Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation
Abstract
:1. Introduction
2. Composition of Vegetable Oils Used for Frying
2.1. Vegetable Oils Rich in MUFA
2.2. Vegetable Oils Rich in PUFA
2.3. Vegetable Oils Rich in SFA
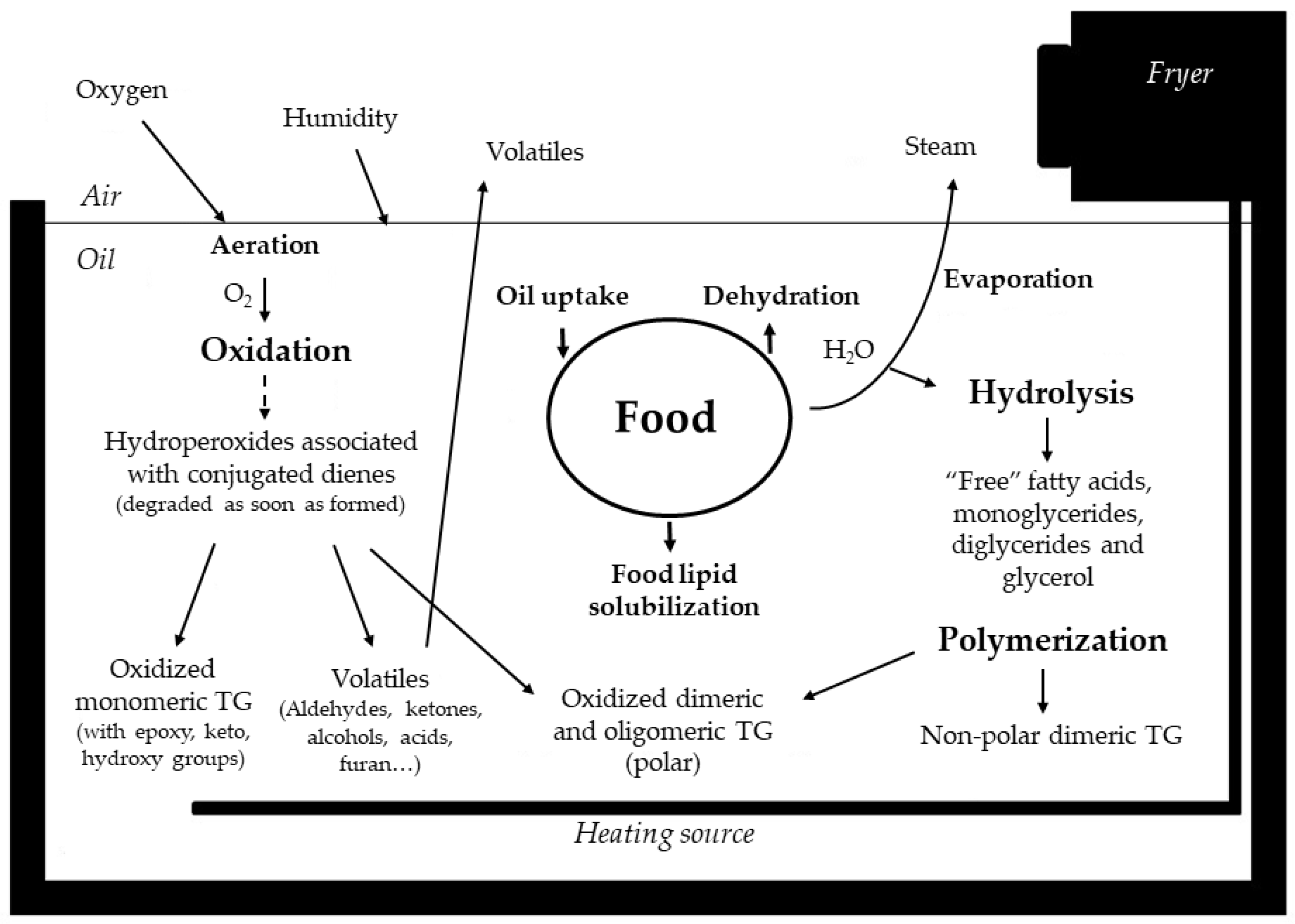
3. Chemical Reactions Occurring in the Oil During Frying
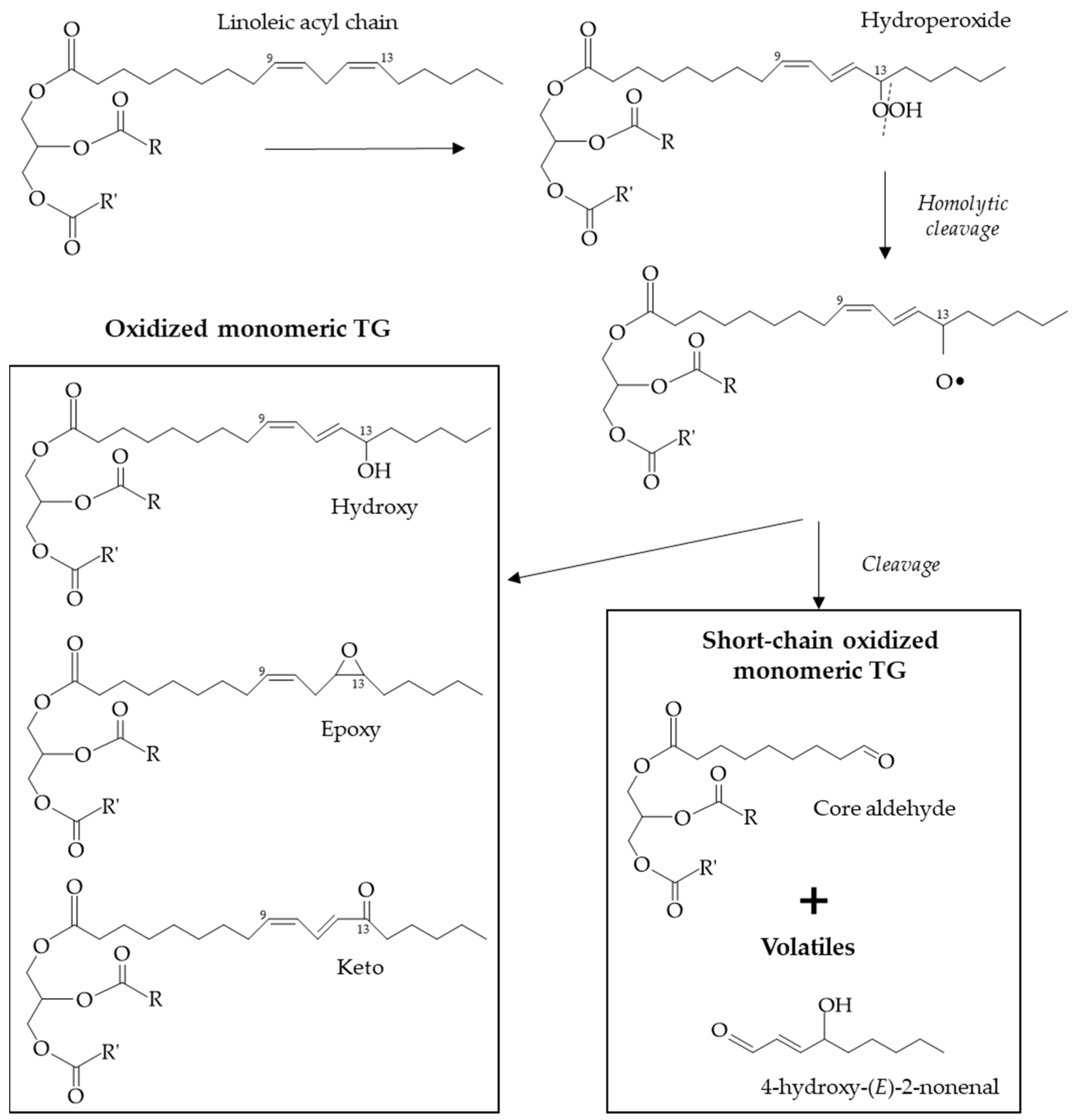
3.1. Thermoxidation
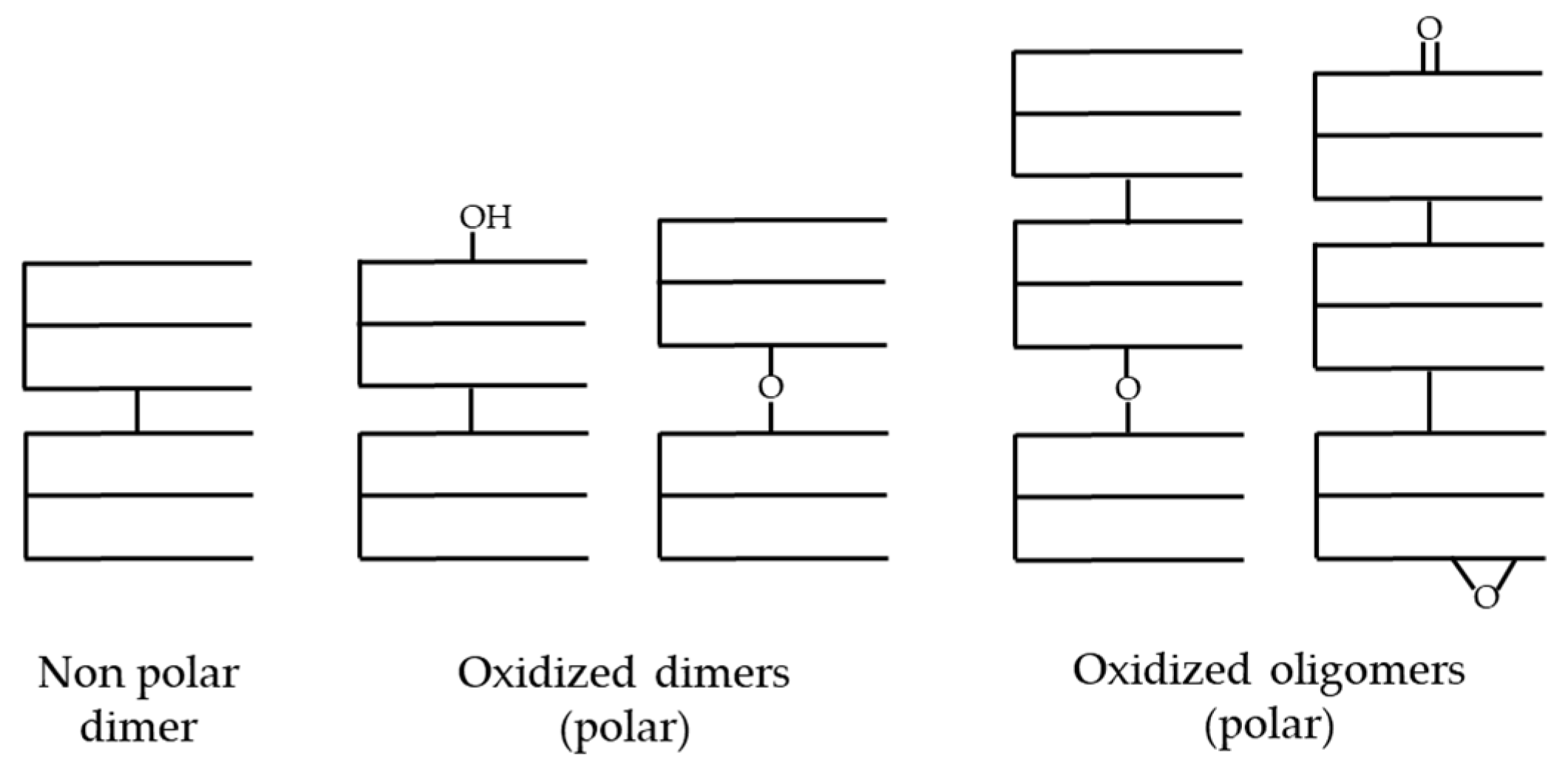
3.2. Polymerization
3.3. Hydrolysis
3.4. Other Reactions
4. Factors Conditioning Frying Medium Degradation
4.1. Oil Composition
4.1.1. Unsaturation Degree of FA and Its Influence on Oil Oxidative Stability
4.1.2. Length of FA and Content of “Free” Fatty Acids: Influence on Oil Smoke Point
4.1.3. Oil Minor Components and Their Influence on Oil Oxidative Stability
4.2. Frying Conditions
4.3. Food
5. Fried Food Quality and Health Implications
5.1. Fried Food Lipids: A Result of Oil Uptake and Food Lipid Solubilization
5.2. Sensory Properties of Fried Food
5.3. Health Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firestone, D. Chapter 21—Regulation of frying fat and oil. In Deep Frying, 2nd ed.; Erickson, M.D., Ed.; AOCS Press: Urbana, IL, USA, 2007; pp. 373–385. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Martini, S. Chapter 14—Chemical and physical deterioration of bulk oils and shortenings, spreads and frying oils. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 413–438. [Google Scholar] [CrossRef]
- Frankel, E.N. Chapter 12—Frying fats. In Lipid Oxidation, 2nd ed.; Frankel, E.N., Ed.; Oily Press Lipid Library Series; Woodhead Publishing: Bridgwater, UK, 2005; pp. 355–389. [Google Scholar] [CrossRef]
- Martinez-Yusta, A.; Guillen, M.D. Deep-frying. A study of the influence of the frying medium and the food nature on the lipidic composition of the fried food, using 1H Nuclear Magnetic Resonance. Food Res. Int. 2014, 62, 998–1007. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Food Balance Sheets; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/faostat/en/#data/FBS/report (accessed on 8 November 2024).
