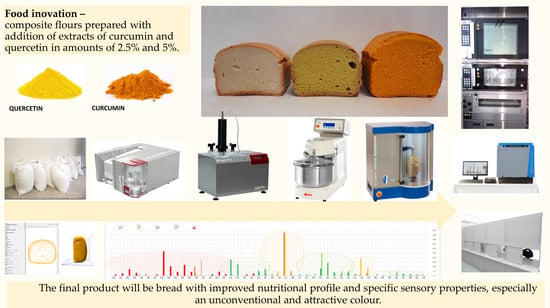
Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Used
2.2. Characterisation of the Antioxidant Ability of Extracts
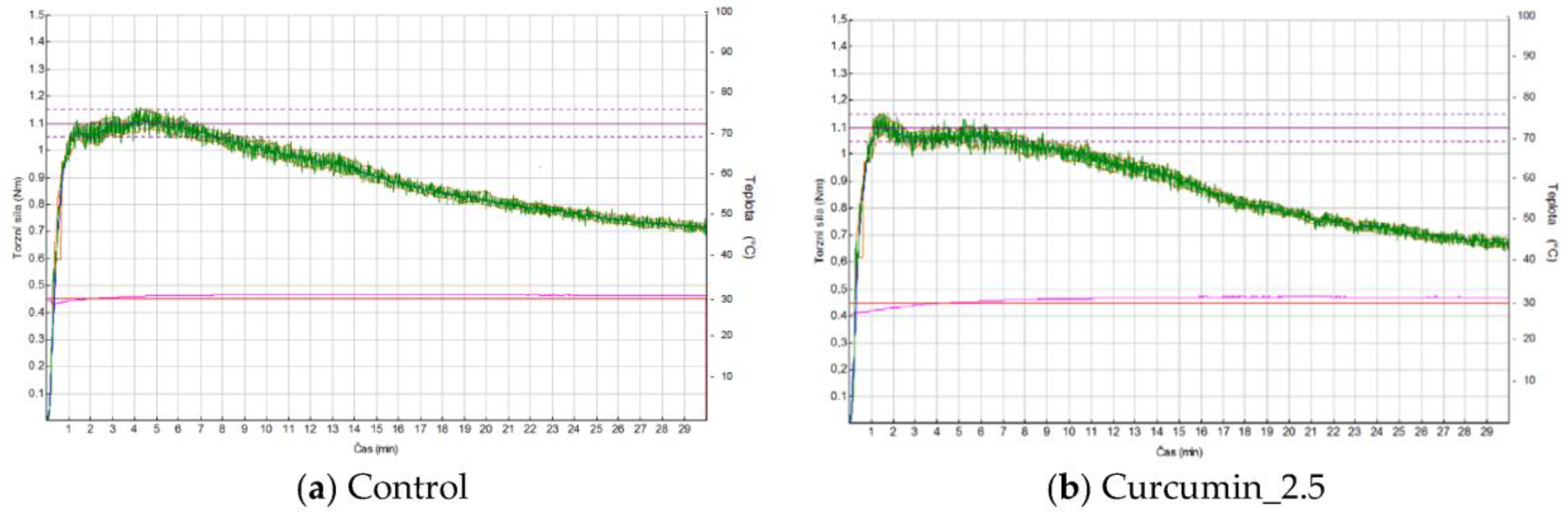
2.3. Mixolab Measurements
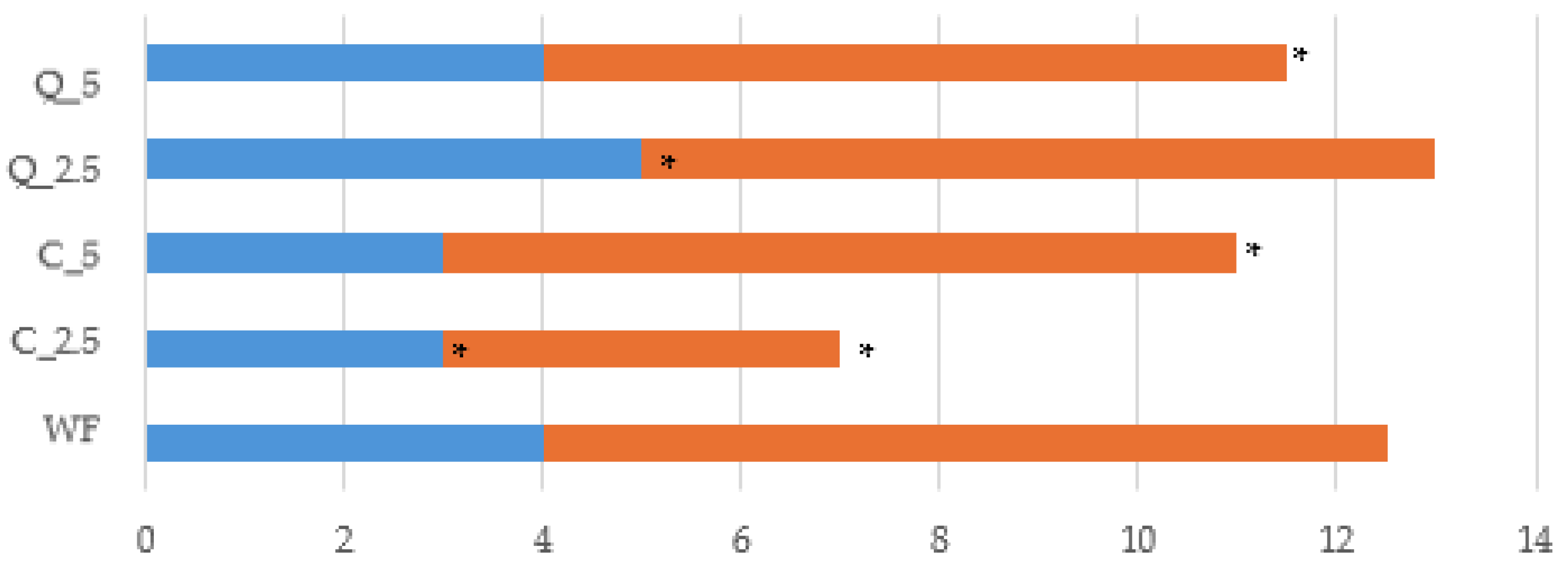
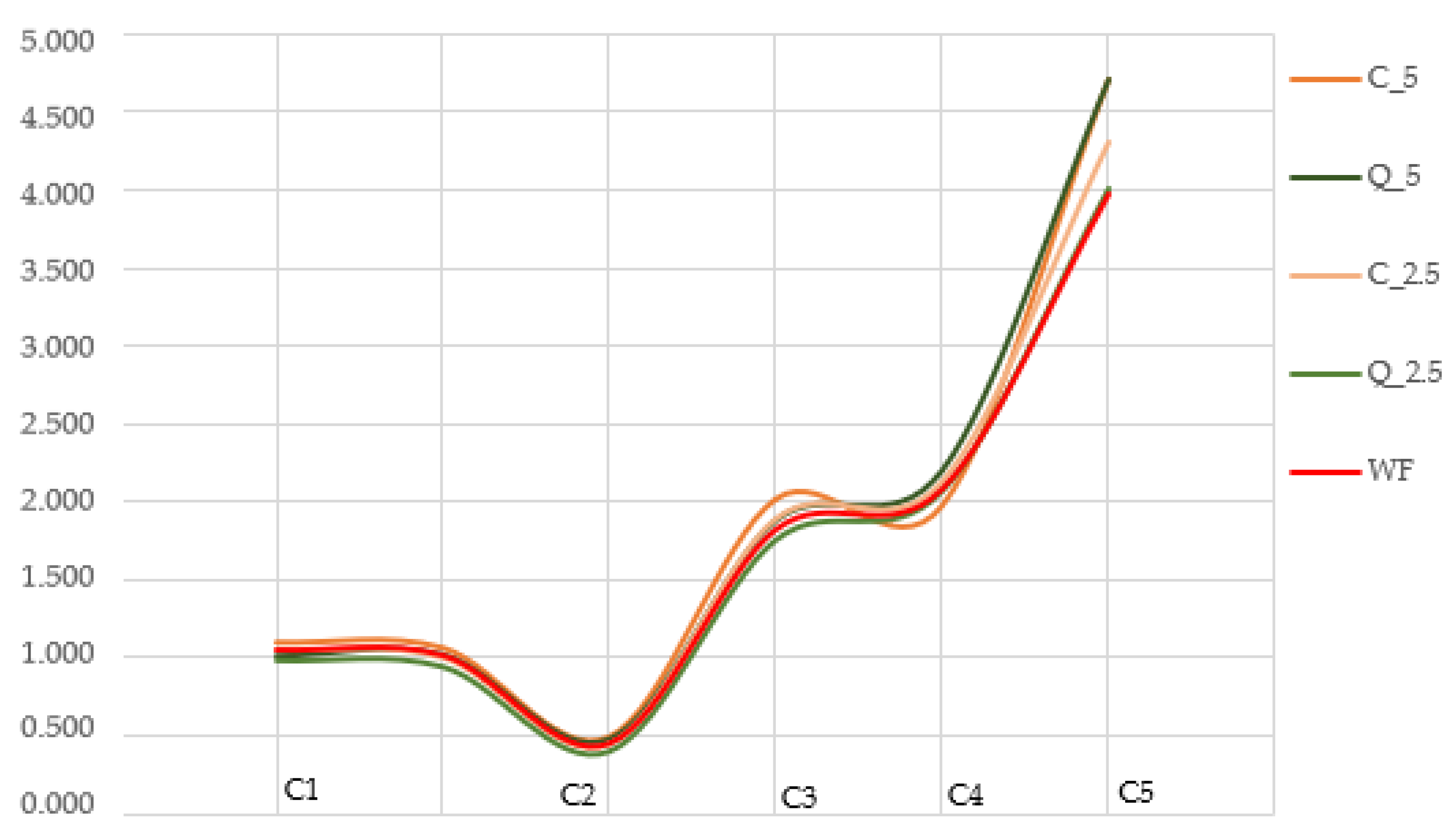
- C1 (used to determine water absorption);
- C2 (measures protein weakening as a function of mechanical work and temperature);
- C3 (measures starch gelatinisation);
- C4 (measures high-temperature gel stability);
- C5 (measures starch retrogradation in the cooling phase);
- slope α (slope of curve between end of period at 30 °C and C2);
- slope β (slope of curve between C2 and C3);
- slope λ (slope of curve between C3 and C4).
