Heterotrophic Selenium Incorporation into Chlorella vulgaris K-01: Selenium Tolerance, Assimilation, and Removal through Microalgal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heterotrophic Culture and Selenium Treatment
2.2. Measurement of Microalgal Biomass
2.3. Growth Parameters Calculation
2.4. Gaussian Process (GP) Regression Analysis
2.5. Glucose Detection and Evaluation
2.6. SEM Microscopy
2.7. Intracellular Selenium Detection through ICP-MS
2.8. Extracellular Residual Selenium Determination through ICP-OES
2.9. Statistical Analysis
3. Results and Discussion
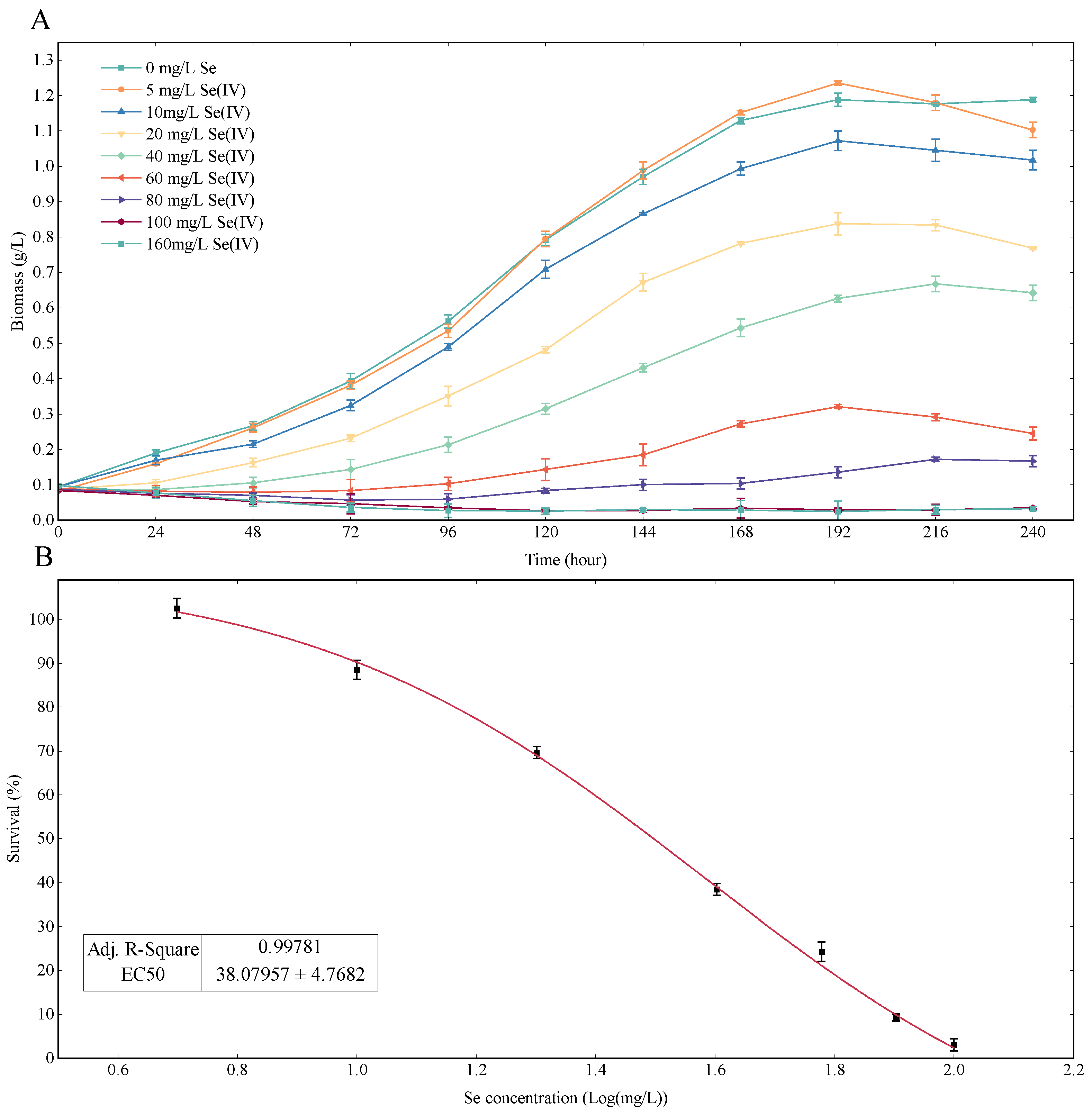
3.1. Se Dose–Response of Chlorella vulgaris K-01 under Heterotrophic Medium
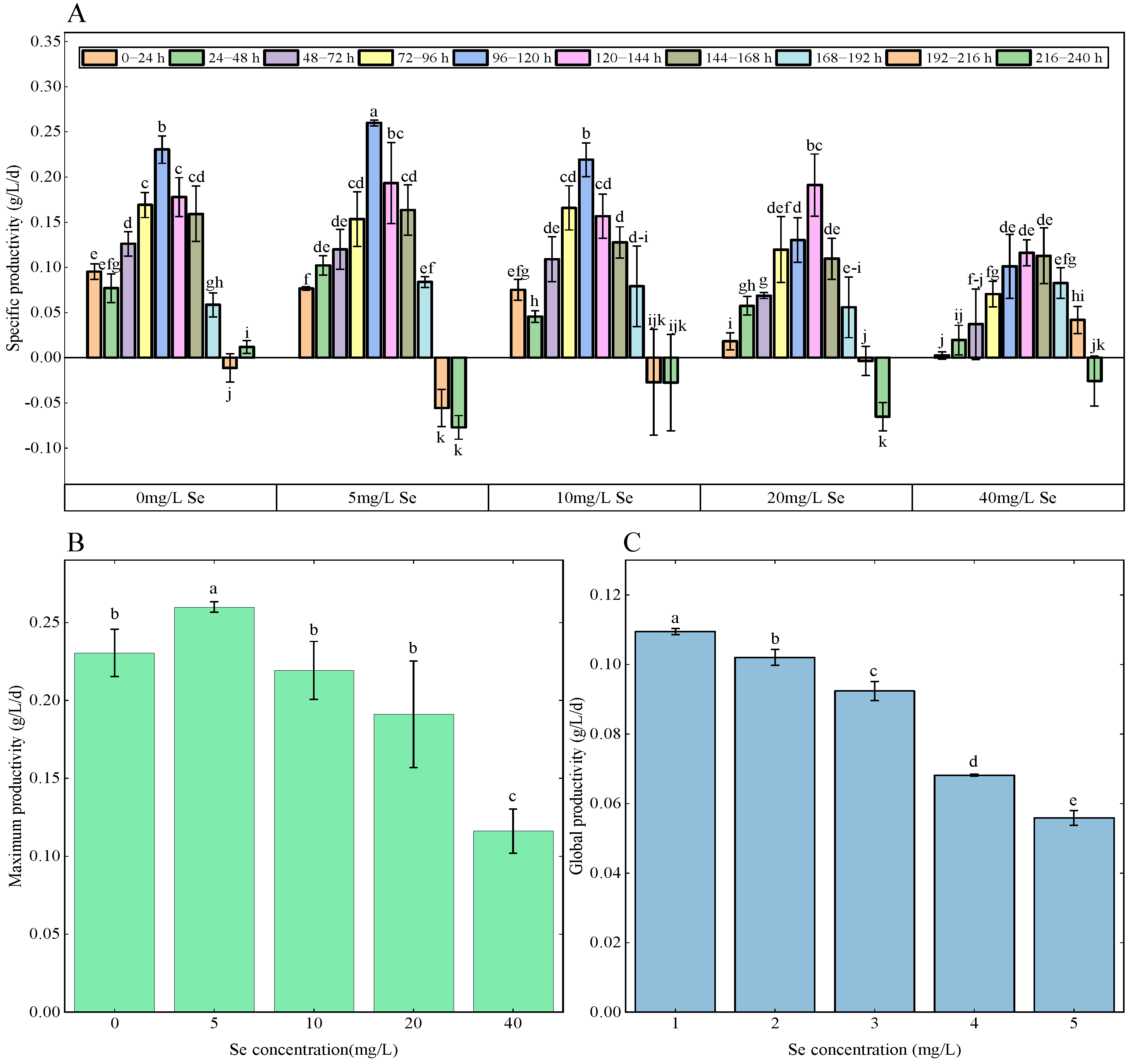
3.2. Growth Rate Analysis
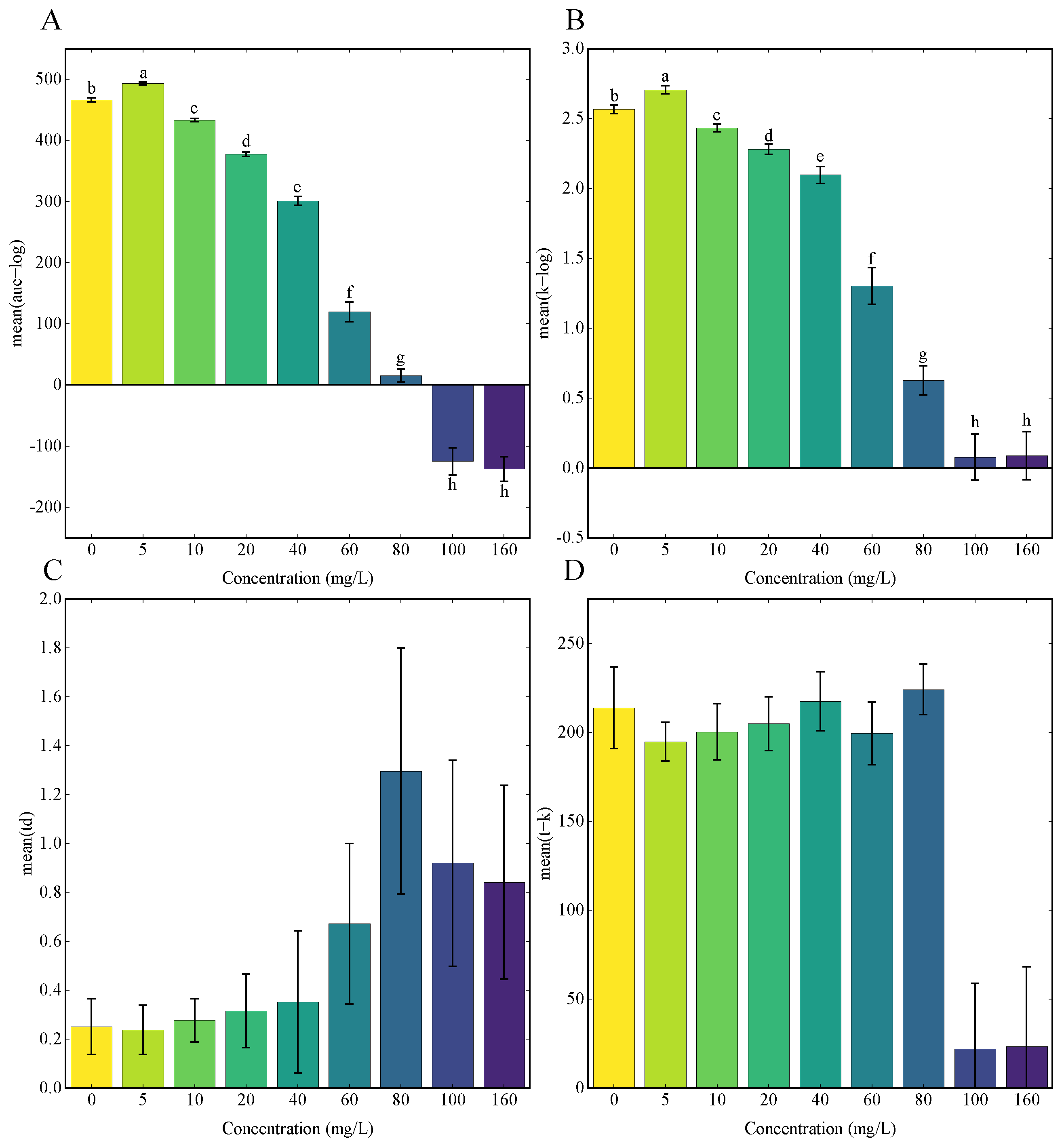
3.3. Growth Curves Analysis Using GP Regression
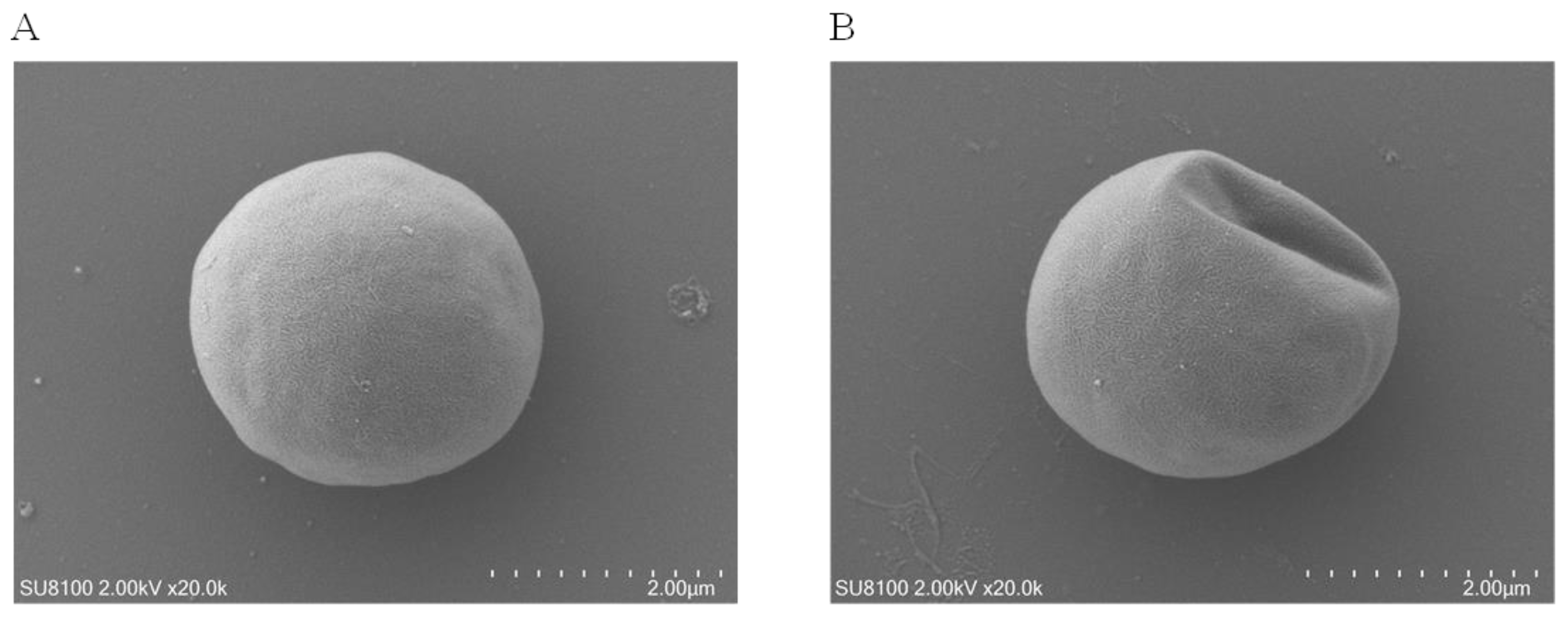
3.4. Microalgal Morphology Disruption under Se Stress
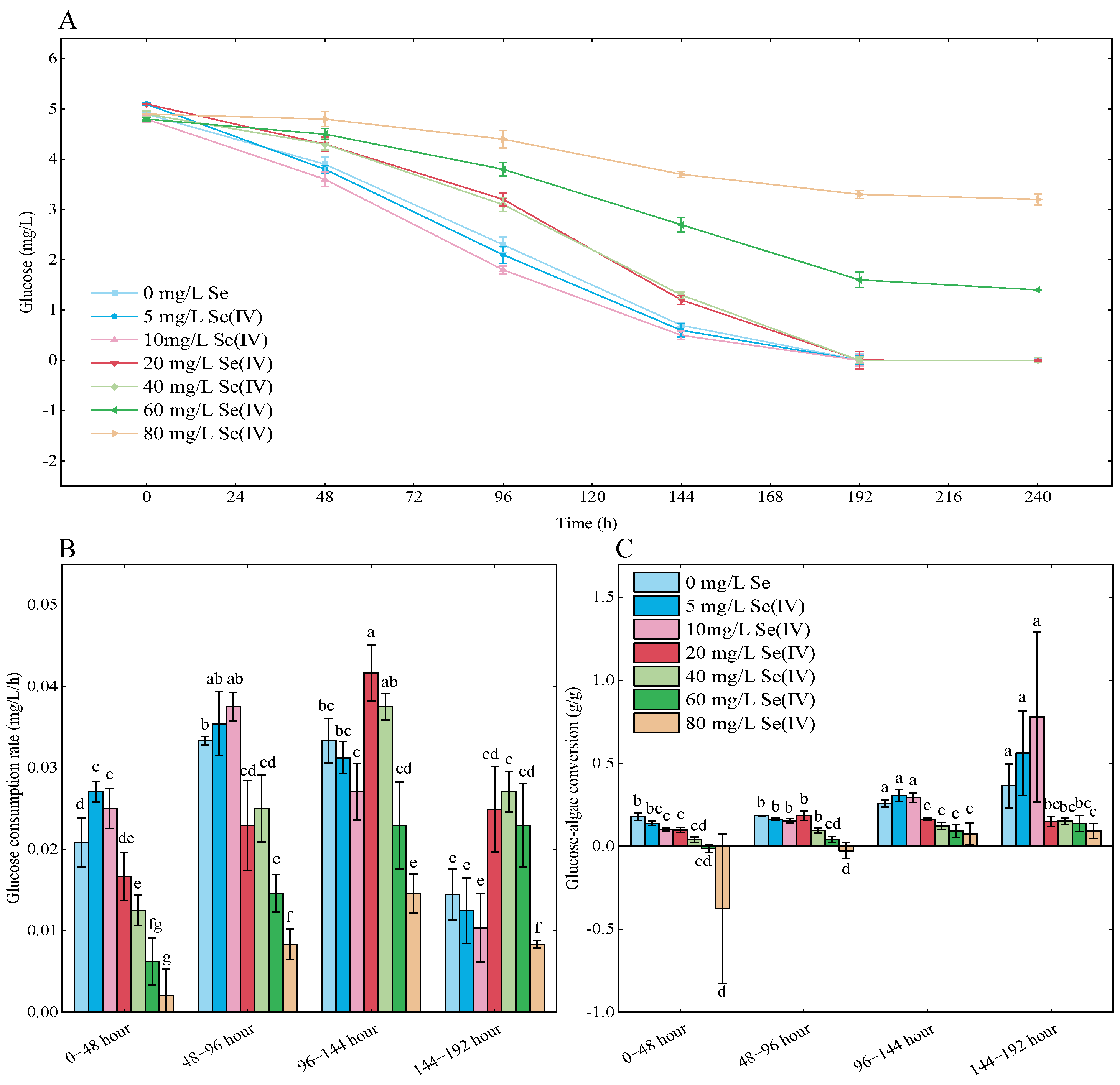
3.5. Glucose Consumption Analysis
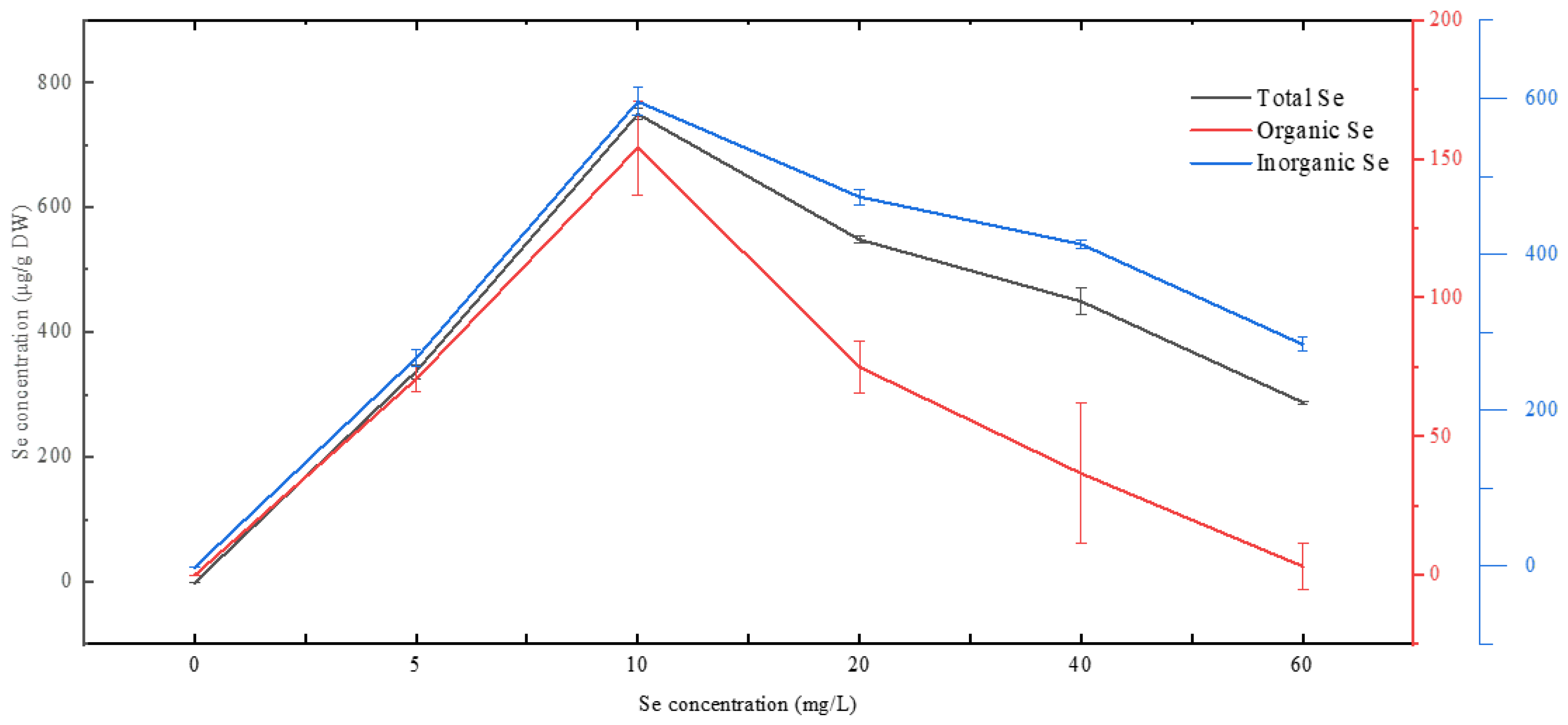
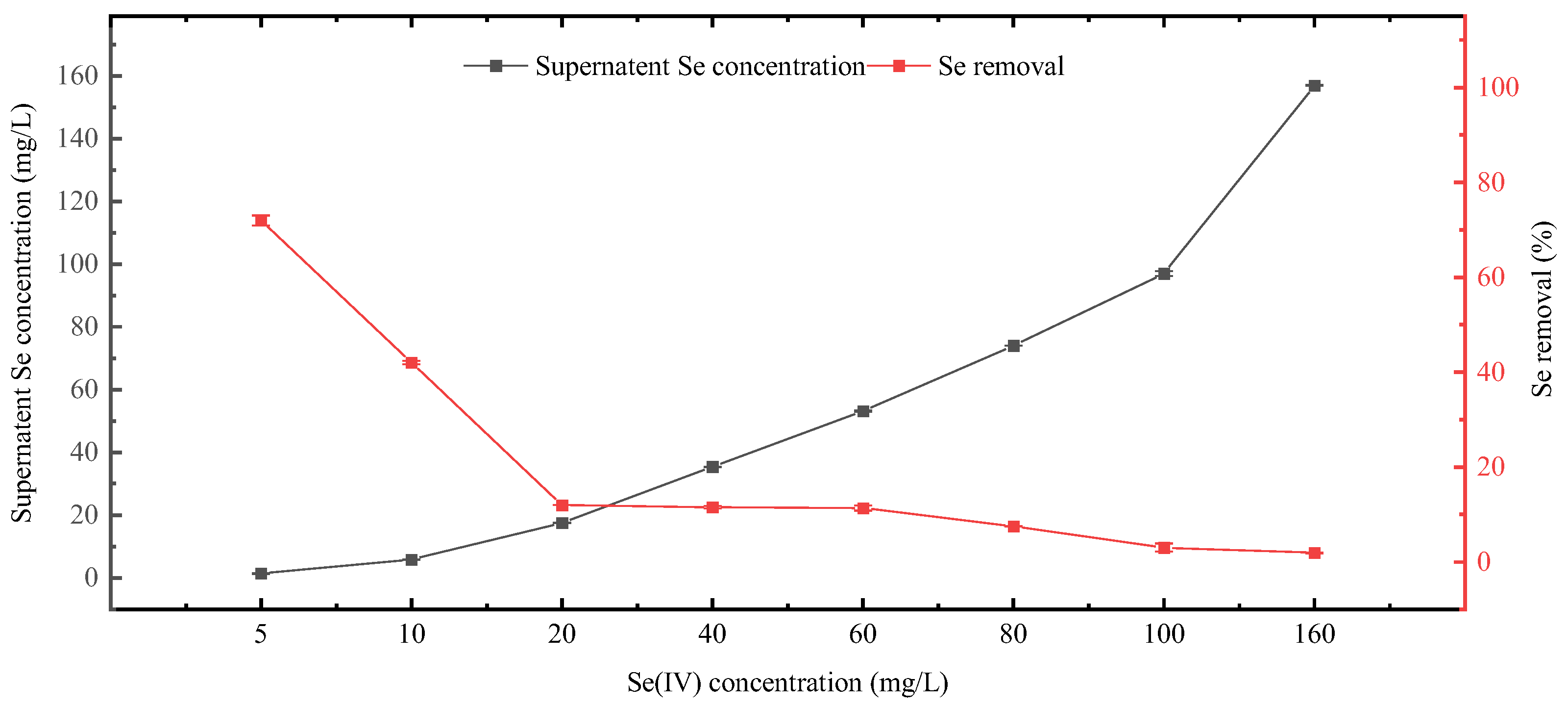
3.6. Se Incorporation and Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of Nano-Selenium in Health and Environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and Reproduction: Boars and Semen Quality—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 730–746. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Sztrik, A.; Fári, M.; El-Marsafawy, S.; Shams, M.S. Selenium in Soils under Climate Change, Implication for Human Health. Environ. Chem. Lett. 2015, 13, 1–19. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Acute Human Toxicity and Mortality after Selenium Ingestion: A Review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef]
