Improving the Edible and Nutritional Quality of Roasted Duck Breasts through Variable Pressure Salting: Implications for Protein Anabolism and Digestion in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Processing of Salted Roasted Duck
2.3. Animal Treatment
2.4. Animal Experiment Design
2.5. Determination of Serum Biochemical Indicators in Rats
2.6. Analysis of In Vivo Digestion Properties in Rats
2.6.1. Determination of Pepsin and Trypsin Activities
2.6.2. Apparent Protein Digestibility Analysis
2.7. Low-Field Nuclear Magnetic Resonance (LF-NMR) Analysis
2.8. Textural Properties and Warner–Bratzler Shear Force (WBSF) Testing
2.9. Analysis of Microstructure
2.10. Determination of Protein Structure
2.10.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.10.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.11. Statistical Analysis
3. Results and Discussion
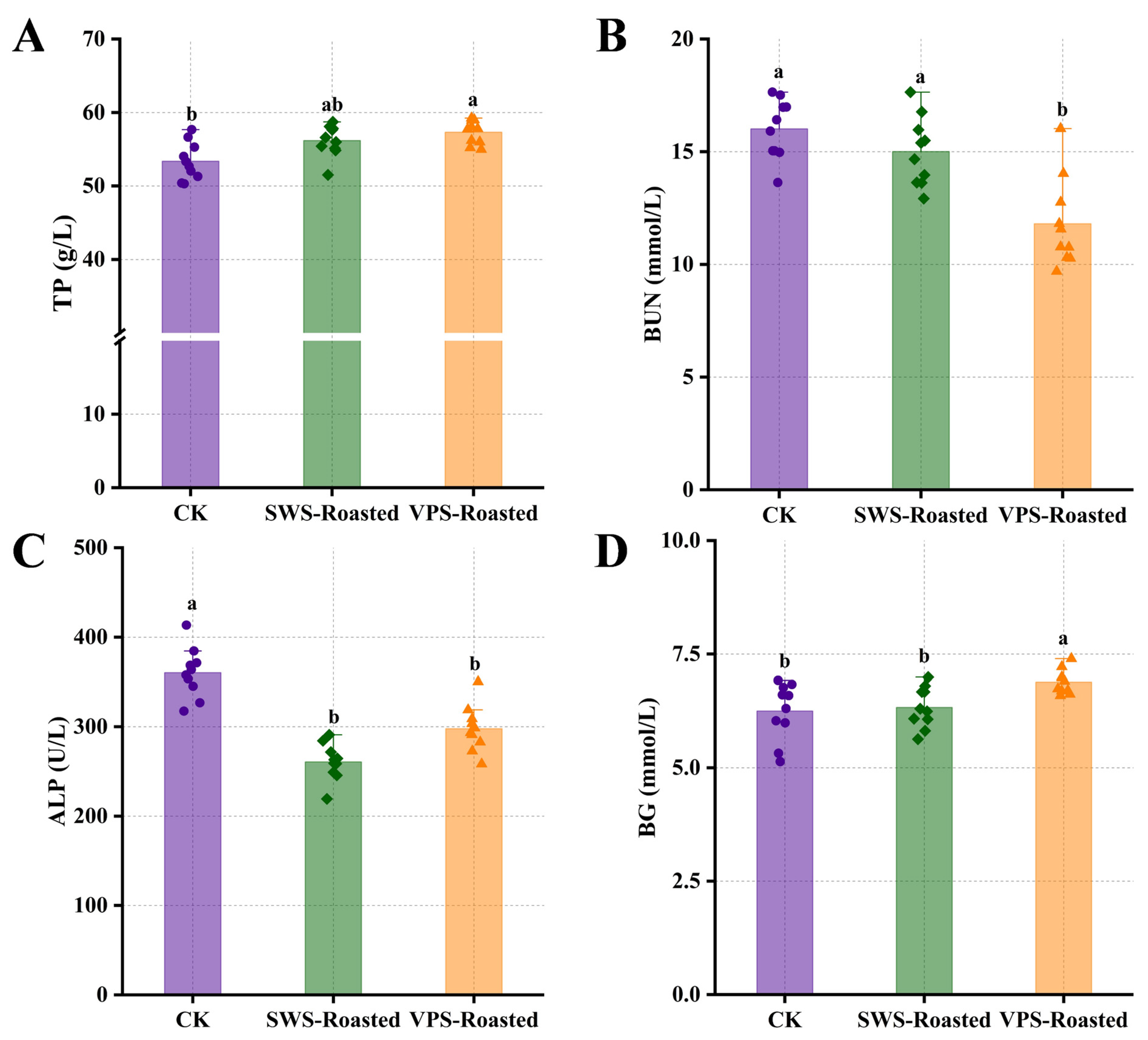
3.1. Analysis of Differences in Serum Biochemical Indicators in Rats
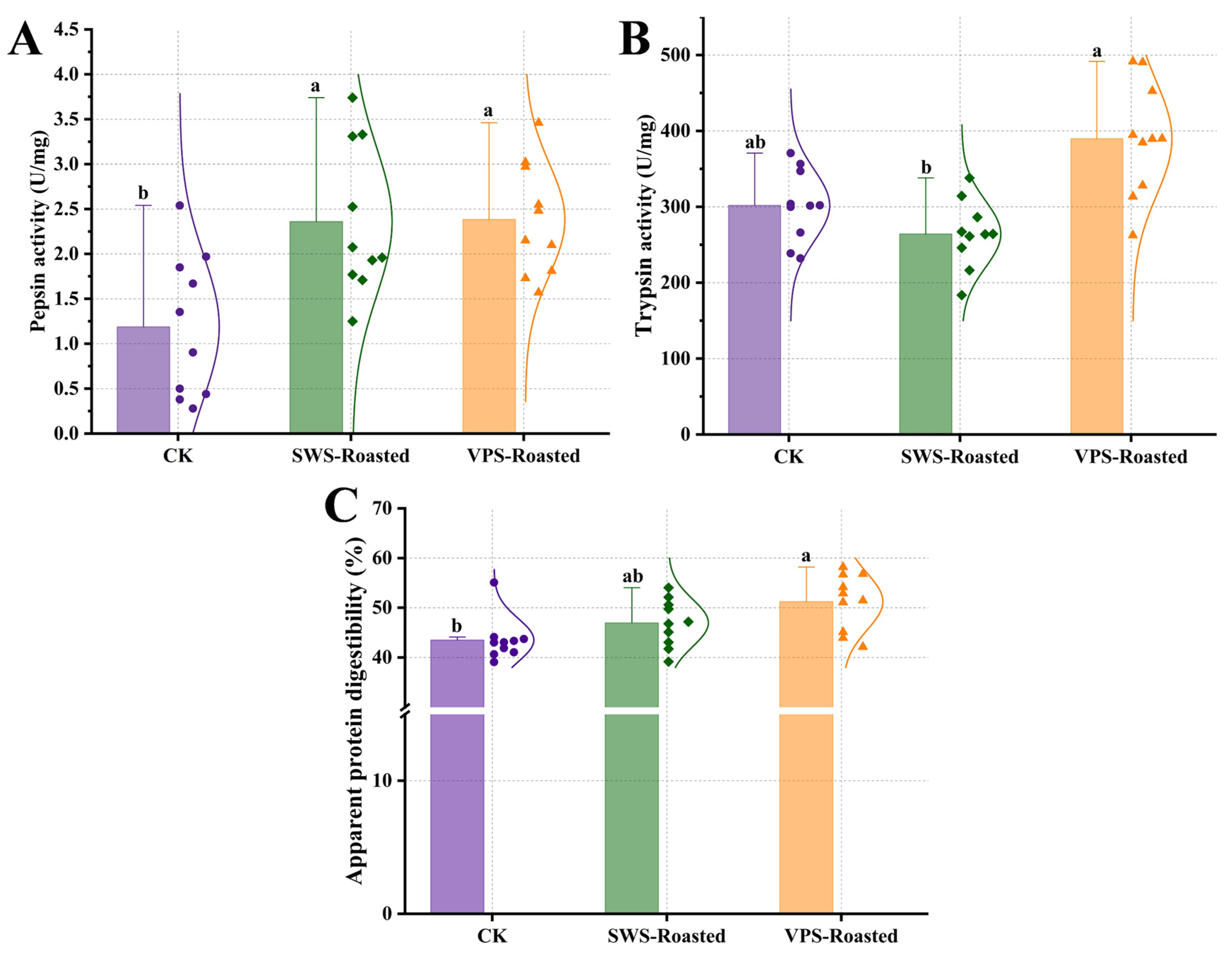
3.2. Differences in the Digestion Properties In Vivo
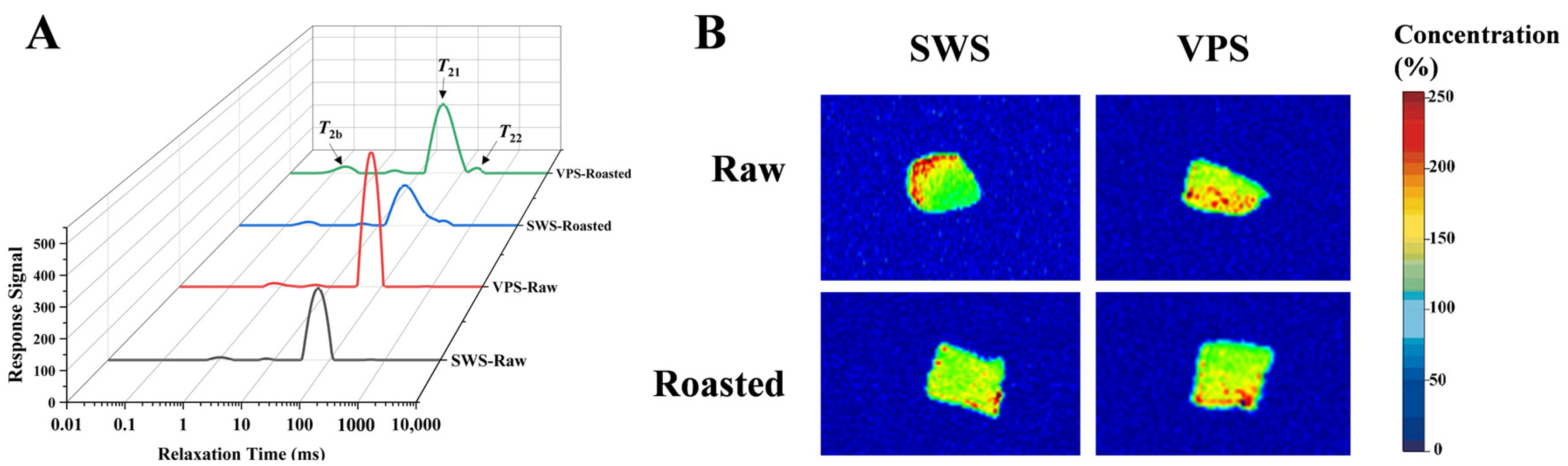
3.3. Changes in Water Distribution
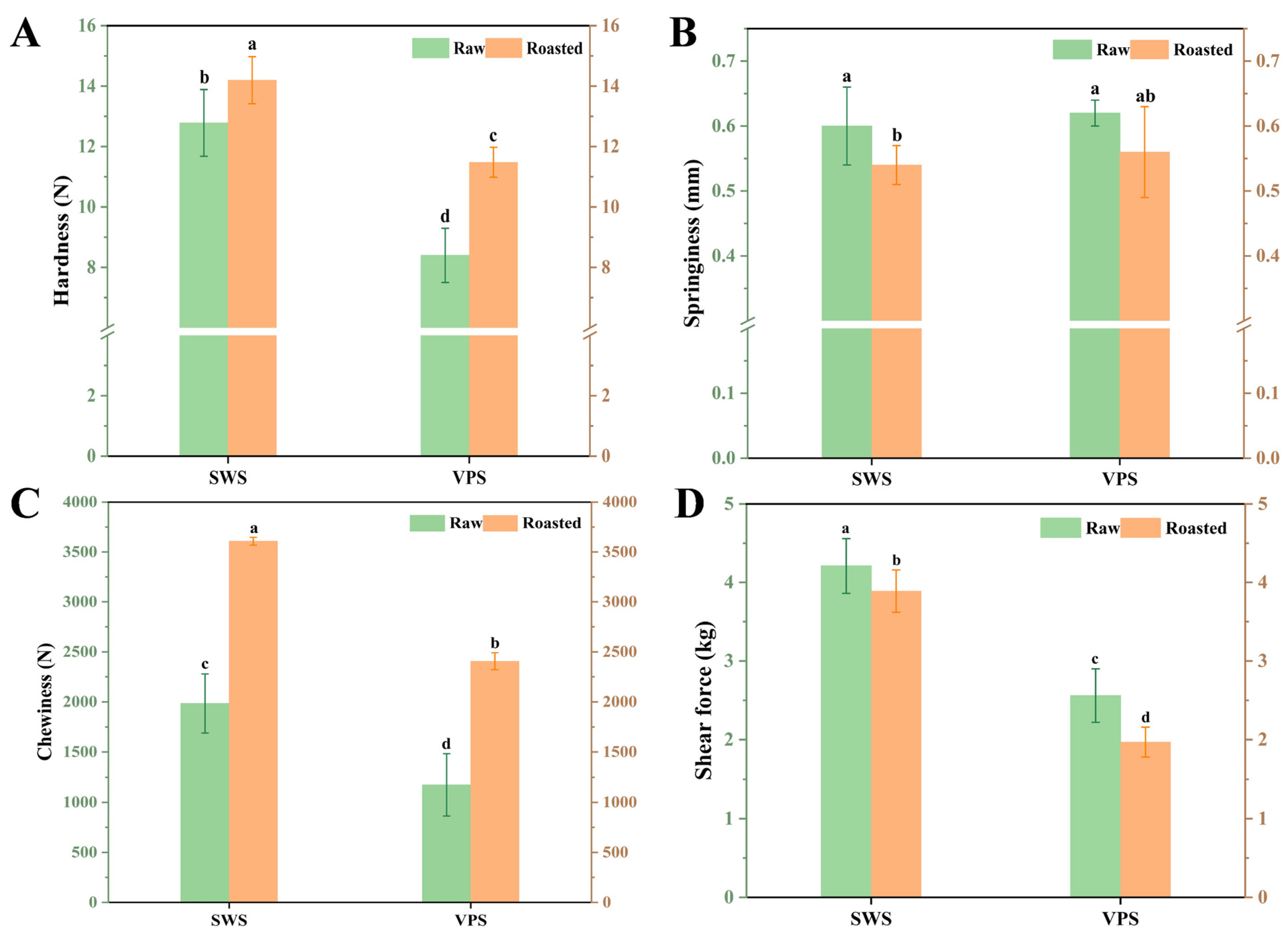
3.4. Changes in Textural Properties
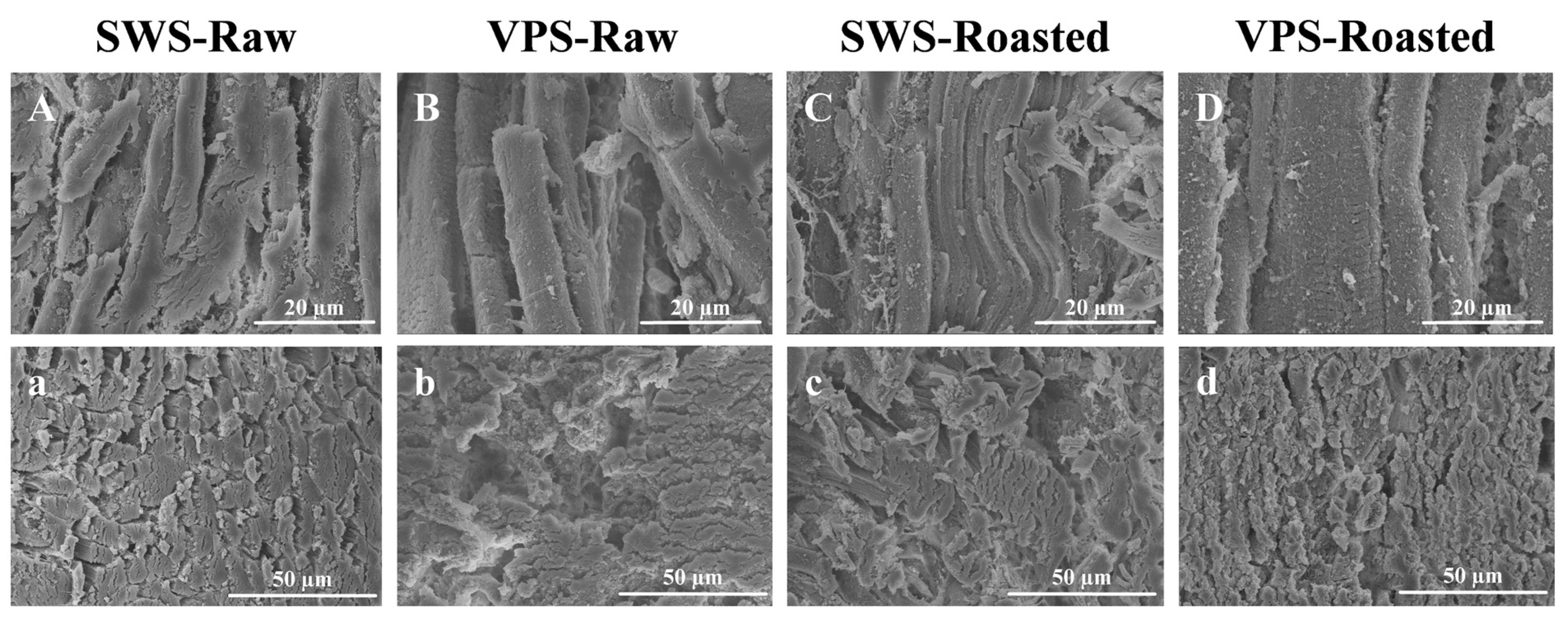
3.5. Analysis of Microstructure
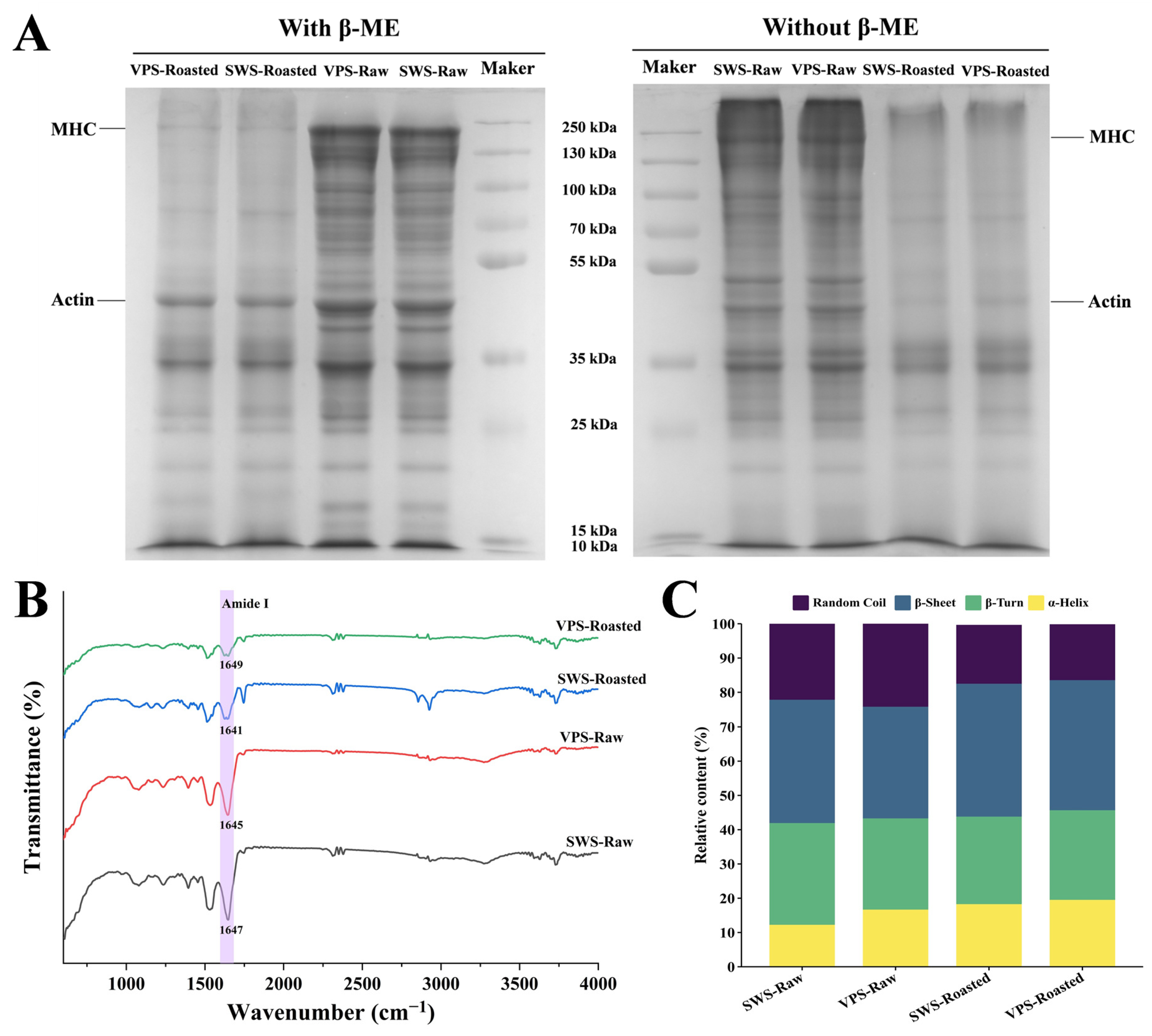
3.6. Changes in Protein Structure
3.7. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of key aroma compounds in beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Jury, V.; de Lamballerie, M.; Duranton, F.; Pottier, L.; Martin, J.L. Salt intake from processed meat products: Benefits, risks and evolving practices. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1453–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Zhu, Y.Z.; Xu, W.M. Composition of intramuscular phospholipids and free fatty acids in three kinds of traditional Chinese duck meat products. Poult. Sci. 2009, 88, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zhang, D.; Chen, R.; Yang, X.; Shi, H.; Liu, H.; Wang, Z.; Lu, L.; Hui, T. A developed variable pressure-assisted salting process: Improving the textural, flavor, and sensory attributes in roasted duck breast. LWT 2022, 167, 113800. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Wang, Q.; Liu, C.; Jin, Y.; Huang, F.; Ma, M. Pulsed pressure assisted brining of porcine meat. Innov. Food Sci. Emerg. Technol. 2014, 22, 76–80. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Li, C.; Zhao, Y.; Chen, C.; Zhang, Y.; Zhang, J.; Ma, M. Effect of pulsed pressure-assisted brining on lipid oxidation and volatiles development in pork bacon during salting and drying-ripening. LWT 2015, 64, 1099–1106. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Zhu, J.; Peng, W.; Chen, Y.; Luo, X.; Chen, C.; Li, L. Effects of salt-induced changes in protein network structure on the properties of surimi gels: Computer simulation and digestion study. Int. J. Food Sci. Technol. 2021, 56, 3914–3923. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Jayawardena, S.R.; Mungure, T.; Bekhit, A.E.D.A. Cooking does not impair the impact of pulsed electric field on the protein digestion of venison (Cervus elaphus) during in-vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 3026–3033. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T. Optimization of dynamic variable pressure curing of duck carcass for roast duck. Meat Res. 2021, 35, 14–22. [Google Scholar] [CrossRef]