2.4. Rheofermentometer Analysis
2.5. Breadmaking Procedure
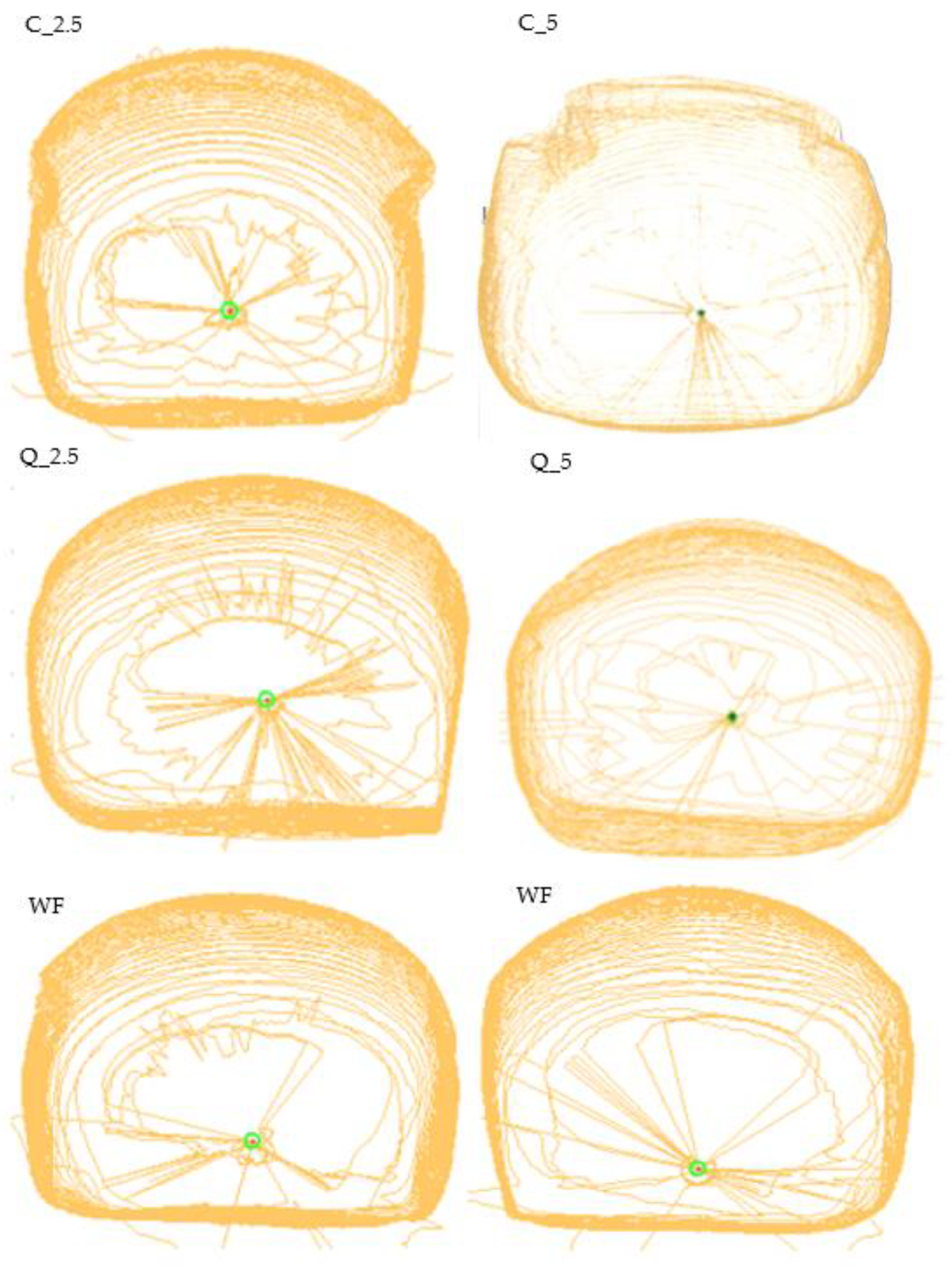
2.6. Physical Characteristics of Bread
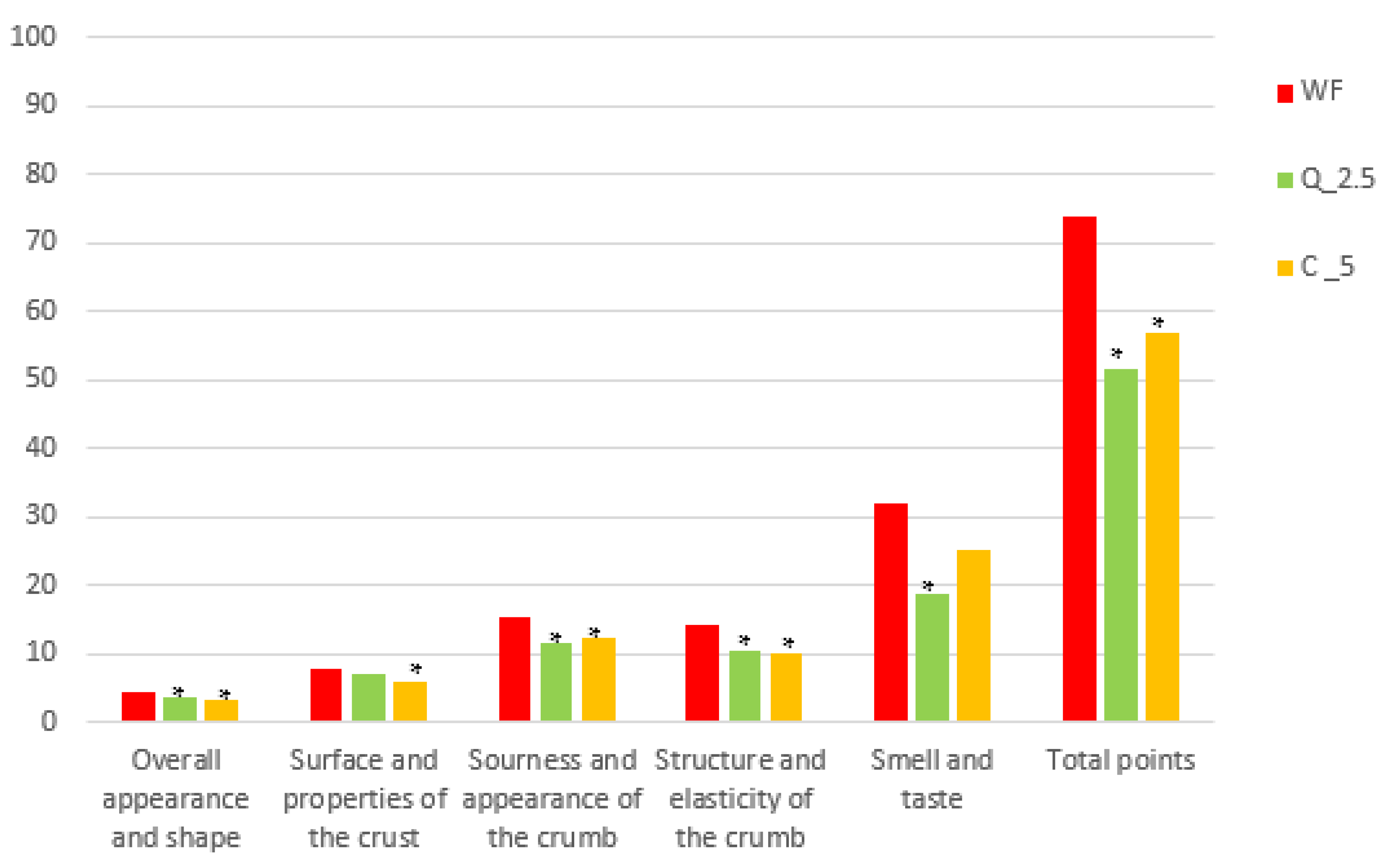
2.7. Sensory Evaluation
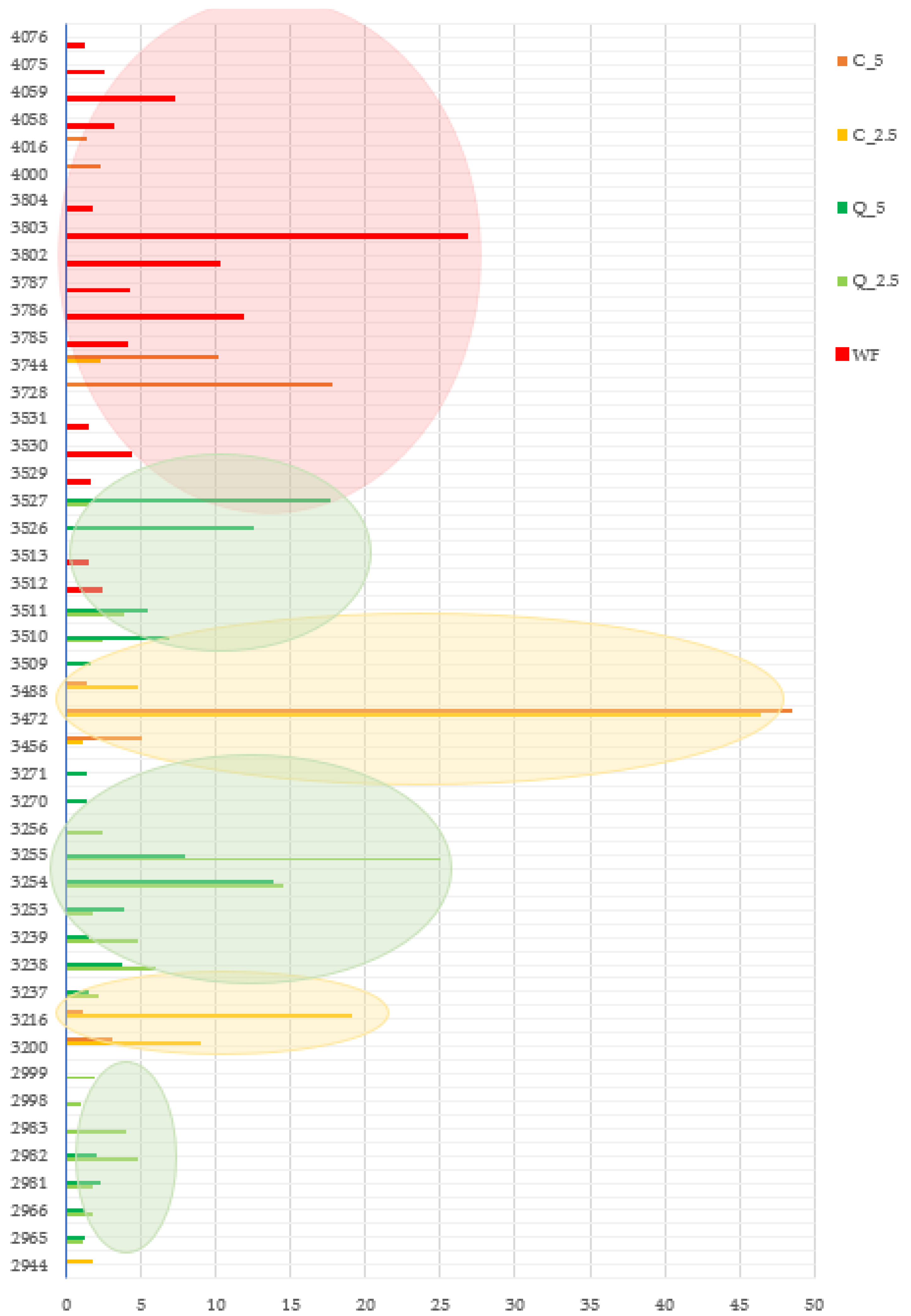
2.8. Presence of Microscopic Fungi
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Applied Extracts
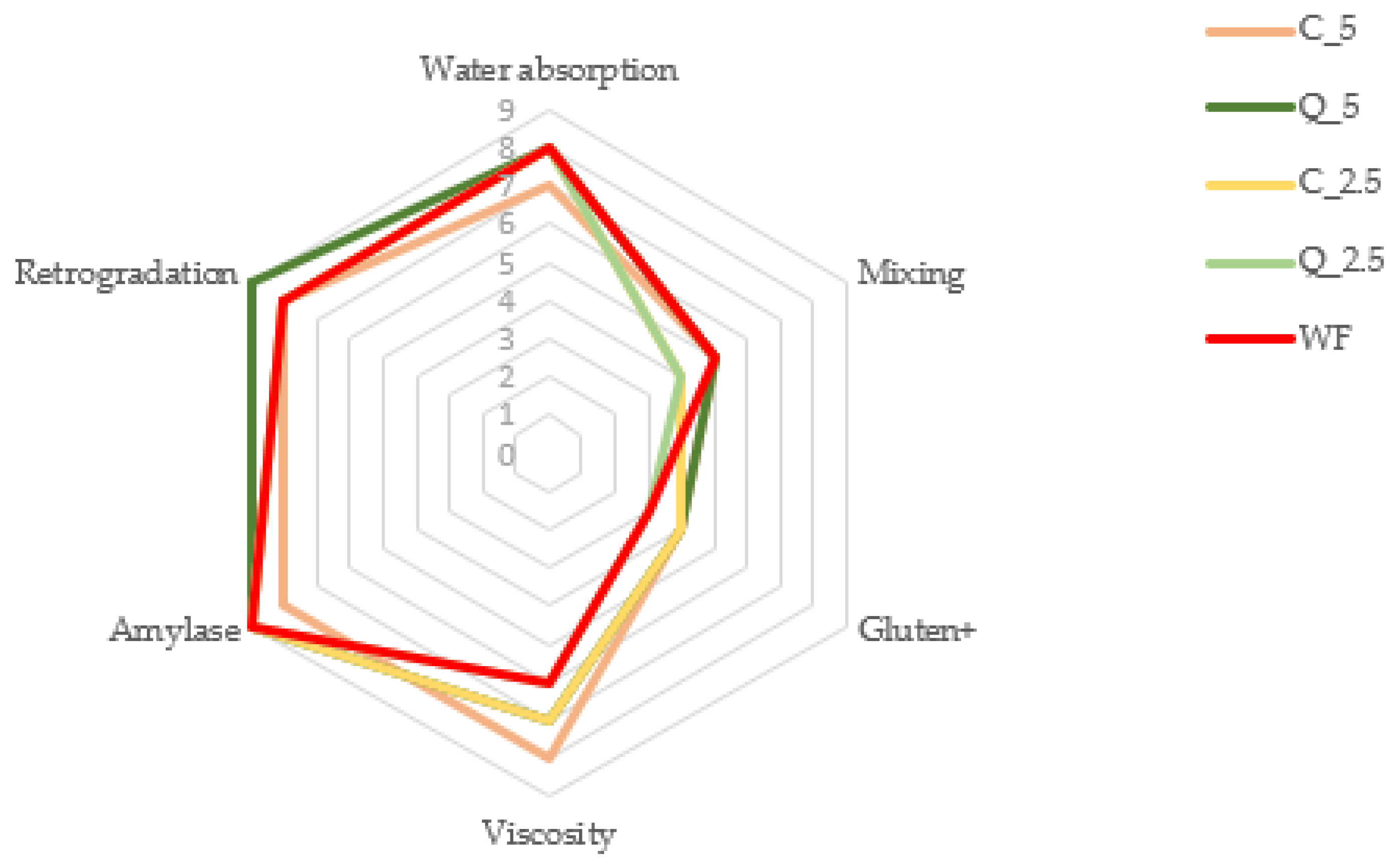
3.2. Rheological Properties of Composite Flours
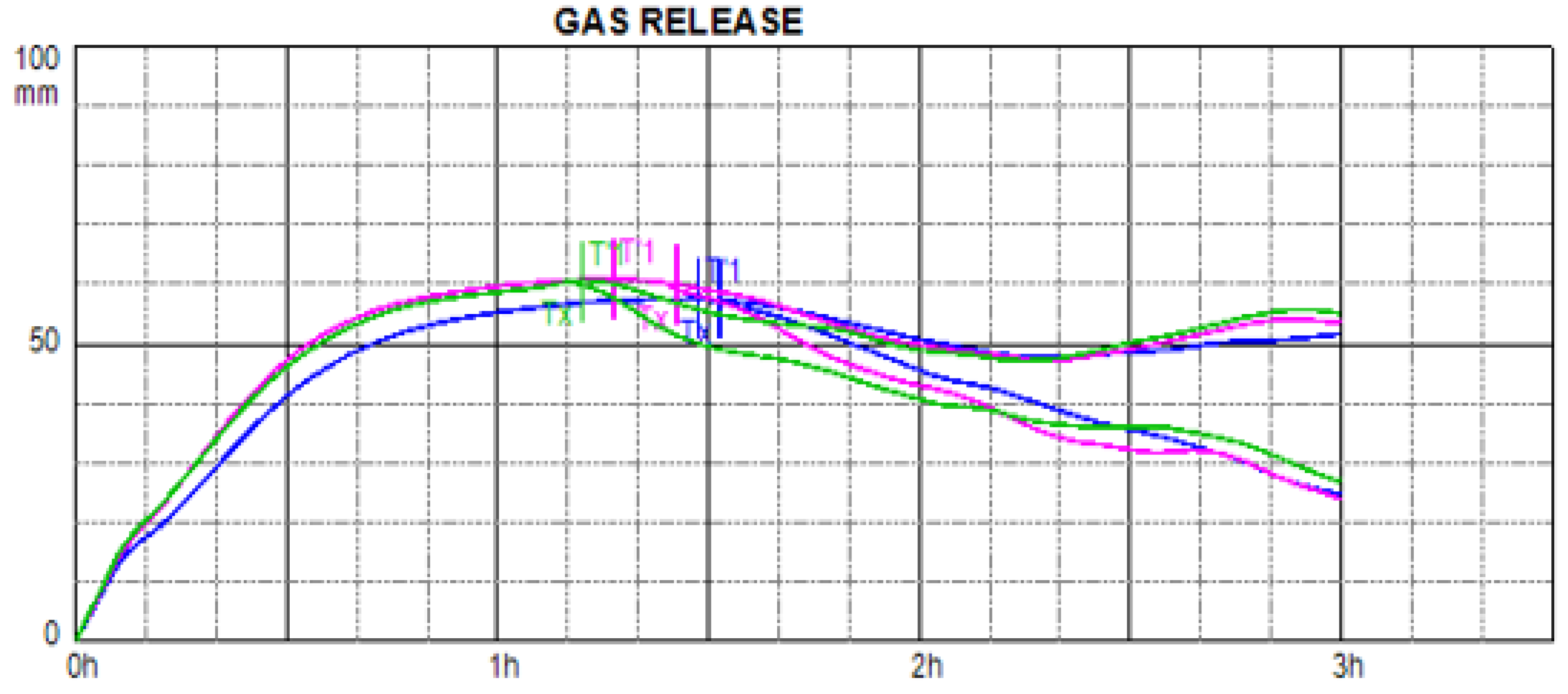
3.3. Ability of Experimental Doughs to Form and Conserve Fermentation Gases
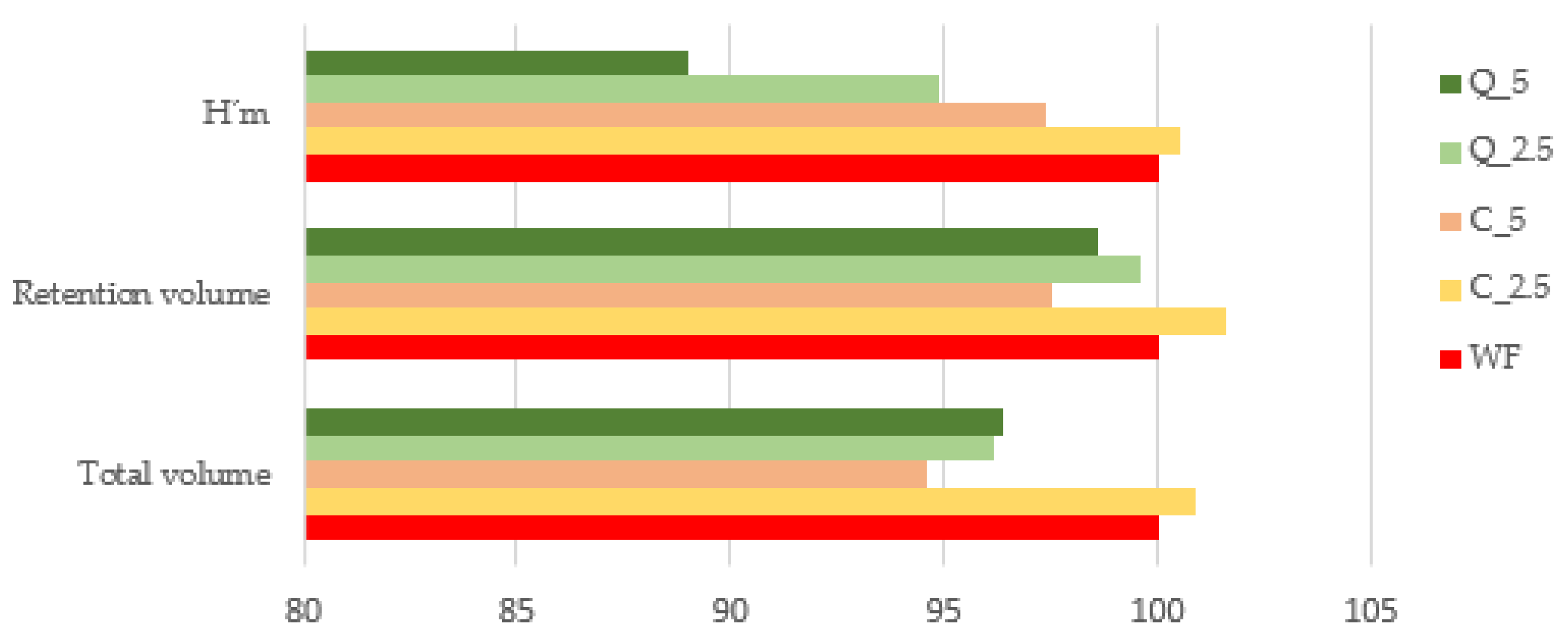
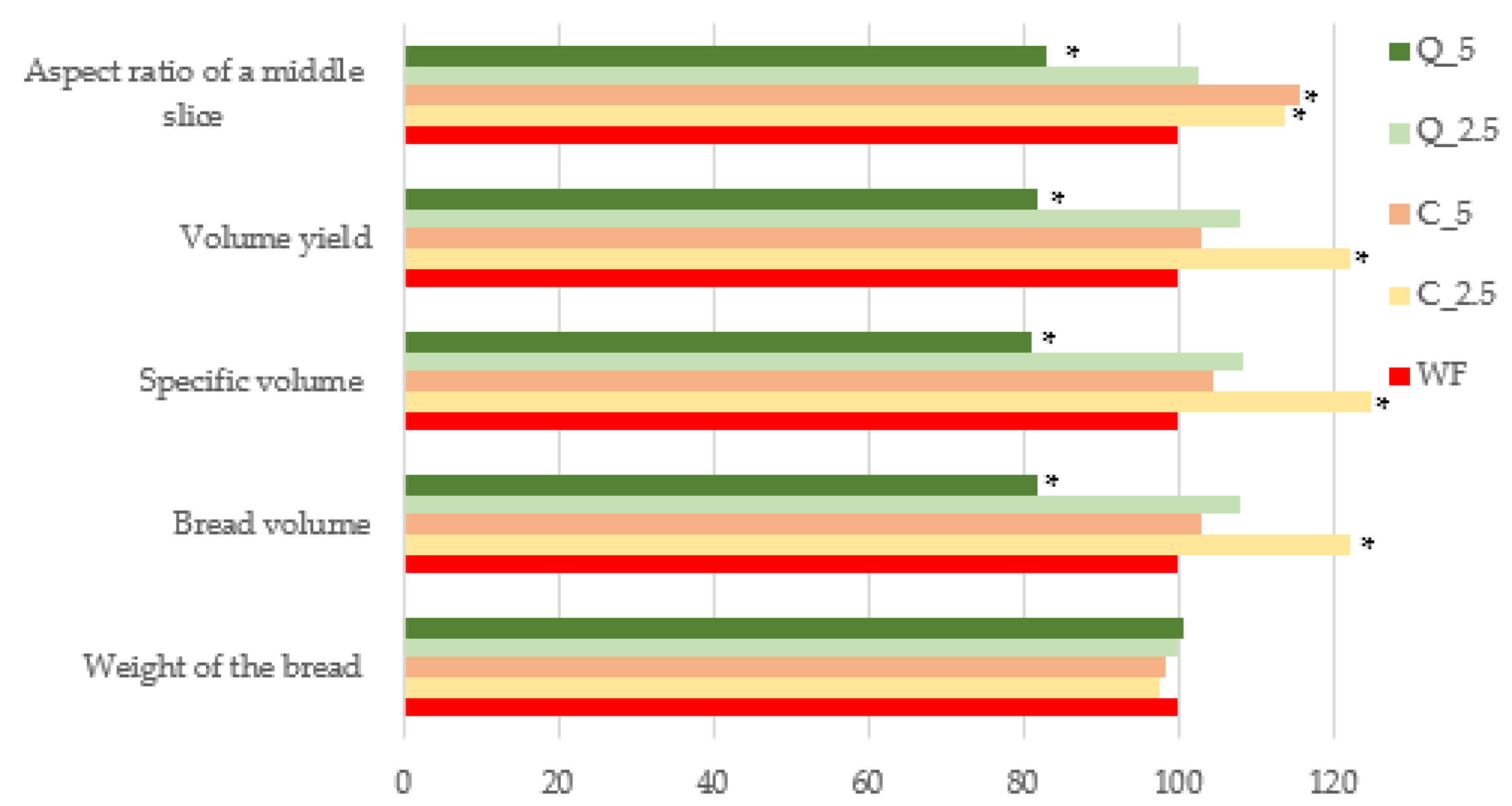
3.4. Bread Evaluation
Presence of Microscopic Fungi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DATACube, Statistical Office of Slovak Republic. 2023. Available online: https://datacube.statistics.sk/ (accessed on 10 December 2023).
- Bender, D.; Schönlechner, R. Innovative Approaches towards Improved Gluten-Free Bread Properties. J. Cereal Sci. 2020, 91, 102904. [Google Scholar] [CrossRef]
- Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Popa, M.E. Trends of Innovation in Bread and Bakery Production. In Trends in Wheat and Bread Making; Academic Press: Cambridge, MA, USA, 2021; pp. 199–226. