. [Google Scholar] [CrossRef]
- Nehme, N.; Mathieu, F.; Taillandier, P. Impact of the co-culture of Saccharomyces cerevisiae–Oenococcus oeni on malolactic fermentation and partial characterization of a yeast-derived inhibitory peptidic fraction. Food Microbiol. 2010, 27, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Marsit, S.; Dequin, S. Diversity and adaptive evolution of Saccharomyces wine yeast: A review. FEMS Yeast Res. 2015, 15, fov067. [Google Scholar] [CrossRef] [PubMed]
- de Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional Yeast Species for Lowering Ethanol Content of Wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Fresno, J.M.D.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina Can Reduce Acetic Acid Produced by Saccharomyces cerevisiae in Sweet Wine Fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Tofalo, R. Editorial: Sparkling wines: Current trends and future evolution. Front. Microbiol. 2023, 14, 1199578. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Franco, W.; De Lecco, C.C.; Durán, A.; Urtubia, A. Evaluation of Indigenous Candida oleophila and Candida boidinii in Monoculture and Sequential Fermentations: Impact on Ethanol Reduction and Chemical Profile in Chilean Sauvignon Blanc Wines. J. Fungi 2022, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Blesi, M. Alcoholic Fermentation as a Source of Congeners in Fruit Spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, R.K.R.; Capone, D.L.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. A Review of Wine Authentication Using Spectroscopic Approaches in Combination with Chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.T.; van Mastrigt, O.; Block, D.E.; Notebaart, R.A.; Smid, E.J. Nitrogenous Compound Utilization and Production of Volatile Organic Compounds among Commercial Wine Yeasts Highlight Strain-Specific Metabolic Diversity. Microbiol. Spectr. 2021, 9, e0048521. [Google Scholar] [CrossRef]
- Rojas, P.; Lopez, D.; Ibañez, F.; Urbina, C.; Franco, W.; Urtubia, A.; Valencia, P. Recycling and Conversion of Yeasts into Organic Nitrogen Sources for Wine Fermentation: Effects on Molecular and Sensory Attributes. Fermentation 2021, 7, 313. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Moldes, A.B.; Domínguez, J.M. Tartaric Acid Recovery from Distilled Lees and Use of the Residual Solid as an Economic Nutrient for Lactobacillus. J. Agric. Food Chem. 2006, 54, 7904–7911. [Google Scholar] [CrossRef]