- Bax, M.L.; Buffière, C.; Hafnaoui, N.; Gaudichon, C.; Savary-Auzeloux, I.; Dardevet, D.; Santé-Lhoutellier, V.; Rémond, D. Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: A study in minipigs. PLoS ONE 2013, 8, e61252. [Google Scholar] [CrossRef]
- GB14924.3-2010; Laboratory Animals-Nutrients for Formula Feeds. Standardization Administration of the People’s Republic of China, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2010.
- Xu, Z.; Zhao, F.; Chen, H.; Xu, S.; Fan, F.; Shi, P.; Tu, M.; Wang, Z.; Du, M. Nutritional properties and osteogenic activity of enzymatic hydrolysates of proteins from the blue mussel (Mytilus edulis). Food Funct. 2019, 10, 7745–7754. [Google Scholar] [CrossRef]
- Terrazas-Fierro, M.; Civera-Cerecedo, R.; Ibarra-Martínez, L.; Goytortúa-Bores, E.; Herrera-Andrade, M.; Reyes-Becerra, A. Apparent digestibility of dry matter, protein, and essential amino acid in marine feedstuffs for juvenile whiteleg shrimp Litopenaeus vannamei. Aquaculture 2010, 308, 166–173. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Xie, F.; Li, X.; Schroyen, M.; Chen, L.; Hou, C. Changes in water holding capacity of chilled fresh pork in controlled freezing-point storage assisted by different modes of electrostatic field action. Meat Sci. 2023, 204, 109269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, R.; Ge, Q.; Wu, M.; Xu, B.; Xi, J.; Yu, H. Effects of branched-chain amino acids and Lactobacillus plantarum CGMCC18217 on volatiles formation and textural properties of dry-cured fermented sausage. Int. J. Food Sci. Technol. 2021, 56, 374–383. [Google Scholar] [CrossRef]