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Monteiro, M.; Nunes, R.; Baião, A.; Braga, S.S.; Sarmento, B.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Bread Enriched with Resveratrol: Influence of the Delivery Vehicles on Its Bioactivity. Food Biosci. 2022, 49, 101887. [Google Scholar] [CrossRef]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current Trends in the Enhancement of Antioxidant Activity of Wheat Bread by the Addition of Plant Materials Rich in Phenolic Compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Mushtaq, B.S. Characterization of moringa oleifera leaves and its utilization as value added ingredient in unleavened flat bread (Chapatti). J. Microbiol. Biotechnol. Food Sci. 2018, 8, 751–755. [Google Scholar] [CrossRef]
- Ak, B.; Avşaroğlu, E.; Isik, O.; Ozyurt, G.; Kafkas, E.; Etyemez, M.; Uslu, L. Nutritional and Physicochemical Characteristics of Bread Enriched with Microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 30–38. [Google Scholar]
- Souza, S.V.S.; Borges, N.; Vieira, E.F. Vitamin D-Fortified Bread: Systematic Review of Fortification Approaches and Clinical Studies. Food Chem. 2022, 372, 131325. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Gouvea, S.E.; Barroso, S.; Teixeira, P.; Mendes, S.; Gil, M.M. Development and Optimization of High-Protein and Low-Saturated Fat Bread Formulations Enriched with Lupin and Microalgae. LWT 2023, 191, 115612. [Google Scholar] [CrossRef]