- Turło, J.; Gutkowska, B.; Herold, F.; Klimaszewska, M.; Suchocki, P. Optimization of Selenium-Enriched Mycelium of Lentinula edodes (Berk.) Pegler as a Food Supplement. Food Biotechnol. 2010, 24, 180–196. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What Do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, H.; Cheng, W.-H. Beneficial and Paradoxical Roles of Selenium at Nutritional Levels of Intake in Healthspan and Longevity. Free Radic. Biol. Med. 2018, 127, 3–13. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, J.J.; Combs, G.F., Jr. Selenium in Bone Health: Roles in Antioxidant Protection and Cell Proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium Biochemistry and Its Role for Human Health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Zhang, J.; Li, H.; Liu, X. Selenium-Enriched Peptides Identified from Selenium-Enriched Soybean Protein Hydrolysate: Protective Effects against Heat Damage in Caco-2 Cells. Food Funct. 2023, 14, 7882–7896. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K.; Kotrbáček, V.; Zachleder, V. Production of Chlorella Biomass Enriched by Selenium and Its Use in Animal Nutrition: A Review. Appl. Microbiol. Biotechnol. 2009, 83, 1001–1008. [Google Scholar] [CrossRef]
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella Vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella Vulgaris Biomass Used as Colouring Source in Traditional Butter Cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Csatlos, N.-I.; Simon, E.; Teleky, B.