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses; Blackwell Publishing: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Rossell, J.B. Chapter 7—Factors affecting the quality of frying oils and fats. In Frying; Rossell, J.B., Ed.; Woodhead Publishing: Cambridge, UK, 2001; pp. 115–164. [Google Scholar] [CrossRef]
- Fine, F.; Brochet, C.; Gaud, M.; Carre, P.; Simon, N.; Ramli, F.; Joffre, F. Micronutrients in vegetable oils: The impact of crushing and refining processes on vitamins and antioxidants in sunflower, rapeseed, and soybean oils. Eur. J. Lipid Sci. Technol. 2016, 118, 680–697. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Healthy fats and oils. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- Garcimartin, A.; Macho-Gonzalez, A.; Caso, G.; Benedi, J.; Bastida, S.; Sanchez-Muniz, F.J. Chapter 19—Frying a cultural way of cooking in the Mediterranean diet and how to obtain improved fried foods. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2020; pp. 191–207. [Google Scholar] [CrossRef]
- Astrup, A.; Teicholz, N.; Magkos, F.; Bier, D.M.; Brenna, J.T.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; Yusuf, S.; et al. Dietary saturated fats and health: Are the U.S. guidelines evidence-based? Nutrients 2021, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Heileson, J.L. Dietary saturated fat and heart disease: A narrative review. Nutr. Rev. 2020, 78, 474–485. [Google Scholar] [CrossRef]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of utilizing healthy fats in foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef] [PubMed]
- Codex Standards for Fats and Oils from Vegetable Sources (33-1981, Rev. 1-1989. and 210-1999); FAO (Food and Agriculture Organization): Rome, Italy, 1999.
- Boskou, D.; Blekas, G.; Tsimidou, M. Chapter 4—Olive oil composition. In Olive Oil, 2nd ed.; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar] [CrossRef]
- Muzammil, S.; Inamuddin; Boddula, R.; Asiri, A.M. Chapter 2—Olive oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 17–29. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, A.; Quintero-Florez, A.; Ruiz-Mendez, M.V.; Perona, J.S. Virgin olive oil ranks first in a new nutritional quality score due to its compositional profile. Nutrients 2023, 15, 2127. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.R.S.J.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant, and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, P. Chapter 5—Understanding oil absorption during deep-fat frying. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 57, pp. 209–234. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Frying of nutritious foods: Obstacles and feasibility. Food Sci. Technol. Res. 2001, 7, 265–279. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Boskou, G.; Salta, F.N. Migration of health promoting microconstituents from frying vegetable oils to french fries. Food Chem. 2012, 133, 1255–1263. [Google Scholar] [CrossRef]
- De Carvalho, A.G.A.; Olmo-Garcia, L.; Gaspar, B.R.A.; Carrasco-Pancorbo, A.; Castelo-Branco, V.N.; Torres, A.G. Evolution of the metabolic profile of virgin olive oil during deep-frying: Assessing the transfer of bioactive compounds to the fried food. Food Chem. 2022, 380, 132205. [Google Scholar] [CrossRef]
- Lozano-Castellon, J.; Rinaldi de Alvarenga, J.F.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. Cooking with extra-virgin olive oil: A mixture of food components to prevent oxidation and degradation. Trends Food Sci. Technol. 2022, 123, 28–36. [Google Scholar] [CrossRef]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried Food Consumption and Cardiovascular Health: A Review of Current Evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Fried Food and Prostate Cancer Risk: Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2015, 66, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of Fried Food Consumption with All Cause, Cardiovascular, and Cancer Mortality: Prospective Cohort Study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Leon-Munoz, L.M.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Buckland, G.; Chirlaque, M.-D.; et al. Consumption of Fried Foods and Risk of Coronary Heart Disease: Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition Study. BMJ 2012, 344, e363. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Talamini, R.; Levi, F.; Pelucchi, C.; Negri, E.; Giacosa, A.; Montella, M.; Franceschi, S.; La Vecchia, C. Fried Foods, Olive Oil, and Colorectal Cancer. Ann. Oncol. 2006, 18, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Sayon-Orea, C.; Bes-Rastrollo, M.; Basterra-Gortari, F.J.; Beunza, J.J.; Guallar-Castillon, P.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A. Consumption of Fried Foods and Weight Gain in a Mediterranean Cohort: The SUN Project. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Casla, A.; García-Esquinas, E.; Lopez-Garcia, E.; Sotos-Prieto, M.; Struijk, E.A.; Caballero, F.F.; Rodríguez-Artalejo, F.; Ortolá, R. Consumption of Food Fried in Olive Oil and Unhealthy Aging in a Mediterranean Country. Clin. Nutr. 2021, 40, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Pokorny, J. The development and application of novel vegetable oils tailor-made for specific human dietary needs. Eur. J. Lipid Sci. Technol. 2003, 105, 769–778. [Google Scholar] [CrossRef]
- Smith, S.A.; King, R.E.; Min, D.B. Oxidative and thermal stabilities of genetically modified high oleic sunflower oil. Food Chem. 2007, 102, 1208–1213. [Google Scholar] [CrossRef]
- Rauf, S.; Jamil, N.; Tariq, S.A.; Khan, M.; Kausar, M.; Kaya, Y. Progress in modification of sunflower oil to expand its industrial value. J. Sci. Food Agric. 2017, 97, 1997–2006. [Google Scholar] [CrossRef]
- Zambelli, A. Current status of high oleic seed oils in food processing. J. Am. Oil Chem. Soc. 2021, 98, 129–137. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A comprehensive review of health-benefiting components in rapeseed oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef]