- Shuvonkar, K.B.; Sumita, T.T.; Tapsia, A.H.; Haque, N.; Islam, S.; Faruque, M.F. Formulation and Evaluation of Cereal-Based Breads Fortified with Natural Prebiotics from Green Banana, Moringa Leaves Powder and Soya Powder. Appl. Food Res. 2023, 4, 100377. [Google Scholar] [CrossRef]
- Amjad, A.; Sohaib, M.; Nawaz, H.; Javed, M.S.; Shah, M.; Shah, F.-U.-H.; Tariq, M.R.; Sajid, M.W.; Khan, A.A.; Bilal, M.; et al. Assessment of Rheological and Quality Characteristics of Bread Made by the Addition of Ginger Powder in Wheat Flour. Food Sci. Technol. 2021, 42, e47820. [Google Scholar] [CrossRef]
- Liu, W.; Brennan, M.A.; Tu, D.; Brennan, C.S.; Huang, W. Effect of Enzyme Compositions on the Rheological Properties of Bread Dough Enriched in Buckwheat Flour. Food Sci. Technol. 2023, 43, e114322. [Google Scholar] [CrossRef]
- Koksel, F.; Scanlon, M.G. Effects of Composition on Dough Development and Air Entrainment in Doughs Made from Gluten-Starch Blends. J. Cereal Sci. 2012, 56, 445–450. [Google Scholar] [CrossRef]