- Yin, Y.T.; Zhou, L.; Cai, J.M.; Feng, F.; Xing, L.J.; Zhang, W.G. Effect of malondialdehyde on the digestibility of beef myofibrillar protein: Potential mechanisms from structure to modification site. Foods 2022, 11, 2176. [Google Scholar] [CrossRef] [PubMed]
- Gangidi, R.R.; Proctor, A.; Pohlman, F.W. Rapid determination of spinal cord content in ground beef by attenuated total reflectance Fourier transform infrared spectroscopy. J. Food Sci. 2003, 68, 124–127. [Google Scholar] [CrossRef]
- D’Souza, J.M.P.; Pai, V.R.; Harish, S.; Shriyan, C. Relation of serum and salivary total sialic acid and total sialic acid/total protein ratio with clinical parameters of disease progression in preeclampsia. Indian J. Clin. Biochem. 2022, 37, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Auer, M.; Zeileis, A.; Deisenhammer, F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: Implications for increased clinical specificity. Clin. Chem. Lab. Med. 2016, 54, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.S.; Quayle, K.S.; McManemy, J.K.; Nigrovic, L.E.; Tzimenatos, L.; Stoner, M.J.; Bennett, J.E.; Trainor, J.L.; Rewers, A.; Schunk, J.E.; et al. Clinical characteristics of children with cerebral injury preceding treatment of diabetic ketoacidosis. J. Pediatr. 2022, 250, 100–104. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Y.; Yan, L.; Yang, S.; Yang, D. Correlation analysis of microbial contamination and alkaline phosphatase activity in raw milk and dairy products. Int. J. Environ. Res. Public Health 2023, 20, 1825. [Google Scholar] [CrossRef] [PubMed]
- Zaher, D.M.; El-Gamal, M.I.; Omar, H.A.; Aljareh, S.N.; Al-Shamma, S.A.; Ali, A.J.; Zaib, S.; Iqbal, J. Recent advances with alkaline phosphatase isoenzymes and their inhibitors. Arch. Pharm. 2020, 353, e2000011. [Google Scholar] [CrossRef] [PubMed]