-E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.-C.; Ciont Nagy, C.; Pop, O.-L. Development of a Fermented Beverage with Chlorella Vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina Platensis and Chlorella Vulgaris Algae into Probiotic Fermented Milks. Compr. Rev. Food Sci. Food Safe 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Dantas, D.M.M.; Cahú, T.B.; Oliveira, C.Y.B.; Abadie-Guedes, R.; Roberto, N.A.; Santana, W.M.; Gálvez, A.O.; Guedes, R.C.A.; Bezerra, R.S. Chlorella vulgaris Functional Alcoholic Beverage: Effect on Propagation of Cortical Spreading Depression and Functional Properties. PLoS ONE 2021, 16, e0255996. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, Antitumor Activities, and Encapsulation of Polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, Structures and Biological Activities of Polysaccharides from Chlorella: A Review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Tao, M.; Li, J.; Hu, Z. Effects of Selenite on Green Microalga Haematococcus pluvialis: Bioaccumulation of Selenium and Enhancement of Astaxanthin Production. Aquat. Toxicol. 2017, 183, 21–27. [Google Scholar] [CrossRef]
- Li, J.; Otero-Gonzalez, L.; Michiels, J.; Lens, P.N.L.; Du Laing, G.; Ferrer, I. Production of Selenium-Enriched Microalgae as Potential Feed Supplement in High-Rate Algae Ponds Treating Domestic Wastewater. Bioresour. Technol. 2021, 333, 125239. [Google Scholar] [CrossRef]