- Warner, K.; Gupta, M. Frying quality and stability of low-and ultra-low-linolenic acid soybean oils. J. Am. Oil Chem. Soc. 2003, 80, 275–280. [Google Scholar] [CrossRef]
- Matthäus, B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur. J. Lipid Sci. Technol. 2006, 108, 200–211. [Google Scholar] [CrossRef]
- Carrin, M.E.; Carelli, A.A. peanut oil: Compositional data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A.R. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef]
- Bhattacharya, S. Chapter 10—Fats and oils. In Snack Foods; Bhattacharya, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 251–281. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Molina-Garcia, L.; Cunha, S.C.; Casal, S. Fried potatoes: Impact of prolonged frying in monounsaturated oils. Food Chem. 2018, 243, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Busse, W.W.; Sachs, M.I.; Parker, J.L.; Yunginger, J.W. Peanut oil is not allergenic to peanut-sensitive individuals. J. Allergy Clin. Immunol. 1981, 68, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Arellano, D.; Badan-Ribeiro, A.P.; Serna-Saldivar, S.O. Chapter 21—Corn oil: Composition, processing, and utilization. In Corn; Serna-Saldivar, S.O., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 593–613. [Google Scholar] [CrossRef]
- Kiralan, M.; Ketenoglu, O.; Kiralan, S.S. Chapter 10—Trans fatty acids—Occurrence, technical aspects, and worldwide regulations. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; Volume 70, pp. 313–343. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to a notification from FEDIOL and IMACE on fully refined soybean oil and fat pursuant to Article 6, Paragraph 11 of Directive 2000/13/EC—For permanent exemption from labelling. EFSA J. 2007, 5, 570. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1219–1237. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, Z.; Chen, Y.; Lai, O.M.; Tan, C.P.; Akoh, C.C. Chapter 16—Minor constituents of palm oil: Characterization, processing, and application. In Palm Oil; Lai, O.M., Tan, C.P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 471–526. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry–A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orru, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Meijaard, E.; Brooks, T.M.; Carlson, K.M.; Slade, E.M.; Garcia-Ulloa, J.; Gaveau, D.L.A.; Lee, J.S.H.; Santika, T.; Juffe-Bignoli, D.; Struebig, M.J.; et al. The environmental impacts of palm oil in context. Nat. Plants 2020, 6, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Oey, S.B.; van der Fels-Klerx, H.J.; Fogliano, V.; van Leeuwen, S.P.J. Mitigation strategies for the reduction of 2- and 3-MCPD esters and glycidyl esters in the vegetable oil processing industry. Comp. Rev. Food Sci. Food Saf. 2019, 18, 349–361. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Some Chemicals Present in Industrial and Consumer Products. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 101, pp. 349–374. [Google Scholar]
- Sankararaman, S.; Sferra, T.J. Are we going nuts on coconut oil? Curr. Nutr. Rep. 2018, 7, 107–115. [Google Scholar] [CrossRef]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, A.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Dobarganes, M.C. Chapter 3—Chemistry of frying. In Advances in Deep-Fat Frying of Foods; Sahin, S., Sumnu, S.G., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Chen, B.; McClements, D.J.; Decker, E.A. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef]
- Fritsch, C.W. Measurements of frying fat deterioration: A brief review. J. Am. Oil Chem. Soc. 1981, 58, 272–274. [Google Scholar] [CrossRef]
- Warner, K. Chapter 5—Impact of high-temperature food processing on fats and oils. In Impact of Processing on Food Safety; Jackson, L.S., Knize, M.G., Morgan, J.N., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Li, M.; Shen, Q.; Saleh, A.S.M. Application of chromatographic techniques in the detection and identification of constituents formed during food frying: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 601–633. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, Y.; Zhang, N.; Gao, Y.; Yu, X. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 2021, 364, 130451. [Google Scholar] [CrossRef] [PubMed]
- Marmesat, S.; Velasco, J.; Dobarganes, M.C. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J. Chromatogr. A. 2008, 1211, 129–134. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and nutritional quality changes of oil during frying. J. Am. Oil Chem. Soc. 2009, 86, 149–156. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative fingerprint changes of toxic volatiles in low PUFA vegetable oils under deep-frying. J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.; Barlow, P.J.; Joshi, P.S.; Lo, H.L.; Chung, Y.K. Review of rapid tests available for measuring the quality changes in frying oils and comparison with standard methods. Crit. Rev. Food Sci. Nutr. 2010, 50, 503–514. [Google Scholar] [CrossRef]
- Osawa, C.C.; Gonçalves, L.A.G.; Gumerato, H.F.; Mendes, F.M. Study of the effectiveness of quick tests based on physical properties for the evaluation of used frying oil. Food Control 2012, 26, 525–530. [Google Scholar] [CrossRef]
- Fatima, S.; Kumar, V.; Bhadauria, G.; Verma, H. Quality indicators based rapid test kits for detection of frying oil quality: A review. Food Chem. Adv. 2023, 2, 100305. [Google Scholar] [CrossRef]
- Guillen, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Weisshaar, R. Quality control of used deep-frying oils. Eur. J. Lipid Sci. Technol. 2014, 116, 716–722. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Dobarganes, C.; Márquez-Ruiz, G. Analysis of used frying oils. Lipid Technol. 2013, 25, 159–162. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Salta, F.N.; Chiou, A.; Andrikopoulos, N.K. Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur. J. Lipid Sci. Technol. 2007, 109, 1111–1123. [Google Scholar] [CrossRef]
- Koch, E.; Löwen, A.; Nikolay, S.; Willenberg, I.; Schebb, N.H. Trans-hydroxy, trans-epoxy, and erythro-dihydroxy fatty acids increase during deep-frying. J. Agric. Food Chem. 2023, 71, 7508–7513. [Google Scholar] [CrossRef]