- Nansel, T.R.; Gellar, L.; McGill, A. Effect of varying glycemic index meals on blood glucose control assessed with continuous glucose monitoring in youth with type 1 diabetes on basalm-bolus insulin regimens. Diabetes Care 2008, 31, 695–697. [Google Scholar] [CrossRef]
- Burgess, S.C.; Jeffrey, F.M.H.; Storey, C.; Milde, A.; Hausler, N.; Merritt, M.E.; Mulder, H.; Holm, C.; Sherry, A.D.; Malloy, C.R. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E53–E61. [Google Scholar] [CrossRef]
- Liu, Y.G.; Yang, L.L.; Zhang, Y.; Liu, X.C.; Wu, Z.J.; Gilbert, R.G.; Deng, B.; Wang, K.P. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.; Lan, A.; Khodorova, N.; Santé-Lhoutellier, V.; Walker, F.; Piedcoq, J.; Davila, A.M.; Blachier, F.; Tomé, D.; Fromentin, G.; et al. Compared with raw bovine meat, boiling but not grilling, barbecuing, or roasting decreases protein digestibility without any major consequences for intestinal mucosa in rats, although the daily ingestion of bovine meat induces histologic modifications in the colon. J. Nutr. 2016, 146, 1506–1513. [Google Scholar] [CrossRef]
- Jeng, S.-S.; Liao, H.-J.; Wang, M.-S. High Zn concentration in the digestive tract tissues of common carp cyprinus carpio. Fish. Sci. 2011, 68, 1181–1184. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.B.L.; van Dijk, J.W.; de Lange, A.; Kiskini, A.; Kuklinski, M.; Senden, J.M.G.; van Loon, L.J.C. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older Men. Am. J. Clin. Nutr. 2013, 98, 121–128. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Relationship of feeds protein structural makeup in common Prairie feeds with protein solubility, in situ ruminal degradation and intestinal digestibility. Anim. Feed. Sci. Technol. 2014, 194, 58–70. [Google Scholar] [CrossRef]