- Górska, S.; Maksymiuk, A.; Turło, J. Selenium-Containing Polysaccharides—Structural Diversity, Biosynthesis, Chemical Modifications and Biological Activity. Appl. Sci. 2021, 11, 3717. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.; Wang, C.; Yu, L.; Zhao, M.; Liu, X. Research Progress on the Biological Activities of Selenium Polysaccharides. Food Funct. 2020, 11, 4834–4852. [Google Scholar] [CrossRef]
- Qi, Z.; Duan, A.; Ng, K. Selenoproteins in Health. Molecules 2023, 29, 136. [Google Scholar] [CrossRef]
- de Alcantara, S.; Lopes, C.C.; Wagener, K. Controlled Introduction of Selenium into Chlorella Cells. Indian J. Exp. Biol. 1998, 36, 1286–1288. [Google Scholar] [PubMed]
- Zhang, Z.; Wang, L.; Wu, Y.; Li, C.; Fu, P.; Liu, J. Isolation and identification of green alga Chlorella vulgaris HNUFU001 for environmental selenium enrichment in heterotrophic growth regime. Algal Res. 2023, 75, 103299. [Google Scholar] [CrossRef]
- Viniarska, H.B.; Bodnar, O.I.; Vasilenko, O.V.; Stanislavchuk, A.V. Accumulation and Effects of Selenium and Zinc on Chlorella vulgaris Beij. (Chlorophyta) Metabolism. In Heavy Metals and Other Pollutants in the Environment; Apple Academic Press: Palm Bay, FL, USA, 2017; ISBN 978-1-315-36602-9. [Google Scholar]
- Ruiz, J.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs. Autotrophic Production of Microalgae: Bringing Some Light into the Everlasting Cost Controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Jareonsin, S.; Pumas, C. Advantages of Heterotrophic Microalgae as a Host for Phytochemicals Production. Front. Bioeng. Biotechnol. 2021, 9, 628597. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, A.P.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Filipiak, M.; Mungunkhuyag, K.; Jedynak, P.; Burczyk, J.; Fu, P.; Malec, P. Fast and Efficient Cadmium Biosorption by Chlorella Vulgaris K-01 Strain: The Role of Cell Walls in Metal Sequestration. Algal Res. 2021, 60, 102497. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Deruelles, J.; Rippka, R.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Pires, R.; Costa, M.; Silva, J.; Pedras, B.; Concórdio-Reis, P.; Lapa, N.; Ventura, M. Se-Enrichment of Chlorella Vulgaris Grown under Different Trophic States for Food Supplementation. Algal Res. 2022, 68, 102876. [Google Scholar] [CrossRef]
- Tonner, P.D.; Darnell, C.L.; Engelhardt, B.E.; Schmid, A.K. Detecting Differential Growth of Microbial Populations with Gaussian Process Regression. Genome Res. 2017, 27, 320–333. [Google Scholar] [CrossRef]
- Sun, M.; Liu, G.; Wu, Q. Speciation of Organic and Inorganic Selenium in Selenium-Enriched Rice by Graphite Furnace Atomic Absorption Spectrometry after Cloud Point Extraction. Food Chem. 2013, 141, 66–71. [Google Scholar] [CrossRef]
- Mylenko, M.; Vu, D.L.; Kuta, J.; Ranglová, K.; Kubáč, D.; Lakatos, G.; Grivalský, T.; Caporgno, M.P.; da Câmara Manoel, J.A.; Kopecký, J.; et al. Selenium Incorporation to Amino Acids in Chlorella Cultures Grown in Phototrophic and Heterotrophic Regimes. J. Agric. Food Chem. 2020, 68, 1654–1665. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Hlavová, M.; Zachleder, V.; Rucki, M.; Čížková, M. Glutathione Peroxidase Activity in the Selenium-Treated Alga Scenedesmus Quadricauda. Aquat. Toxicol. 2011, 102, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.O.; de Boer, K.; Gremmen, P.; Drinkwaard, A.; Wieggers, R.