- Guillen, M.D.; Uriarte, P.S. Monitoring by 1H Nuclear Magnetic Resonance of the changes in the composition of virgin linseed oil heated at frying temperature. Comparison with the evolution of other edible oils. Food Control 2012, 28, 59–68. [Google Scholar] [CrossRef]
- Guillen, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Guillen, M.D.; Uriarte, P.S. Simultaneous control of the evolution of the percentage in weight of polar compounds, iodine value, acyl groups proportions and aldehydes concentrations in sunflower oil submitted to frying temperature in an industrial fryer. Food Control 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Petersen, K.D.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Chemical and sensory assessment of deep-frying oil alternatives for the processing of french fries. Eur. J. Lipid Sci. Technol. 2013, 115, 935–945. [Google Scholar] [CrossRef]
- Peng, C.Y.; Lan, C.H.; Lin, P.C.; Kuo, Y.C. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard. Mater. 2017, 324, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.S.; Cho, H.; Hwang, K.T. Physicochemical properties and oxidative stability of frying oils during repeated frying of potato chips. Food Sci. Biotechnol. 2018, 27, 651–659. [Google Scholar] [CrossRef]
- Hammouda, I.; Triki, M.; Matthäus, B.; Bouaziz, M. A comparative study on formation of polar components, fatty acids and sterols during frying of refined olive pomace oil pure and its blend coconut oil. J. Agric. Food Chem. 2018, 66, 3514–3523. [Google Scholar] [CrossRef]
- Hammouda, I.; Márquez-Ruiz, G.; Holgado, F.; Freitas, F.; Da Silva, M.D.R.G.; Bouaziz, M. Comparative study of polymers and total polar compounds as indicators of refined oil degradation during frying. Eur. Food Res. Technol. 2019, 245, 967–976. [Google Scholar] [CrossRef]
- Xu, T.; Li, J.; Fan, Y.-W.; Zheng, T.; Deng, Z.-Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Marmesat, S.; Morales, A.; Velasco, J.; Dobarganes, M.C. Action and fate of natural and synthetic antioxidants during frying. Grasas y Aceites 2010, 61, 333–340. [Google Scholar] [CrossRef]
- Wang, L.; Csallany, A.S.; Kerr, B.J.; Shurson, G.C.; Chen, C. Kinetics of forming aldehydes in frying oils and their distribution in french fries revealed by LC–MS-based chemometrics. J. Agric. Food Chem. 2016, 64, 3881–3889. [Google Scholar] [CrossRef]
- Marquez-Ruiz, G.; Velasco, J.; Holgado, F. Chapter 1—Major dietary lipids in nutrition and health. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 105, pp. 1–49. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Marquez-Ruiz, G.; Dobarganes, M.C. Formation of short-chain glycerol-bound oxidation products and oxidised monomeric triacylglycerols during deep-frying and occurrence in used frying fats. Eur. J. Lipid Sci. Technol. 2004, 106, 728–735. [Google Scholar] [CrossRef]
- Xia, W.; Budge, S.M. Techniques for the analysis of minor lipid oxidation products derived from triacylglycerols: Epoxides, alcohols, and ketones. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Xie, Z.; Gao, B.; Yu, L. Chemical structures, analytical approaches, and toxicological effects of oxidative derivatives of triglycerides as potential hazards in lipid thermal processing: A review. Grain Oil Sci. Technol. 2024, 7, 270–279. [Google Scholar] [CrossRef]
- Chang, S.S.; Peterson, R.J.; Ho, C.-T. Chemical reactions involved in the deep-fat frying of foods. J. Am. Oil Chem. Soc. 1978, 55, 718–727. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Household Use of Solid Fuels and High-Temperature Frying. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 95, pp. 310–393. [Google Scholar]
- Dobarganes, M.C.; Márquez-Ruiz, G. Chapter 6—Formation and analysis of oxidized monomeric, dimeric, and higher oligomeric triglycerides. In Deep Frying, 2nd ed.; Erickson, M.D., Ed.; AOCS Press: Urbana, IL, USA, 2007; pp. 87–110. [Google Scholar] [CrossRef]
- Marquez-Ruiz, G.; Ruiz-Mendez, M.V.; Velasco, J. Antioxidants in frying: Analysis and evaluation of efficacy. Eur. J. Lipid Sci. Technol. 2014, 116, 1441–1450. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Perez-Camino, M.C. Non-polar dimer formation during thermoxidation of edible fats. Lipid/Fett 1987, 89, 216–220. [Google Scholar] [CrossRef]
- Bastida, S.; Sánchez-Muniz, F.J. Thermal oxidation of olive oil, sunflower oil and a mix of both oils during forty discontinuous domestic fryings of different foods. Food Sci. Technol. Int. 2001, 7, 15–21. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Full, G.H.; Dao, L.T. Effect of heating on the characteristics and chemical composition of selected frying oils and fats. J. Agric. Food Chem. 1997, 45, 3244–3249. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Review: Mechanism of oil uptake during deep-fat frying and the surfactant effect—Theory and myth. Adv. Colloid Interface Sci. 2006, 128, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lioumbas, J.S.; Ampatzidis, C.; Karapantsios, T.D. Effect of potato deep-fat frying conditions on temperature dependence of olive oil and palm oil viscosity. J. Food Eng. 2012, 113, 217–225. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Sheikh, H.; Sayeed, S.A. Deterioration of olive, corn and soybean oils due to air, light, heat and deep-frying. Food Res. Int. 2005, 38, 127–134. [Google Scholar] [CrossRef]
- Bhat, S.; Reddy, S.Y.; Gowda, S.G.R.; Hariprasad, D.S.; Jamuna, B. Influence of heating during cooking on trans fatty acid content of edible oils: A systematic review and meta-analysis. Nutrients 2022, 14, 1489. [Google Scholar] [CrossRef]
- Guo, Q.; Li, T.; Qu, Y.; Liang, M.; Ha, Y.; Zhang, Y.; Wang, Q. New research development on trans fatty acids in food: Biological effects, analytical methods, formation mechanism, and mitigating measures. Prog. Lipid Res. 2023, 89, 101199. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Dobson, G. Formation of cyclic fatty acids during the frying process. Eur. J. Lipid Sci. Technol. 2000, 102, 515–520. [Google Scholar] [CrossRef]