- Franco, W.; Benavides, S.; Valencia, P.; Ramírez, C.; Urtubia, A. Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile). Foods 2021, 10, 1737. [Google Scholar] [CrossRef]
- Salgado, J.M.; Rodríguez, N.; Cortés, S.; Domínguez, J.M. Improving downstream processes to recover tartaric acid, tartrate and nutrients from vinasses and formulation of inexpensive fermentative broths for xylitol production. J. Sci. Food Agric. 2010, 90, 2168–2177. [Google Scholar] [CrossRef]
- Jian, H.; Zhang, M.; Ye, J.; Xiangluan, X.; Zhao, W.; Bai, W. HS-SPME-GC-MS and OAV analyses of characteristic volatile flavour compounds in salt-baked drumstick. Food Sci. Technol. 2022, 170, 114041. [Google Scholar]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Childs, B.; Bohlscheid, J.; Edwards, C. Impact of available nitrogen and sugar concentration in musts on alcoholic fermentation and subsequent wine spoilage by Brettanomyces bruxellensis. J. Sci. Food Agric. 2015, 46, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, G.; Wang, S.; Zhao, P.; Cao, X.; Cheng, C.; Liu, H.; Xue, Y.; Wang, X. Investigating the role of tartaric acid in wine astringency. Food. Chem. 2023, 403, 134385. [Google Scholar] [CrossRef]
- Santos, A. Olfatory Perception Threshold Assessment of Volatile Acidity in Madeira Wines. Master’s Thesis, Universidade da Madeira, Funchal, Portugal, 2015. [Google Scholar]
- Bely, M.; Masneuf-Pomarède, I.; Dubourdieu, D. Influence of physiological state of inoculum on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Int. Des Sci. La Vigne Du Vin 2005, 39, 191–197. [Google Scholar] [CrossRef]
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of red grapes co-winemaking in polyphenols and color of wines. J. Agric. Food Chem. 2005, 53, 7609–7616. [Google Scholar] [CrossRef]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The impact of single amino acids on growth and volatile aroma production by Saccharomyces cerevisiae strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Bogaki, T. Mechanisms of production and control of acetate esters in yeasts. J. Biosci. Bioeng. 2023, 136, 261–269. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Carrau, F.; Boido, E.; Dellacassa, E. Yeast Diversity and Flavor Compounds. In Fungal Metabolites; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Kövilein, A.; Zadravec, L.; Hohmann, S.; Umpfenbach, J.; Ochsenreither, K. Effect of process mode, nitrogen source and temperature on L-malic acid production with Aspergillus oryzae DSM 1863 using acetate as carbon source. Front. Bioeng. Biotechnol. 2022, 10, 1033777. [Google Scholar] [CrossRef] [PubMed]