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Selenium Enrichment in the Marine Microalga Nannochloropsis Oceanica. Algal Res. 2021, 59, 102427. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Cao, X.; Wang, Y.; Si, Z.; Chen, Y. Toxic Effect and Bioaccumulation of Selenium in Green Alga Chlorella Pyrenoidosa. J. Appl. Phycol. 2019, 31, 1733–1742. [Google Scholar] [CrossRef]
- Morlon, H.; Fortin, C.; Floriani, M.; Adam, C.; Garnier-Laplace, J.; Boudou, A. Toxicity of Selenite in the Unicellular Green Alga Chlamydomonas Reinhardtii: Comparison between Effects at the Population and Sub-Cellular Level. Aquat. Toxicol. 2005, 73, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth Kinetic Models for Microalgae Cultivation: A Review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Babaei, A.; Ranglová, K.; Malapascua, J.R.; Masojídek, J. The Synergistic Effect of Selenium (Selenite, –SeO32−) Dose and Irradiance Intensity in Chlorella Cultures. AMB Express 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.P.; Ghahramani, Z.; Rasmussen, C.E. Gaussian Processes for Time-Marked Time-Series Data. In Proceedings of the 15th International Conference on Artificial Intelligence and Statistics, La Palma, Canary Islands, Spain, 21–23 April 2012; pp. 255–263. [Google Scholar]
- Morales-Sánchez, D.; Tinoco-Valencia, R.; Kyndt, J.; Martinez, A. Heterotrophic Growth of Neochloris oleoabundans Using Glucose as a Carbon Source. Biotechnol. Biofuels 2013, 6, 100. [Google Scholar] [CrossRef]