- Barros, J.H.; Franco, L. Changes in Rheology, Quality, and Staling of White Breads Enriched with Medium-Polymerized Inulin. Food Sci. Technol. Int. 2021, 28, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu-Iuga, M.; Atudorei, D.; Codină, G.G.; Mironeasa, S. Rheological Approaches of Wheat Flour Dough Enriched with Germinated Soybean and Lentil. Appl. Sci. 2021, 11, 11706. [Google Scholar] [CrossRef]
- Šmídová, Z.; Rysová, J. Gluten-Free Bread and Bakery Products Technology. Foods 2022, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Otegbayo, B.; Adebiyi, O.M.; Bolaji, O.; Olunlade, B. Effect of soy enrichment on bread quality. Int. Food Res. J. 2018, 25, 1120–1125. Available online: http://www.ifrj.upm.edu.my/25%20(03)%202018/(32).pdf (accessed on 5 December 2023).
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The Role of Agave Fructans in Health and Food Applications: A Review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Zhu, M.; Gao, L.; Ni, C.; Cao, W. Spectroscopy Characterization, Theoretical Study and Antioxidant Activities of the Flavonoids-Pb(II) Complexes. J. Mol. Struct. 2020, 1209, 127919. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelínek, L.; Dostálek, P. The Importance of Hop Prenylflavonids for Human Health. Chem. Listy 2012, 106, 1095–1103. Available online: http://www.chemicke-listy.cz/ojs3/index.php/chemicke-listy/article/view/798 (accessed on 2 December 2023).