- Ferreira, V.C.S.; Morcuende, D.; Madruga, M.S.; Silva, F.A.P.; Estévez, M. Role of protein oxidation in the nutritional loss and texture changes in ready-to-eat chicken patties. Int. J. Food Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef]
- Qian, S.; Hu, F.; Mehmood, W.; Li, X.; Zhang, C.; Blecker, C. The rise of thawing drip: Freezing rate effects on ice crystallization and myowater dynamics changes. Food Chem. 2022, 373, 131461. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, F.; Li, X.; Han, D.; Zhao, L.; Liang, H.; Rui, M.; Wang, J.; Zhang, C. Water status evolution of pork blocks at different cooking procedures: A two-dimensional LF-NMR T1-T2 relaxation study. Food Res. Int. 2021, 148, 110614. [Google Scholar] [CrossRef]
- Hu, F.; Qian, S.; Huang, F.; Han, D.; Li, X.; Zhang, C. Combined impacts of low voltage electrostatic field and high humidity assisted-thawing on quality of pork steaks. LWT 2021, 150, 0023–6438. [Google Scholar] [CrossRef]
- Bombrun, L.; Gatellier, P.; Carlier, M.; Kondjoyan, A. The effects of low salt concentrations on the mechanism of adhesion between two pieces of pork semimembranosus muscle following tumbling and cooking. Meat Sci. 2014, 96, 5–13. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.K.; Allen, P.; Duggan, E.; Arimi, J.M.; Casey, E.; Duane, G.; Lyng, J.G. The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: A low field NMR study. Meat Sci. 2013, 95, 51–58. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, L.; Zareef, M.; Wang, L.; Chen, Q. Application of portable visible and near-infrared spectroscopy for rapid detection of cooking loss rate in pork: Comparing spectra from frozen and thawed pork. LWT 2022, 160, 113304. [Google Scholar] [CrossRef]