- Samejima, H.; Myers, J. On the Heterotrophic Growth of Chlorella pyrenoidosa. J. Gen. Microbiol. 1958, 18, 107–117. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Liu, T.; Gao, K.; Zhang, Q.; Cao, L.; Wang, Y.; Wu, X.; Zheng, H.; Peng, H.; et al. Heterotrophic Cultivation of Chlorella Vulgaris Using Broken Rice Hydrolysate as Carbon Source for Biomass and Pigment Production. Bioresour. Technol. 2021, 323, 124607. [Google Scholar] [CrossRef]
- Zhao, F.; Tan, X.; Zhang, Y.; Chu, H.; Yang, L.; Zhou, X. Effect of Temperature on the Conversion Ratio of Glucose to Chlorella Pyrenoidosa Cells: Reducing the Cost of Cultivation. Algal Res. 2015, 12, 431–435. [Google Scholar] [CrossRef]
- Choix, F.J.; Palacios, O.A.; Contreras, C.A.; Espinoza-Hicks, J.C.; Mondragón-Cortez, P.; Torres, J.R. Growth and Metabolism Enhancement in Microalgae Co-Cultured in Suspension with the Bacterium Azospirillum brasilense under Heterotrophic Conditions. J. Appl. Phycol. 2023, 35, 57–71. [Google Scholar] [CrossRef]
- Young, E.B.; Reed, L.; Berges, J.A. Growth Parameters and Responses of Green Algae across a Gradient of Phototrophic, Mixotrophic and Heterotrophic Conditions. PeerJ 2022, 10, e13776. [Google Scholar] [CrossRef]
- Yang, J.-C.; Huang, Y.-X.; Sun, H.; Liu, M.; Zhao, L.; Sun, L.-H. Selenium Deficiency Dysregulates One-Carbon Metabolism in Nutritional Muscular Dystrophy of Chicks. J. Nutr. 2023, 153, 47–55. [Google Scholar] [CrossRef]
- Bhadwal, S.; Sharma, S. Selenium Alleviates Carbohydrate Metabolism and Nutrient Composition in Arsenic Stressed Rice Plants. Rice Sci. 2022, 29, 385–396. [Google Scholar] [CrossRef]
- Bottino, N.R.; Banks, C.H.; Irgolic, K.J.; Micks, P.; Wheeler, A.E.; Zingaro, R.A. Selenium Containing Amino Acids and Proteins in Marine Algae. Phytochemistry 1984, 23, 2445–2452. [Google Scholar] [CrossRef]