- Vion, C.; Muro, M.; Bernard, M.; Richard, B.; Valentine, F.; Yeramian, N.; Masneuf-Pomarède, I.; Tempère, S.; Marullo, P. New malic acid producer strains of Saccharomyces cerevisiae for preserving wine acidity during alcoholic fermentation. Food Microbiol. 2023, 112, 104209. [Google Scholar] [CrossRef] [PubMed]
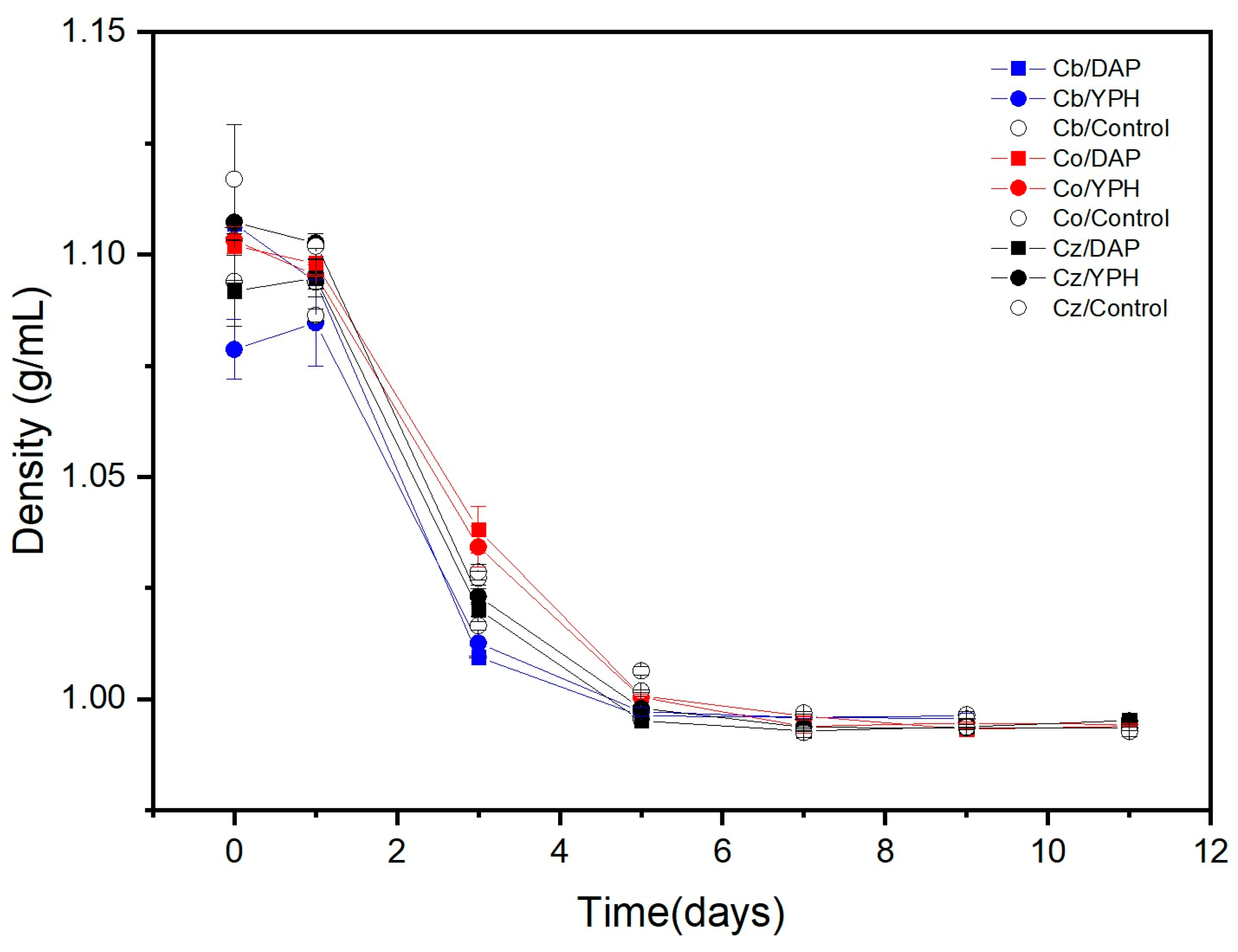

| Yeast | Nitrogen Source | Glucose (g/L) | Fructose (g/L) | Glycerol (g/L) | Tartaric Acid (g/L) | Acetic Acid (g/L) | Ethanol (% v/v) |
|---|---|---|---|---|---|---|---|
| No yeast | Juice grape | 63.40 ± 0.02 | 111.20 ± 0.02 | 0.96 ± 0.00 | 2.91 ± 0.01 | 2.76 ± 0.00 | ND |
| C. boidinii Day 0 | DAP (43 mg/L) | 54.90 ± 0.02 e | 96.75 ± 0.01 d | 0.72 ± 0.00 d | 3.04 ± 0.00 cd | 1.40 ± 0.09 ab | <0.02 |
| YPH (43 mg/L) | 64.25 ± 0.01 cd | 112.50 ± 0.00 c | 0.78 ± 0.00 cd | 4.12 ± 0.00 bc | 1.50 ± 0.01 ab | <0.02 | |
| Control | 63.72 ± 0.00 cd | 113.28 ± 0.08 c | 0.98 ± 0.00 bc | 4.19 ± 0.00 bc | 1.66 ± 0.00 a | <0.02 | |
| C. boidinii Day 9 | DAP (43 mg/L) | <0.10 | <0.10 | 8.61 ± 0.00 d | 2.55 ± 0.02 a | 0.34 ± 0.02 d | 11.15 ± 0.00 c |
| YPH (43 mg/L) | <0.10 | <0.10 | 9.20 ± 0.00 cd | 2.66 ± 0.00 a | 2.26 ± 0.01 b | 12.60 ± 0.01 b | |
| Control | <0.10 | 2.20 ± 0.01 | 9.61 ± 0.00 bc | 1.76 ± 0.02 bc | 3.34 ± 0.01 a | 12.85 ± 0.01 | |
| C. oleophila Day 0 | DAP (43 mg/L) | 63.50 ± 0.12 d | 112.42 ± 0.03 c | 1.07 ± 0.00 ab | 4.33 ± 0.08 b | 1.28 ± 0.06 ab | <0.02 |
| YPH (43 mg/L) | 66.62 ± 0.10 b | 118.22 ± 1.88 b | 1.11 ± 0.00 ab | 4.56 ± 0.01 ab | 1.29 ± 0.00 ab | <0.02 | |
| Control | 64.78 ± 0.09 c | 118.60 ± 0.00 b | 1.23 ± 0.00 a | 5.68 ± 0.22 a | 1.28 ± 0.00 ab | <0.02 | |
| C. oleophila Day 11 | DAP (43 mg/L) | <0.10 | <0.10 | 10.14 ± 0.00 ab | 2.28 ± 0.00 ab | 0.49 ± 0.00 c | 11.91 ± 0.01 abc |
| YPH (43 mg/L) | <0.10 | <0.10 | 10.09 ± 0.05 ab | 2.30 ± 0.01 ab | <0.02 | 12.12 ± 0.04 abc | |
| Control | <0.10 | <0.10 | 10.58 ± 0.02 a | 2.35 ± 0.05 ab | <0.02 | 12.69 ± 0.05 ab | |
| C. zemplinina Day 0 | DAP (43 mg/L) | 42.70 ± 0.02 f | 78.15 ± 0.25 e | 0.85 ± 0.02 cd | 2.26 ± 0.00 d | 1.03 ± 0.03 b | <0.02 |
| YPH (43 mg/L) | 67.44 ± 0.02 b | 118.21 ± 2.90 b | 1.14 ± 0.00 ab | 2.27 ± 0.33 d | 1.30 ± 0.00 ab | <0.02 | |
| Control | 78.55 ± 0.41 a | 142.05 ± 3.13 a | 1.25 ± 0.02 a | 2.15 ± 0.00 d | 1.62 ± 0.06 a | <0.02 | |
| C. zemplinina Day 11 | DAP (43 mg/L) | <0.10 | <0.10 | 9.29 ± 0.12 c | 1.56 ± 0.00 c | 0.08 ± 0.00 f | 11.41 ± 1.16 bc |