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and Biological Activities of Hop Flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef]
- Vollmannová, A.; Musilová, J.; Urminská, D.; Bajčan, D.; Bobková, A.; Bojňanská, T.; Lidiková, J.; Čanigová, M.; Kročko, M.; Mašková, Z.; et al. Chémia Potravín, 2nd ed.; Slovak University of Agriculture: Nitra, Slovakia, 2022; 543p, ISBN 978-80-552-2504-3. [Google Scholar]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, K.M.; Priya, V.V.; Mallika, J. Pioglitazone, quercetin and hydroxy citric acid effect on cytochrome P450 2E1 (CYP2E1) enzyme levels in experimentally induced non alcoholic steatohepatitis (NASH). Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2736–2741. [Google Scholar] [PubMed]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R.M.M. The Potential of Flavonoids in the Treatment of Non-Alcoholic Fatty Liver Disease. Crit. Rev. Food Sci. Nutr. 2015, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The Correlation between Active Oxygens Scavenging and Antioxidative Effects of Flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Gao, L. Mechanism of Antioxidant Properties of Quercetin and Quercetin-DNA Complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative Damage and Protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Guan, X.; Gao, M.; Xu, H.; Zhang, C.; Liu, H.; Lv, L.; Deng, S.; Gao, D.; Tian, Y. Quercetin-Loaded Poly (Lactic-Co-Glycolic Acid)-d-α-Tocopheryl Polyethylene Glycol 1000 Succinate Nanoparticles for the Targeted Treatment of Liver Cancer. Drug Deliv. 2016, 23, 3307–3318. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Choi, K.-C.; Son, J. Quercetin Sensitizes Pancreatic Cancer Cells to TRAIL-Induced Apoptosis through JNK-Mediated CFLIP Turnover. Int. J. Biochem. Cell Biol. 2016, 78, 327–334. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, D.; Zheng, Z.-P.; Li, E.T.S.; Chen, F.; Cheng, K.-W.; Wang, M. 8-C-(E-Phenylethenyl) Quercetin from Onion/Beef Soup Induces Autophagic Cell Death in Colon Cancer Cells through ERK Activation. Mol. Nutr. Food Res. 2017, 61, 1600437. [Google Scholar] [CrossRef]
- Lei, C.-S.; Hou, Y.-C.; Pai, M.-H.; Lin, M.-T.; Yeh, S.-L. Effects of Quercetin Combined with Anticancer Drugs on Metastasis-Associated Factors of Gastric Cancer Cells: In Vitro and in Vivo Studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin Suppresses Proliferation of Liver Cancer Cell Lines in Vitro. Anticancer Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and Antimetastatic Action of Quercetin on A549 Non-Small Cell Lung Cancer Cells through Its Effect on the Cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Divya, S.P.; Wang, L.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Kim, D.; Dai, J.; Asha, P.; et al. Quercetin Inhibits Cr(VI)-Induced Malignant Cell Transformation by Targeting MiR-21-PDCD4 Signaling Pathway. Oncotarget 2016, 8, 52118–52131. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; TanTai, J. Co-Delivery Anticancer Drug Nanoparticles for Synergistic Therapy against Lung Cancer Cells. Drug Des. Dev. Ther. 2020, 14, 4503–4510. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin Induces Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells through Modulation of Foxo3a Activity. Korean J. Physiol. Pharmacol. 2017, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Mahmoud, M.E. Dual Effects of Quercetin in Doxorubicin-Induced Nephrotoxicity in Rats and Its Modulation of the Cytotoxic Activity of Doxorubicin on Human Carcinoma Cells. Environ. Toxicol. 2014, 31, 507–636. [Google Scholar] [CrossRef]
- Abu Ayana, M.; Elmasry, N.A.; Shehata, F.I.; Khalil, N.M. Efficacy of quercetin on alveolar bone structure of rats with induced diabetes. Alex. Dent. J. 2017, 42, 141–146. [Google Scholar] [CrossRef]
- Singh, J.; Mittal, P.; Vasant Bonde, G.; Ajmal, G.; Mishra, B. Design, Optimization, Characterization and In-Vivo Evaluation of Quercetin Enveloped Soluplus®/P407 Micelles in Diabetes Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S546–S555. [Google Scholar] [CrossRef]
- Shaikhomar, A.O.; Bahattab, O.S. Physiological Effect of Quercetin as a Natural Flavonoid to Be Used as Hypoglycemic Agent in Diabetes Mellitus Type II Rats. Saudi J. Biomed. Res. 2021, 6, 10–17. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and Rutin as Potential Agents Antifungal against Cryptococcus spp. Braz. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, S.; Jayachitra, A. Targeting Biofilm Inhibition Using Quercetin—Interaction with Bacterial Cell Membrane and ROS Mediated Biofilm Control. Funct. Foods Health Dis. 2018, 8, 292. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.d.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.d.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal Effects of the Flavonoids Kaempferol and Quercetin: A Possible Alternative for the Control of Fungal Biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-C.; Zhou, Y.-Q.; Ren, D.; Wang, R.; Wang, C.; Lin, L.-G.; Zhang, X.-Q.; Ye, W.-C.; Zhang, Q.-W. Turmeric: A Review of Its Chemical Composition, Quality Control, Bioactivity, and Pharmaceutical Application. In Natural and Artificial Flavoring Agents and Food Dyes; Academic Press: Cambridge, MA, USA, 2018; pp. 299–350. [Google Scholar] [CrossRef]
- Thangavel, K.; Dhivya, K. Determination of Curcumin, Starch and Moisture Content in Turmeric by Fourier Transform near Infrared Spectroscopy (FT-NIR). Eng. Agric. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I Dose Escalation Trial of Docetaxel plus Curcumin in Patients with Advanced and Metastatic Breast Cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined Inhibitory Effects of Soy Isoflavones and Curcumin on the Production of Prostate-Specific Antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef]
- Kim, S.G.; Veena, M.S.; Basak, S.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin Treatment Suppresses IKKβ Kinase Activity of Salivary Cells of Patients with Head and Neck Cancer: A Pilot Study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabrício, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome Complex of Curcumin as Complementary Therapy of Advanced Pancreatic Cancer Improves Safety and Efficacy of Gemcitabine: Results of a Prospective Phase II Trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Yao, Z.; Le, T.H.; Du, Q.; Mu, H.; Liu, C.; Zhu, Y. The Potential Clinical Value of Curcumin and Its Derivatives in Colorectal Cancer. Anti-Cancer Agents Med. Chem. 2021, 21, 1626–1637. [Google Scholar] [CrossRef]
- Lambring, C.; Varga, K.; Livingston, K.; Lorusso, N.; Dudhia, A.; Basha, R. Therapeutic Applications of Curcumin and Derivatives in Colorectal Cancer. Onco Ther. 2022, 9, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin Nanoparticles Potentiate Therapeutic Effectiveness of Acitrein in Moderate-To-Severe Psoriasis Patients and Control Serum Cholesterol Levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The Impact of Curcumin and Its Modified Formulations on Alzheimer’s Disease. J. Cell. Physiol. 2019, 234, 16655–19103. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, E.; Giuffrè, C.; Cicinelli, M.V.; Marchese, A.; Gattinara, M.; Modorati, G.; Bandello, F. Oral Phospholipidic Curcumin in Juvenile Idiopathic Arthritis-Associated Uveitis. Eur. J. Ophthalmol. 2019, 30, 112067211989280. [Google Scholar] [CrossRef] [PubMed]
- de Alvares Goulart, R.; Barbalho, S.M.; Lima, V.M.; de Souza, G.A.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; de Carvalho, A.C.A.; et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2021, 24, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story so Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Shojaei-Zarghani, S.; Rafraf, M. Curcumin and Rheumatoid Arthritis: A Systematic Review of Literature. Int. J. Clin. Pract. 2021, 75, e14280. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Barreto, G.E.; Bland, A.R.; Sahebkar, A. Curcumin: A Small Molecule with Big Functionality against Amyloid Aggregation in Neurodegenerative Diseases and Type 2 Diabetes. Biofactors 2021, 47, 570–586. [Google Scholar] [CrossRef]
- Yang, J.; Miao, X.; Yang, F.-J.; Cao, J.-F.; Liu, X.; Fu, J.-L.; Su, G.-F. Therapeutic Potential of Curcumin in Diabetic Retinopathy (Review). Int. J. Mol. Med. 2021, 47, 75. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hosnedl, V.; Pivec, V.; Orsák, M. Polyphenols in cereals and their positive and negative role in human and animal nutrition. In Proceedings of the Conference Cereals for Human Health and Preventive Nutrition, Brno, Czech Republic, 7–11 July 1998. [Google Scholar]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- ICC. Standard Methods of the International Association for Cereal Chemistry 173; International Association for Cereal Science and Technology: Vienna, Austria, 2010. [Google Scholar]
- AACC. Approved Methods of Analysis, 11th Edition—AACC Method 89-01.01. Cereals & Grains Association. Yeast Activity, Gas Production. 2000. Available online: https://methods.aaccnet.org/methods/89-01.pdf (accessed on 28 November 2023).