- Sebedio, J.-L.; Juaneda, P. Isomeric and cyclic fatty acids as a result of frying. In Deep Frying: Chemistry, Nutrition, and Practical Applications, 2nd ed.; Erickson, M.D., Ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 57–86. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Wu, B.; Wang, G.; Shen, G.; Zhu, Y.; Zhang, Y.; Tao, S. Characteristics of PAHs from deep-frying and frying cooking fumes. Environ. Sci. Pollut. Res. 2015, 22, 16110–16120. [Google Scholar] [CrossRef]
- Asokapandian, S.; Swamy, G.J.; Hajjul, H. Deep fat frying of foods: A critical review on process and product parameters. Crit. Rev. Food Sci. Nutr. 2020, 60, 3400–3413. [Google Scholar] [CrossRef]
- Warner, K.; Knowlton, S. Frying quality and oxidative stability of high-oleic corn oils. J. Am. Oil Chem. Soc. 1997, 74, 1317–1322. [Google Scholar] [CrossRef]
- Santos, C.S.; Garcia, L.M.; Cruz, R.; Cunha, S.C.; Fernandes, J.O.; Casal, S. Impact of potatoes deep-frying on common monounsaturated-rich vegetable oils: A comparative study. J. Food Sci. Technol. 2019, 56, 290–301. [Google Scholar] [CrossRef]
- Wann, A.I.; Percival, B.C.; Woodason, K.; Gibson, M.; Vincent, S.; Grootveld, M. Comparative 1H NMR-based chemometric evaluations of the time-dependent generation of aldehydic lipid oxidation products in culinary oils exposed to laboratory-simulated shallow frying episodes: Differential patterns observed for omega-3 fatty acid-containing soybean oils. Foods 2021, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.D.; Goicoechea, E. Toxic oxygenated alpha, beta-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Daniali, G.; Jinap, S.; Hajeb, P.; Sanny, M.; Tan, C.P. Acrylamide formation in vegetable oils and animal fats during heat treatment. Food Chem. 2016, 212, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Elmadfa, I. (Eds.) Frying of Food: Oxidation, Nutrient and Non-Nutrient Antioxidants, Biologically Active Compounds and High Temperatures, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Guillaume, C.; De Alzaa, F.; Ravetti, L. Evaluation of chemical and physical changes in different commercial oils during heating. Acta Sci. Nutr. Health 2018, 2, 2–11. [Google Scholar]
- Akil, E.; Castelo-Branco, V.N.; Costa, A.M.M.; do Amaral Vendramini, A.L.; Calado, V.; Torres, A.G. Oxidative stability and changes in chemical composition of extra virgin olive oils after short-term deep-frying of french fries. J. Am. Oil Chem. Soc. 2015, 92, 409–421. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Minor components in oils and their effects on frying performance. Lipid Technol. 2013, 25, 87–90. [Google Scholar] [CrossRef]
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Lannes, S.C.D.S. Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview. Food Rev. Int. 2022, 38, 349–372. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Kuang, Y.; Bi, Y.; Wang, H. A review on losses and transformation mechanisms of common antioxidants. J. Am. Oil Chem. Soc. 2023, 100, 259–285. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Li, S.; Yan, J.; Qin, W.; Saleh, A.S.; Zhang, Q. Plant phenolic extracts for the quality protection of frying oil during deep frying: Sources, effects, and mechanisms. Grain Oil Sci. Technol. 2023, 6, 148–161. [Google Scholar] [CrossRef]
- Zeb, A. Food Frying: Chemistry, Biochemistry, and Safety; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mehta, U.; Swinburn, B.A. Review of factors affecting fat absorption in hot chips. Crit. Rev. Food Sci. Nutr. 2001, 41, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Melton, S.L.; Jafar, S.; Sykes, D.; Trigiano, M.K. Review of stability measurements for frying oils and fried food flavor. J. Am. Oil Chem. Soc. 1994, 71, 1301–1308. [Google Scholar] [CrossRef]
- Pokorny, J. Substrate influence on the frying process. Grasas Aceites 1998, 49, 265–270. [Google Scholar] [CrossRef]
- Pokorny, J.; Reblova, Z. Effect of food components on changes in frying oil. Food Technol. Biotechnol. 1999, 37, 139–144. [Google Scholar]
- Koh, E.; Surh, J. Food types and frying frequency affect the lipid oxidation of deep frying oil for the preparation of school meals in korea. Food Chem. 2015, 174, 467–472. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.R.; Ngadi, M. Application of batter coating for modulating oil, texture, and structure of fried foods: A review. Food Chem. 2024, 453, 139655. [Google Scholar] [CrossRef]
- Frakolaki, G.; Kekes, T.; Bizymis, A.-P.; Giannou, V.; Tzia, C. Chapter—9—Fundamentals of food frying processes. In High-Temperature Processing of Food Products; Jafari, S.M., Ed.; Unit Operations and Processing Equipment in the Food Industry; Woodhead Publishing: Cambridge, UK, 2023; pp. 227–291. [Google Scholar] [CrossRef]
- Fillion, L.; Henry, C.J.K. Nutrient losses and gains during frying: A review. Int. J. Food Sci. Nutr. 1998, 49, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Bordin, K.; Kunitake, M.T.; Aracava, K.K.; Trindade, C.S.F. Changes in food caused by deep fat frying—A review. Arch. Latinoam. Nutr. 2013, 63, 5–13. [Google Scholar]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Dangal, A.; Tahergorabi, R.; Acharya, D.R.; Timsina, P.; Rai, K.; Dahal, S.; Acharya, P.; Giuffrè, A.M. Review on deep-fat fried foods: Physical and chemical attributes, and consequences of high consumption. Eur. Food Res. Technol. 2024, 250, 1537–1550. [Google Scholar] [CrossRef]
- Mellema, M. Mechanism and reduction of fat uptake in deep-fat fried foods. Trends Food Sci. Technol. 2003, 14, 364–373. [Google Scholar] [CrossRef]
- Liberty, J.T.; Dehghannya, J.; Ngadi, M.O. Effective strategies for reduction of oil content in deep-fat fried foods: A review. Trends Food Sci. Technol. 2019, 92, 172–183. [Google Scholar] [CrossRef]
- Valle, C.; Echeverría, F.; Chávez, V.; Valenzuela, R.; Bustamante, A. Deep-frying impact on food and oil chemical composition: Strategies to reduce oil absorption in the final product. Food Saf. Health 2024, 2, 414–428. [Google Scholar] [CrossRef]