| YPH (43 mg/L) | <0.10 | <0.10 | 10.40 ± 0.01 a | 1.48 ± 0.14 c | 0.36 ± 0.02 d | 13.32 ± 0.05 a | |
| Control | <0.10 | <0.10 | 10.27 ± 0.00 a | 1.48 ± 0.14 c | 0.27 ± 0.01 e | 13.03 ± 0.01 a |
| Compounds | Cb/DAP µg/L | Cb/YPH µg/L | Co/DAP µg/L | Co/YPH µg/L | Cz/DAP µg/L | Cz/YPH µg/L |
|---|---|---|---|---|---|---|
| Isoamyl alcohol | 6430.91 ± 0.00 b | 7294.80 ± 0.00 a | 2589.34 ± 0.00 e | 2913.96 ± 0.00 c | 2365.57 ± 2.29 f | 2693.09 ± 0.00 d |
| 2-methylbutanol | 2297.21 ± 0.00 b | 2455.46 ± 0.00 a | 529.52 ± 0.00 e | 834.86 ± 0.00 c | 518.29 ± 0.00 f | 782.12 ± 0.00 d |
| Isobutyl alcohol | 612.76 ± 0.00 b | 620.95 ± 0.00 a | 226.14 ± 0.00 f | 343.90 ± 2.90 c | 228.91 ± 0.00 e | 293.13 ± 0.00 d |
| 2-Phenyl ethanol | 330.45 ± 4.23 b | 453.85 ± 0.00 a | 209.43 ± 0.00 e | 223.02 ± 0.00 d | 225.70 ± 0.00 d | 231.62 ± 0.00 c |
| 1-propanol | 155.85 ± 0.00 b | 216.55 ± 3.11 a | 42.33 ± 3.00 d | 46.64 ± 1.16 cd | 48.80 ± 0.00 c | 33.37 ± 0.70 e |
| 2,3-butanediol Isomero 2 | 41.82 ± 0.00 b | 53.90 ± 0.00 a | 27.56 ± 0.00 c | 39.78 ± 0.00 b | 52.69 ± 2.92 a | 52.99 ± 0.00 a |
| 2,3-butanediol Isomero 1 | 16.90 ± 0.08 bc | 14.79 ± 0.86 cd | 12.63 ± 0.70 d | 14.14 ± 0.52 cd | 21.99 ± 0.30 a | 19.39 ± 0.76 ab |
| Ethyl acetate | 4816.51 ± 0.00 a | 4564.31 ± 0.00 b | 1252.76 ± 0.00 e | 1591.66 ± 0.00 c | 1209.30 ± 0.29 f | 1461.92 ± 0.00 d |
| Ethyl hexanoate | 812.85 ± 0.00 b | 917.11 ± 0.00 a | 275.58 ± 2.90 f | 506.46 ± 0.00 c | 299.56 ± 0.00 e | 459.03 ± 0.00 d |
| Isoamyl acetate | 657.70 ± 0.00 b | 684.50 ± 0.00 a | 289.75 ± 1.13 e | 298.90 ± 0.02 d | 248.05 ± 0.00 f | 369.00 ± 0.00 c |
| Ethyl octanoate | 639.59 ± 0.00 d | 678.22 ± 0.00 a | 290.14 ± 0.05 f | 667.76 ± 0.00 b | 387.80 ± 0.00 e | 642.09 ± 0.00 c |
| Ethyl lactate | 462.62 ± 0.00 b | 576.65 ± 0.00 a | 276.73 ± 3.23 f | 342.37 ± 0.00 d | 305.45 ± 0.00 e | 364.52 ± 0.00 c |
| Ethyl butanoate | 143.09 ± 0.00 b | 160.73 ± 0.00 a | 39.41 ± 0.97 cd | 41.52 ± 3.52 c | 38.15 ± 0.00 cd | 37.75 ± 0.39 d |
| Diethyl butanedioate | 130.58 ± 0.00 b | 136.45 ± 0.00 a | 93.33 ± 1.29 e | 103.26 ± 0.00 d | 95.30 ± 0.00 e | 117.28 ± 0.53 c |
| Ethyl decanoate | 36.10 ± 0.00 d | 41.80 ± 0.50 cd | 42.01 ± 8.04 c | 81.08 ± 8.51 a | 65.64 ± 0.00 b | 81.63 ± 0.00 a |
| Compounds | Cb/DAP | Cb/YPH | Co/DAP | Co/YPH | Cz/DAP | Cz/YPH | OT |
|---|---|---|---|---|---|---|---|
| Ethyl 3-methylpentanoate | - | 3.8 | 1.6 | 3.2 | 1.4 | 2.6 | 0.5 |
| Ethyl hexanoate | 13.1 | 14.8 | 4.4 | 8.2 | 4.8 | 7.4 | 62 |
| Ethyl butanoate | 7.2 | 8.0 | 2.0 | 2.1 | 1.9 | 1.9 | 20 |