- Kayrouz, C.M.; Huang, J.; Hauser, N.; Seyedsayamdost, M.R. Biosynthesis of Selenium-Containing Small Molecules in Diverse Microorganisms. Nature 2022, 610, 199–204. [Google Scholar] [CrossRef]
- Araie, H.; Shiraiwa, Y. Selenium Utilization Strategy by Microalgae. Molecules 2009, 14, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- Morlon, H.; Fortin, C.; Adam, C.; Garnier-Laplace, J. Selenite Transport and Its Inhibition in the Unicellular Green Alga Chlamydomonas Reinhardtii. Environ. Toxicol. Chem. 2006, 25, 1408–1417. [Google Scholar] [CrossRef]
- Ponton, D.E.; Graves, S.D.; Fortin, C.; Janz, D.; Amyot, M.; Schiavon, M. Selenium Interactions with Algae: Chemical Processes at Biological Uptake Sites, Bioaccumulation, and Intracellular Metabolism. Plants 2020, 9, 528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Y.; Hua, Y.; Chen, G.; Fu, P.; Liu, J. Heterotrophic Selenium Incorporation into Chlorella vulgaris K-01: Selenium Tolerance, Assimilation, and Removal through Microalgal Cells. Foods 2024, 13, 405. https://doi.org/10.3390/foods13030405
Zhang Z, Zhang Y, Hua Y, Chen G, Fu P, Liu J. Heterotrophic Selenium Incorporation into Chlorella vulgaris K-01: Selenium Tolerance, Assimilation, and Removal through Microalgal Cells. Foods. 2024; 13(3):405. https://doi.org/10.3390/foods13030405
Chicago/Turabian StyleZhang, Zhenyu, Yan Zhang, Yanying Hua, Guancheng Chen, Pengcheng Fu, and Jing Liu. 2024. "Heterotrophic Selenium Incorporation into Chlorella vulgaris K-01: Selenium Tolerance, Assimilation, and Removal through Microalgal Cells" Foods 13, no. 3: 405. https://doi.org/10.3390/foods13030405