- Fallah-Joshaqani, S.; Hamdami, N.; Keramat, J. Qualitative attributes of button mushroom (Agaricus bisporus) frozen under high voltage electrostatic field. J. Food Eng. 2021, 293, 110384. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of high intensity ultrasound on gelation properties of silver carp surimi with different salt contents. Ultrason. Sonochem. 2021, 70, 105326. [Google Scholar] [CrossRef]
- Yang, H.; Chen, L.; Tang, H.; Wang, H.; Zhang, J.; Xiao, C.; Tao, F.; Cao, G.; Shen, Q. Tenderness improvement of reduced-fat and reduced-salt meat gels as affected by high pressure treating time. Innov. Food Sci. Emerg. Technol. 2021, 70, 102687. [Google Scholar] [CrossRef]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Li, Y.; Feng, T.; Sun, J.; Guo, L.; Wang, B.; Huang, M.; Xu, X.; Yu, J.; Ho, H. Physicochemical and microstructural attributes of marinated chicken breast influenced by breathing ultrasonic tumbling. Ultrason. Sonochem. 2020, 64, 1350–4177. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Nian, L.; Cao, A.; Zhang, Y.; Li, X. Effect of carboxymethyl chitosan magnetic nanoparticles plus herring antifreeze protein on conformation and oxidation of myofibrillar protein from red sea bream (Pagrosomus major) after freeze-thaw treatment. Food Bioprocess Technol. 2020, 13, 355–366. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yang, Y.-L.; Tang, X.-Z.; Ni, W.-X.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017, 38, 225–233. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Beattie, J.R.; Bell, S.E.J.; Borggaard, C.; Moss, B.W. Preliminary investigations on the effects of ageing and cooking on the Raman spectra of porcine longissimus dorsi. Meat Sci. 2008, 80, 1205–1211. [Google Scholar] [CrossRef]