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019; 481p, ISBN 978-94-91751-18-9. [Google Scholar]
- Lumivero. XLSTAT Statistical and Data Analysis Solution. New York, USA. 2023. Available online: https://www.xlstat.com/en (accessed on 30 November 2023).
- Microsoft Corporation. Microsoft Excel 365. Redmond, WA, USA. 2018. Available online: https://office.microsoft.com/excel (accessed on 30 November 2023).
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.; Al-Humaid, L.; Aldawsari, M.; Abid, I.F.; Jhanani, G.K.; Shanmuganathan, R. Quercetin Extraction from Small Onion Skin (Allium cepa L. Var. Aggregatum Don.) and Its Antioxidant Activity. Environ. Res. 2023, 224, 115497. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Mahernia, S.; Amanlou, M. Comparison of Different Methods in Quercetin Extraction from Leaves of Raphanus sativus L. Pharm. Sci. 2017, 23, 59–65. [Google Scholar] [CrossRef]
- Guzmán, C.; Posadas-Romano, G.; Hernández-Espinosa, N.; Morales-Dorantes, A.; Peña, R.J. A new standard water absorption criteria based on solvent retention capacity (SRC) to determine dough mixing properties, viscoelasticity, and bread-making quality. J. Cereal Sci. 2015, 66, 59–65. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of Quercetin in the Physicochemical Properties, Antioxidant and Antiglycation Activities of Bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Miś, A.; Grundas, S.; Dziki, D.; Laskowski, J. Use of Farinograph Measurements for Predicting Extensograph Traits of Bread Dough Enriched with Carob Fibre and Oat Wholemeal. J. Food Eng. 2012, 108, 1–12. [Google Scholar] [CrossRef]
- Park, S.; Lim, H.; Hwang, S. Evaluation of Antioxidant, Rheological, Physical and Sensorial Properties of Wheat Flour Dough and Cake Containing Turmeric Powder. Food Sci. Technol. Int. 2012, 18, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hadnađev, T.D.; Torbica, A.; Hadnađev, M. Rheological properties of wheat flour substitutes/alternative crops assessed by Mixolab. Procedia Food Sci. 2011, 1, 328–334. [Google Scholar] [CrossRef]
- Dubat, A.; Rosell, C.; Gallagher, E. Mixolab: A New Approach to Rheology; AACC International: St. Paul, MN, USA, 2013; ISBN 978-1-891127-77-9. [Google Scholar]
- Barrera, G.N.; Bustos, M.C.; Iturriaga, L.; Flores, S.K.; León, A.E.; Ribotta, P.D. Effect of damaged starch on the rheological properties of wheat starch suspensions. J. Food Eng. 2013, 116, 233–239. [Google Scholar] [CrossRef]
- Bojňanská, T.; Vollmannová, A.; Musilová, J. Milk thistle flour effect on dough rheological properties. Potravin. Slovak J. Food Sci. 2020, 14, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Chenlo, F.; Torres, M.; Prieto, D. Influence of the particle size on the rheological behaviour of chestnut flour doughs. J. Food Eng. 2010, 100, 270–277. [Google Scholar] [CrossRef]
- Rosell, C.M.; Altamirano-Fortoul, R.; Don, C.; Dubat, A. Thermomechanically Induced Protein Aggregation and Starch Structural Changes in Wheat Flour Dough. Cereal Chem. J. 2013, 90, 89–100. [Google Scholar] [CrossRef]
- Bojňanská, T.; Musilová, J.; Vollmannová, A. Effects of Adding Legume Flours on the Rheological and Breadmaking Properties of Dough. Foods 2021, 10, 1087. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Xu, B.; Ren, G.; Peiyan, L.; Li, X.; Sihai, H.; Liu, J. Effects of Inulin with Different Degree of Polymerization on Gelatinization and Retrogradation of Wheat Starch. Food Chem. 2017, 229, 35–43. [Google Scholar] [CrossRef]
- Park, E.Y.; Jang, S.-B.; Lim, S.-T. Effect of Fructo-Oligosaccharide and Isomalto-Oligosaccharide Addition on Baking Quality of Frozen Dough. Food Chem. 2016, 213, 157–162. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Ding, B.; Ding, W.; Xiao, S.; Song, J.; Lyu, Q.; Ke, Y.; Wang, X.; Fu, Y. Effect of Hydrocolloids on Physical, Thermal and Microstructure Properties of Par-Baked Baguette during Frozen Storage. Int. J. Biol. Macromol. 2020, 163, 1866–1874. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E. Impact of Fibers on Physical Characteristics of Fresh and Staled Bake off Bread. J. Food Eng. 2010, 98, 273–281. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of Sourdough at Different Concentrations on Quality and Shelf Life of Bread. LWT—Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Cho, Y.J.; Bae, I.Y.; Inglett, G.E.; Lee, S. Utilization of Tartary Buckwheat Bran as a Source of Rutin and Its Effect on the Rheological and Antioxidant Properties of Wheat-Based Products. Ind. Crops Prod. 2014, 61, 211–216. [Google Scholar] [CrossRef]
- Koksel, H.; Kahraman, K.; Sanal, T.; Ozay, D.S.; Dubat, A. Potential Utilization of Mixolab for Quality Evaluation of Bread Wheat Genotypes. Cereal Chem. J. 2009, 86, 522–526. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Martoriello, T.; Mocci, G.; La Nottem, E. Modeling the Starch Retrogradation Kinetic of Durum Wheat Bread. J. Food Eng. 2003, 59, 123–128. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Kinetic Study of Staling in Breads with High-Amylose Resistant Starch. Food Hydrocoll. 2020, 106, 105879. [Google Scholar] [CrossRef]
- Hayes, A.M.R.; Okoniewska, M.; Martinez, M.M.; Zhao, B.; Hamaker, B.R. Investigating the Potential of Slow-Retrograding Starches to Reduce Staling in Soft Savory Bread and Sweet Cake Model Systems. Food Res. Int. 2020, 138, 109745. [Google Scholar] [CrossRef]
- Hwang, E.-I.; Ahn, B.-T.; Lee, H.-B.; Kim, Y.-K.; Lee, K.-S.; Bok, S.-H.; Kim, Y.-T.; Kim, S.-U. Inhibitory Activity for Chitin Synthase II from Saccharomyces Cerevisiae by Tannins and Related Compounds. Planta Med. 2004, 67, 501–504. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Minear, S.; O’Donnell, A.F.; Ballew, A.; Giaever, G.; Nislow, C.; Stearns, T.; Cyert, M.S. Curcumin Inhibits Growth of Saccharomyces Cerevisiae through Iron Chelation. Eukaryot. Cell 2011, 10, 1574–1581. [Google Scholar] [CrossRef]
- Lin, J.; Gwyneth Tan, Y.X.; Leong, L.P.; Zhou, W. Steamed Bread Enriched with Quercetin as an Antiglycative Food Product: Its Quality Attributes and Antioxidant Properties. Food Funct. 2018, 9, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, C.; Albrecht, A.; Elgeti, D.; Jekle, M.; Becker, T. Impact of gas formation kinetics on dough development and bread quality. Food Res. Int. 2015, 76, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Codină, G.; Mironeasa, S.; Voica, D.; Mironeasa, C. Multivariate analysis of wheat flour dough sugars, gas production, and dough development at different fermentation times. Czech J. Food Sci. 2013, 31, 222–229. [Google Scholar] [CrossRef]
- Babin, P.; Della Valle, G.; Chiron, H.; Cloetens, P.; Hoszowska, J.; Pernot, P.; Réguerre, A.L.; Salvo, L.; Dendievel, R. Fast X-ray Tomography Analysis of Bubble Growth and Foam Setting during Breadmaking. J. Cereal Sci. 2006, 43, 393–397. [Google Scholar] [CrossRef]