- Mallikarjunan, P.; Ngadi, M.O.; Chinnan, M.S. Breaded Fried Foods; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Achir, N.; Vitrac, O.; Trystram, G. Chapter 2—Heat and mass transfer during frying. In Advances in Deep-Fat Frying of Foods; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Saguy, I.S. Oil Uptake during deep-fat frying: Factors and mechanism. Food Technol. 1995, 49, 142–145. [Google Scholar]
- Dobarganes, C.; Marquez-Ruiz, G.; Velasco, J. Interactions between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Blumenthal, M.M.; Stier, R.F. Optimization of deep-fat frying operations. Trends Food Sci. Technol. 1991, 2, 144–148. [Google Scholar] [CrossRef]
- Lamberg, I.; Hallstroem, B.; Olsson, H. Fat uptake in a potato drying/frying process. Lebensm.-Wiss. Technol. 1990, 223, 295–300. [Google Scholar]
- Greenfield, H.; Makinson, J.; Wills, R.B.H. Lipids in french fries: A retail and laboratory study. Int. J. Food Sci. Technol. 1984, 19, 239–245. [Google Scholar] [CrossRef]
- Baumann, B.; Escher, F. Mass and heat transfer during deep-fat frying of potato slices—I. Rate of drying and oil uptake. LWT 1995, 28, 395–403. [Google Scholar] [CrossRef]
- Makinson, J.H.; Greenfield, H.; Wong, M.L.; Wills, R.B.H. Fat uptake during deep-fat frying of coated and uncoated foods. J. Food Comp. Anal. 1987, 1, 93–101. [Google Scholar] [CrossRef]
- Nieva-Echevarria, B.; Goicoechea, E.; Manzanos, M.J.; Guillen, M.D. The influence of frying technique, cooking oil, and fish species on the changes occurring in fish lipids and oil during shallow-frying, studied by 1H NMR. Food Res. Int. 2016, 84, 150–159. [Google Scholar] [CrossRef]
- Llorca, E.; Hernando, I.; Pérez-Munuera, I.; Quiles, A.; Fiszman, S.M.; Lluch, M.A. effect of batter formulation on lipid uptake during frying and lipid fraction of frozen battered squid. Eur. Food Res. Technol. 2003, 216, 297–302. [Google Scholar] [CrossRef]
- Wang, X.; McClements, D.J.; Xu, Z.; Meng, M.; Qiu, C.; Long, J.; Jin, Z.; Chen, L. Recent advances in the optimization of the sensory attributes of fried foods: Appearance, flavor, and texture. Trends Food Sci. Technol. 2023, 138, 297–309. [Google Scholar] [CrossRef]
- Kita, A.; Lisinska, G. The influence of oil type and frying temperatures on the texture and oil content of french fries. J. Sci. Food Agric. 2005, 85, 2600–2604. [Google Scholar] [CrossRef]
- Kita, A.; Lisinska, G.; Gołubowska, G. The effects of oils and frying temperatures on the texture and fat content of potato crisps. Food Chem. 2007, 102, 1–5. [Google Scholar] [CrossRef]
- Gillatt, P. Flavour and aroma development in frying and fried food. In Flavour in Food; Woodhead Publishing: Cambridge, UK, 2001; pp. 266–336. [Google Scholar] [CrossRef]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Thürer, A.; Granvogl, M. Generation of desired aroma-active as well as undesired toxicologically relevant compounds during deep-frying of potatoes with different edible vegetable fats and oils. J. Agric. Food Chem. 2016, 64, 9107–9115. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C.; Holgado, F.; Gonzalez-Mulero, L.; Morales, F.J. Effect of consumer’s decisions on acrylamide exposure during the preparation of french fries. Part 2: Color Analysis. Food Chem. Toxicol. 2021, 154, 112321. [Google Scholar] [CrossRef]
- Baixauli, R.; Salvador, A.; Fiszman, S.M.; Calvo, C. Effect of oil degradation during frying on the color of fried, battered squid rings. J. Am. Oil Chem. Soc. 2002, 79, 1127–1131. [Google Scholar] [CrossRef]
- Paul, S.; Mittal, G.S. Dynamics of fat/oil degradation during frying based on optical properties. J. Food Eng. 1996, 30, 389–403. [Google Scholar] [CrossRef]
- Dobarganes, C.; Márquez-Ruiz, G. Possible adverse effects of frying with vegetable oils. Br. J. Nutr. 2015, 113 (Suppl. S2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Sayon-Orea, C.; Carlos, S.; Martínez-Gonzalez, M.A. Does cooking with vegetable oils increase the risk of chronic diseases?: A systematic review. Br. J. Nutr. 2015, 113 (Suppl. S2), S36–S48. [Google Scholar] [CrossRef]
- Marquez-Ruiz, G.; Dobarganes, M.C. Chapter 9—Nutritional and physiological effects of used frying fats. In Deep Frying: Chemistry, Nutrition, and Practical Applications, 2nd ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 173–203. [Google Scholar]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.G.; Costa, H.S.; Oliveira, M.B.P.P. 4-Hydroxy-2-alkenals in foods: A review on risk assessment, analytical methods, formation, occurrence, mitigation and future challenges. Crit. Rev. Food Sci. Nutr. 2021, 62, 3569–3597. [Google Scholar] [CrossRef]
- Metayer, C.; Wang, Z.; Kleinerman, R.A.; Wang, L. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer 2002, 35, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, F.; Li, H.; Lin, Y.; Zhao, K.; Fang, L. The content and emission form of volatile organic compounds from cooking oils: A Gas Chromatography-Mass Spectrometry (GC-MS) Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1796. [Google Scholar] [CrossRef]

| Vegetable Oils | Olive | High-Oleic Sunflower | Rapeseed * | Peanut | Rice Bran | Sunflower | Maize (Corn) | Soybean | Cottonseed | Palm | Palm Kernel | Coconut | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acyl chains (FA) | |||||||||||||
| Monounsaturated fatty acyl chains (MUFA) | |||||||||||||
| Palmitoleic | 16:1ω7 | 0.3–3.5 | nd–0.1 | nd–0.6 | nd–0.2 | nd–0.5 | nd–0.3 | nd–0.5 | nd–0.2 | nd–1.2 | nd–0.6 | nd–0.2 | nd |
| Oleic | 18:1ω9 | 55.0–83.0 | 75.0–90.7 | 51.0–70.0 | 35.0–80.0 | 38.0–48.0 | 14.0–43.0 | 20.0–42.2 | 17.0–30.0 | 14.7–21.7 | 36.0–44.0 | 12.0–19.0 | 5.0–10.0 |
| Eicosenoic | 20:1ω9 | nd–0.4 | 0.1–0.5 | 0.1–4.3 | 0.7–3.2 | nd–0.8 | nd–0.3 | 0.2–0.6 | nd–0.5 | nd–0.1 | nd–0.4 | nd–0.2 | nd–0.2 |
| Erucic | 22:1ω9 | nd | nd–0.3 | nd–2.0 | nd–0.6 | nd | nd–0.3 | nd–0.3 | nd–0.3 | nd–0.3 | nd | nd | nd |
| Polyunsaturated fatty acyl chains (PUFA) | |||||||||||||
| Linoleic | 18:2ω6 | 3.5–21.0 | 2.1–17.0 | 15.0–30.0 | 4.0–43.0 | 21.0–42.0 | 45.4–74.0 | 34.0–65.6 | 48.0–59.0 | 46.7–58.2 | 9.0–12.0 | 1.0–3.5 | 1.0–2.5 |
| Linolenic | 18:3ω3 | nd–1.5 | nd–0.3 | 5.0–14.0 | nd–0.5 | 0.1–2.9 | nd–0.3 | nd–2.0 | 4.5–11.0 | nd–0.4 | nd–0.5 | nd–0.2 | nd–0.2 |
| Saturated fatty acyl chains (SFA) | |||||||||||||