| Ethyl 2-methylbutanoate | 1.0 | 1.2 | 0.1 | 0.1 | 0.2 | 0.2 | 18 |
| Ethyl 3-methylbutanoate | 6.3 | 7.6 | 0.7 | 0.9 | 0.7 | 0.8 | 3 |
| Ethyl octanoate | 1.1 | 1.2 | 0.5 | 1.2 | 0.7 | 1.1 | 580 |
| Isoamyl acetate | 21.9 | 22.8 | 9.7 | 10.0 | 8.3 | 12.3 | 30 |
| Yeast | Nitrogen Source | Malic Acid (g/L) | pH |
|---|---|---|---|
| No yeast | Juice grape | 1.27 ± 0.00 | 3.62 ± 0.00 |
| C. boidinii Day 0 | DAP (43 mg/L) | 1.26 ± 0.00 bc | 3.73 ± 0.00 a |
| YPH (43 mg/L) | 1.23 ± 0.36 bc | 3.65 ± 0.00 ab | |
| Control | 1.24 ± 0.00 bc | 3.65 ± 0.01 ab | |
| C. boidinii Day 9 | DAP (43 mg/L) | 1.35 ± 0.00 a | 3.71 ± 0.00 a |
| YPH (43 mg/L) | 1.13 ± 0.00 c | 3.66 ± 0.00 bc | |
| Control | 1.24 ± 0.00 b | 3.70 ± 0.00 ab | |
| C. oleophila Day 0 | DAP (43 mg/L) | 1.25 ± 0.00 b | 3.60 ± 0.00 ab |
| YPH (43 mg/L) | 1.26 ± 0.00 bc | 3.62 ± 0.00 b | |
| Control | 1.23 ± 0.00 bc | 3.62 ± 0.00 b | |
| C. oleophila Day 11 | DAP (43 mg/L) | 0.03 ± 0.00 fg | 3.62 ± 0.00 c |
| YPH (43 mg/L) | 0.08 ± 0.00 e | 3.63 ± 0.00 bc | |
| Control | 0.04 ± 0.00 fg | 3.64 ± 0.00 c | |
| C. zemplinina Day 0 | DAP (43 mg/L) | 1.15 ± 0.00 c | 3.63 ± 0.00 b |
| YPH (43 mg/L) | 1.30 ± 0.00 b | 3.62 ± 0.00 b | |
| Control | 1.35 ± 0.00 a | 3.63 ± 0.00 b | |
| C. zemplinina Day 11 | DAP (43 mg/L) | 0.02 ± 0.00 g | 3.91 ± 0.00 c |
| YPH (43 mg/L) | 0.32 ± 0.00 d | 3.91 ± 0.00 c | |
| Control | 0.04 ± 0.00 f | 3.91 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Lira, C.; Valencia, P.; Urtubia, A.; Landaeta, E.; Tapia, R.A.; Franco, W. Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina. Foods 2024, 13, 4166. https://doi.org/10.3390/foods13244166
López-Lira C, Valencia P, Urtubia A, Landaeta E, Tapia RA, Franco W. Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina. Foods. 2024; 13(24):4166. https://doi.org/10.3390/foods13244166
Chicago/Turabian StyleLópez-Lira, Claudia, Pedro Valencia, Alejandra Urtubia, Esteban Landaeta, Ricardo A. Tapia, and Wendy Franco. 2024. "Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina" Foods 13, no. 24: 4166. https://doi.org/10.3390/foods13244166
APA StyleLópez-Lira, C., Valencia, P., Urtubia, A., Landaeta, E., Tapia, R. A., & Franco, W. (2024). Influence of Organic Nitrogen Derived from Recycled Wine Lees and Inorganic Nitrogen on the Chemical Composition of Cabernet Sauvignon Wines Fermented in the Presence of Non-Saccharomyces Yeasts Candida boidinii, C. oleophila, and C. zemplinina. Foods, 13(24), 4166. https://doi.org/10.3390/foods13244166