- Bojňanská, T.; Šmitalová, J. The Influence of Additional Fluors on the Retention Ability of Dough and the Technological Quality of Bakery Products. Potravin. Slovak J. Food Sci. 2015, 9, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Ananingsih, V.K.; Gao, J.; Zhou, W. Impact of Green Tea Extract and Fungal Alpha-Amylase on Dough Proofing and Steaming. Food Bioprocess Technol. 2012, 6, 3400–3411. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Yu, H.-H.; Chow, W.-F. Effects of Green Tea Extract on the Quality of Bread Made from Unfrozen and Frozen Dough Processes. J. Sci. Food Agric. 2006, 86, 857–864. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of Dietary Fibre on Dough Rheology and Bread Quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and Antioxidant Properties of Bread Containing Turmeric (Curcuma longa L.) Cultivated in South Korea. Food Chem. 2011, 124, 1577–1582. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Sivam, A.S.; Cooney, J.; Zhou, J.; Perera, C.O.; Waterhouse, G.I.N. Effects of Added Fruit Polyphenols and Pectin on the Properties of Finished Breads Revealed by HPLC/LC-MS and Size-Exclusion HPLC. Food Res. Int. 2011, 44, 3047–3056. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread Enriched with Quinoa Leaves—The Influence of Protein–Phenolics Interactions on the Nutritional and Antioxidant Quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread Fortified with Anthocyanin-Rich Extract from Black Rice as Nutraceutical Sources: Its Quality Attributes and in Vitro Digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef]
- Bojňanská, T.; Vollmannová, A.; Lidiková, J.; Musilová, J. Use of common buckwheat in the production of baked and pasta products. In Pseudocereals; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2022; pp. 11–34. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble Drug Delivery Strategies: Review of Recent Advances and Business Prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Trask, A.; Motherwell, W.; Jones, W. Physical Stability Enhancement of Theophylline via Cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef]
- Almansa, C.; Mercè, R.; Tesson, N.; Farran, J.; Tomàs, J.; Plata-Salamán, C.R. Co-Crystal of Tramadol Hydrochloride–Celecoxib (Ctc): A Novel API–API Co-Crystal for the Treatment of Pain. Cryst. Growth Des. 2017, 17, 1884–1892. [Google Scholar] [CrossRef]
- Bojňanská, T.; Kolesárová, A.; Ivanišová, E.; Bojňanský, J. The application of non-bakery raw materials to bakery flours, their effect on the technological quality and the cost of innovative products. J. Microbiol. Biotechnol. Food Sci. 2023; in press. [Google Scholar] [CrossRef]
- Abdellatif, A.M.; Ziena, H.M.; Rozan, M.A. Turmeric Powder Enhances the Chemical, Microbiological, Sensorial, and Shelflife Quality of Bun-Bread. Food Res. 2023, 7, 271–279. [Google Scholar] [CrossRef]
- Garcia, M.V.; Copetti, M.V. Alternative methods for mould spoilage control in bread and bakery products. Int. Food Res. J. 2019, 26, 737–749. [Google Scholar]
- Legan, J.D. Mould Spoilage of Bread: The Problem and Some Solutions. Int. Biodeterior. Biodegrad. 1993, 32, 33–53. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold Spoilage of Bread and Its Biopreservation: A Review of Current Strategies for Bread Shelf Life Extension. Crit. Rev. Food Sci. Nutr. 2016, 57, 3528–3542. [Google Scholar] [CrossRef]
- Pateras, I. Bread Spoilage and Staling. In Technology of Breadmaking; Springer Ebooks: Boston, MA, USA, 1999; pp. 240–261. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Ecology of Fungal Food Spoilage. In Fungi Food Spoilage; Springer: Cham, Switzerland, 2022; pp. 3–12. [Google Scholar] [CrossRef]
- Ribeiro Oliveira, A.; Emannuele, A.; Oliveira, É.R.; Ribeiro, K.O.; Garcia, M.C.; Careli-Gondim, Í.; Soares, M.; Caliari, M. Physicochemical, Microbiological and Sensory Characteristics of Snacks Developed from Broken Rice Grains and Turmeric Powder. Int. J. Food Sci. Technol. 2020, 55, 2719–2729. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Wolska, A.; Remaley, A.T.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Bread Enriched with Phytosterols with or without Curcumin Modulates Lipoprotein Profiles in Hypercholesterolaemic Individuals. A Randomised Controlled Trial. Food Funct. 2019, 10, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.S.; Carini, J.P.; Honorato, S.B.; Ayala, A.P.; Moreira, J.C.F.; Bassani, V.L. Physicochemical Properties and Thermal Stability of Quercetin Hydrates in the Solid State. Thermochim. Acta 2012, 539, 109–114. [Google Scholar] [CrossRef]
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent Development of Plant Products with Anti-Glycation Activity: A Review. RSC Adv. 2015, 5, 31113–31138. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and Antiglycation Activity of Selected Dietary Polyphenols in a Cookie Model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef]




| Wheat Flour T650 | Curcumin | Quercetin | Identification of the Sample | |
|---|---|---|---|---|
| 100% | 0% | 0% | WF | |
| 97.5% | 2.5% | 2.5% | C_2.5 | Q_2.5 |
| 95% | 5% | 5% | C_5 | Q_5 |
| Extract | Methanol Solvent | Ethanol Solvent |
|---|---|---|
| a. | ||
| Curcumin 0.25% | 1.389 ± 0.016 a | 1.289 ± 0.008 a |
| Quercetin dihydrate 0.25% | 2.656 ± 0.020 b | 3.274 ± 0.034 b |
| b. | ||
| Curcumin 0.25% | 2.890 ± 0.042 a | 3.081 ± 0.080 a |
| Quercetin dihydrate 0.25% | 3.045 ± 0.039 b | 3.118 ± 0.023 a |
| c. | ||
| Curcumin 0.25% | 94.43 ± 0.72 a | 87.32 ± 0.46 a |
| Quercetin dihydrate 0.25% | 96.57 ± 0.53 b | 100.0 ± 0.01 b |
| Identified Mould | DRBC | |||||
|---|---|---|---|---|---|---|
| WF1 | Q_2.5 | C_2.5 | WF2 | Q_5 | C_5 | |
| Penicillium sp. | + | + | + | + | + | |
| Alternaria sp. | + | + | ||||
| Nigrospora oryzae | + | |||||
| Aspergillus (form Eurotium) | + | |||||
| Aspergillus sp. | ||||||
| Aspergillus Sektion Circumdati | ||||||
| Aspergillus Sektion Nigri | + | |||||
| Epicoccum nigrum | + | |||||
| Chaetomuim sp. | + | |||||
| Cladosporium sp. | + | |||||
| In a Closed Package | ||||||
| WF1 | Q_2.5 | C_2.5 | WF2 | Q_5 | C_5 | |
| Penicillium sp. | + | + | + | + | ||
| Alternaria sp. | + | |||||
| Nigrospora oryzae | ||||||
| Aspergillus (form Eurotium) | + | |||||
| Aspergillus sp. | + | + | ||||
| Aspergillus Sektion Circumdati | + | |||||
| Aspergillus Sektion Nigri | + | |||||
| Epicoccum nigrum | ||||||
| Chaetomuim sp. | ||||||
| Cladosporium sp. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojňanská, T.; Kolesárová, A.; Čech, M.; Tančinová, D.; Urminská, D. Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread. Foods 2024, 13, 382. https://doi.org/10.3390/foods13030382
Bojňanská T, Kolesárová A, Čech M, Tančinová D, Urminská D. Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread. Foods. 2024; 13(3):382. https://doi.org/10.3390/foods13030382
Chicago/Turabian StyleBojňanská, Tatiana, Anna Kolesárová, Matej Čech, Dana Tančinová, and Dana Urminská. 2024. "Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread" Foods 13, no. 3: 382. https://doi.org/10.3390/foods13030382
APA StyleBojňanská, T., Kolesárová, A., Čech, M., Tančinová, D., & Urminská, D. (2024). Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread. Foods, 13(3), 382. https://doi.org/10.3390/foods13030382