| Caprylic | 8:0 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.4–6.2 | 4.6–10.0 |
| Capric | 10:0 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.6–5.0 | 5.0–8.0 |
| Lauric | 12:0 | nd | nd | nd | nd–0.1 | nd–0.2 | nd–0.1 | nd–0.3 | nd–0.1 | nd–0.2 | nd–0.5 | 45.0–55.0 | 45.1–53.2 |
| Myristic | 14:0 | nd–0.1 | nd–0.1 | nd–0.2 | nd–0.1 | nd–1.0 | nd–0.2 | nd–0.3 | nd–0.2 | 0.6–1.0 | 0.5–2.0 | 14.0–18.0 | 16.8–21.0 |
| Palmitic | 16:0 | 7.5–20.0 | 2.6–5.0 | 2.5–7.0 | 5.0–14.0 | 14.0–23.0 | 5.0–7.6 | 8.6–16.5 | 8.0–13.5 | 21.4–26.4 | 39.3–47.5 | 6.5–10.0 | 7.5–10.2 |
| Stearic | 18:0 | 0.5–5.0 | 2.9–6.2 | 0.8–3.0 | 1.0–4.5 | 0.9–4.0 | 2.7–6.5 | nd–3.3 | 2.0–5.4 | 2.1–3.3 | 3.5– 6.0 | 1.0–3.0 | 2.0–4.0 |
| Arachidic | 20:0 | nd–0.6 | 0.2–0.5 | 0.2–1.2 | 0.7–2.0 | nd–0.9 | 0.1–0.5 | 0.3–1.0 | 0.1–0.6 | 0.2–0.5 | nd–1.0 | nd–0.2 | nd–0.2 |
| Behenic | 22:0 | nd–0.2 | 0.5–1.6 | nd–0.6 | 1.5–4.5 | nd–1.0 | 0.3–1.5 | nd–0.5 | nd–0.7 | nd–0.6 | nd–0.2 | nd–0.2 | nd |
| Lignoceric | 24:0 | nd–0.2 | nd–0.5 | nd–0.3 | 0.5–2.5 | nd–0.6 | nd–0.5 | nd–0.5 | nd–0.5 | nd–0.1 | nd | nd | nd |
| Minor components (mg/kg) | |||||||||||||
| Total sterols | 1000–2000 | 1700–5200 | 4500–11,300 | 900–2900 | 10,500–31,000 | 2400–5000 | 7000–22,100 | 1800–4500 | 2700–6400 | 300–700 | 700–1400 | 400–1200 | |
| Total tocopherols and tocotrienols | 55–320 | 450–1120 | 430–2680 | 170–1300 | 191–2349 | 440–1520 | 330–3720 | 600–3370 | 380–1200 | 150–1500 | nd–260 | nd–50 | |
| Oil | Fried Food | Frying Conditions | Parameters or Compounds Studied in Oils | Methodology | Ref. |
|---|---|---|---|---|---|
| Cottonseed, sunflower, palm, shortening, virgin olive (2 L) | Potato (400 g) | 170 °C, 8–9 min, 8 cycles | Polymerized TG Oxidized FA (epoxystearates, epoxyoleates, ketostearates) | HPSEC FAME and GC/MS | [76] |
| Refined canola (3.75 L) | Frozen par-fried French fries (200 g) | 185 °C, 215 °C, 5 min, 56 cycles | TPC (DG, oxidized TG, dimers and polymers) FA composition, trans FA AnV Oil color | Gravimetric method HPSEC FAME and GC/MS UV-Vis UV-Vis | [67] |
| EVOO, peanut, canola (1.5 L) | French fries (50 g) | 175 °C, 6 min, 16 cycles | TPC (dimeric, polymeric and oxidized monomeric TG) Volatiles (aldehydes, hydrocarbons, ketones, alcohols, carboxylic acids, furans) AnV | Dielectric constant, HPSEC HS-SPME-GC/MS UV-Vis | [68] |
| High-oleic sunflower (3.3 kg) | Potato chips (200 g) | 175 °C, 3 min, 40 cycles | Total oxylipin concentrations (FA with hydroperoxy, hydroxy, epoxy, dihydroxy groups) | LC-MS | [77] |
| EVOO, sunflower, virgin linseed (4 L) | None | 190 °C, 8 h/d, 5 days | FA composition, IV and degradation compounds (aldehydes, epoxides, MG, DG) TPC | 1H NMR Dielectric constant | [78,79,80] |
| EVOO, soybean, sunflower (4 L) | Doughnuts (40 g), pork adipose tissue (250 g), salmon (250 g) | 190 °C, 1 min, 8 h/d for 4 days | FA composition and degradation compounds (aldehydes, epoxides, alcohols, MG, DG) | 1H NMR | [4] |
| EVOO, refined sunflower, virgin linseed (4 L) | None | 190 °C, 20 h (8 h/d) | Aldehydes (alkanals, alkenals, alkadienals, alkatrienals, oxygenated saturated and α,β-unsaturated aldehydes) TPC | HS-SPME-GC/MS Dielectric constant | [72] |
| Sunflower, high-oleic sunflower, rapeseed, high-oleic rapeseed, palm olein (1.5 L) | French fries (175 g) | 170 °C, 4 min; 36 h, 12 cycles | Volatiles (alkanals, 2-alkenals, 2,4-alkadienals, alcohols, ketones) 4-hydroxy-2-(E)-nonenal TPC PV AnV Polymerized TG | HS-SPME-GC DHS-GC/MS DGF C-III 3e DGF C-VI 6a DGF C-VI 6e Gel Permeation Chromatography | [81] |
| Palm, rapeseed, sunflower, soybean (0.6 L) | French fries (160 g), pork loin strips (160 g) | 173–182 °C, 10 min | Volatile aldehydes in cooking oil fumes (alkanals, 2-alkenals, 2,4-alkadienals) | HPLC-UV | [82] |
| Coconut, soybean, olive, vegetable shortening (4 L) | Potato chips | 180 °C, 4 min; 80 cycles | FA composition Tocopherols Free radical scavenging activity Volatiles (alkanals, 2-alkenals, 2,4-alkadienals) Color AV AnV CD (234 nm) TPC (490 nm) | FAME and GC/FID HPLC/FD DPPH method HS-SPME-GC/MS Colorimeter Titration UV-Vis UV-Vis UV-Vis | [83] |
| Olive pomace, and blended with coconut (2.7 L) | French fries (200 g) | 180 °C, 9 min, 60 cycles | FA composition IV Sterols TPC, polymeric TG, oxidized monomeric TG, AnV, AV, color, trans FA Oxidative stability | FAME and GC/FID FT-NIR according to AOCS Cd 1e-01 Thin-layer chromatography FT-NIR according to DGF C-VI 21 Rancimat | [84] |
| Olive pomace, and blended with coconut (2.7 L) | French fries (200 g) | 180 °C, 9 min, 60 cycles | FA composition Tocopherols TPC TG dimers, oligomers, oxidized TG monomers, DG, MG, free FA | FAME and GC/FID GC/FID Dielectric constant, HPSEC HPSEC | [85] |
| Palm, peanut, camellia (2 L) | Potatoes (80 g) | 170 °C, 3 min, 75 cycles | FA composition Tocopherols AV IV PV AnV | FAME and GC/FID HPLC-FD Titration Titration Titration UV-Vis | [86] |
| EVOO, virgin olive, olive, sunflower (1.5 L) | Potato chips (300 g) | 170 °C, hourly, 9 h/d | FA composition Tocopherols and tocotrienols Beta-carotene Total phenols TPC FFA and PV AnV K232 and K270 Oxidative Stability | FAME and GC/FID HPLC-FD UV-Vis (454 nm) Folin–Ciocalteu Dielectric constant Titration UV-Vis UV Rancimat | [87] |
| Component | Main Changes Caused by Frying |
|---|---|
| Water |
|
| Lipids |
|
| Carbohydrates |
|
| Proteins |
|
| Vitamins |
|
| Minerals |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. https://doi.org/10.3390/foods13244186
Abrante-Pascual S, Nieva-Echevarría B, Goicoechea-Oses E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods. 2024; 13(24):4186. https://doi.org/10.3390/foods13244186
Chicago/Turabian StyleAbrante-Pascual, Susana, Barbara Nieva-Echevarría, and Encarnacion Goicoechea-Oses. 2024. "Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation" Foods 13, no. 24: 4186. https://doi.org/10.3390/foods13244186
APA StyleAbrante-Pascual, S., Nieva-Echevarría, B., & Goicoechea-Oses, E. (2024). Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods, 13(24), 4186. https://doi.org/10.3390/foods13244186