| Parameters | Static Wet Salting | Variable Pressure Salting |
|---|---|---|
| Temperature/°C | 4 | 4 |
| Vacuum pressure/KPa | Atmospheric pressure | −70~−80 |
| Mass fraction of salt solution/% | 8.9 | 8.9 |
| Pulse ratio | - | 1.5 (54 min vacuum pressure/36 min atmospheric pressure) |
| Salting time/h | 12 | 12 |
| Roasting time/min | 50 | 50 |
| Index/% | SWS-Raw | VPS-Raw | SWS-Roasted | VPS-Roasted |
|---|---|---|---|---|
| P2b | 4.86 ± 0.95 a | 4.43 ± 0.25 a | 3.49 ± 0.20 b | 3.43 ± 0.37 b |
| P21 | 94.91 ± 1.04 b | 95.30 ± 0.64 a | 89.26 ± 1.87 c | 90.75 ± 0.61 bc |
| P22 | 0.23 ± 0.05 b | 0.27 ± 0.03 b | 7.25 ± 1.67 a | 5.82 ± 0.97 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Zhou, Y.; Zhang, D.; Wu, R.; Ma, J.; He, J.; Wang, Z. Improving the Edible and Nutritional Quality of Roasted Duck Breasts through Variable Pressure Salting: Implications for Protein Anabolism and Digestion in Rats. Foods 2024, 13, 402. https://doi.org/10.3390/foods13030402
Gao Z, Zhou Y, Zhang D, Wu R, Ma J, He J, Wang Z. Improving the Edible and Nutritional Quality of Roasted Duck Breasts through Variable Pressure Salting: Implications for Protein Anabolism and Digestion in Rats. Foods. 2024; 13(3):402. https://doi.org/10.3390/foods13030402
Chicago/Turabian StyleGao, Ziwu, Yinna Zhou, Dequan Zhang, Ruiyun Wu, Jiale Ma, Jinhua He, and Zhenyu Wang. 2024. "Improving the Edible and Nutritional Quality of Roasted Duck Breasts through Variable Pressure Salting: Implications for Protein Anabolism and Digestion in Rats" Foods 13, no. 3: 402. https://doi.org/10.3390/foods13030402






